Chitooligosaccharide as A Possible Replacement for Sulfur Dioxide in Winemaking
Abstract
Featured Application
Abstract
1. Introduction
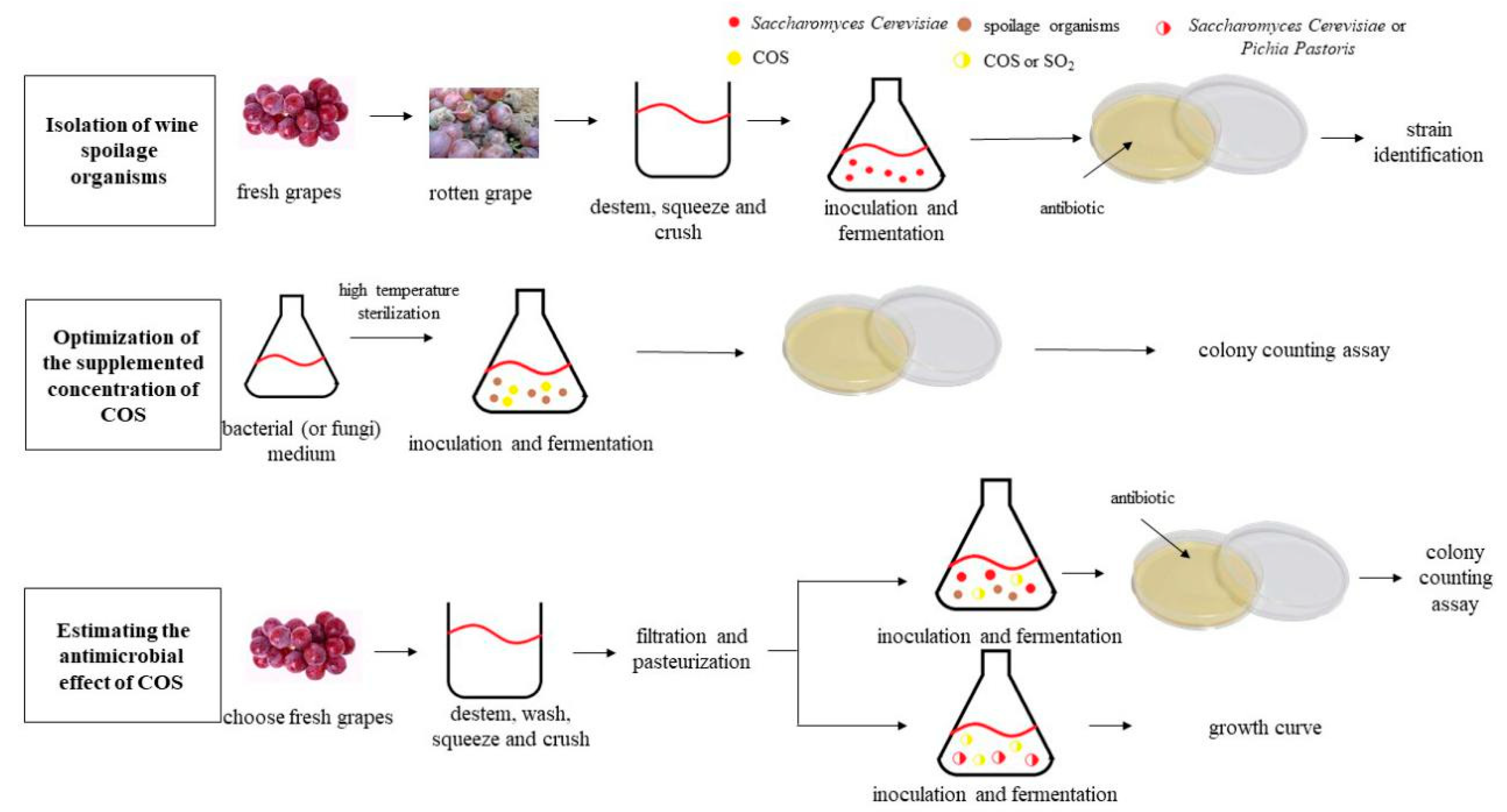
2. Materials and Methods
2.1. Must Preparation
2.2. Isolation of Wine Spoilage Organisms and Culture Conditions
2.3. Vinification
2.4. Colony-Counting Assay
2.5. Application of Known Spoilage Microorganisms to Vinification
2.6. Growing Curves of Saccharomyces Cerevisiae and PichiaPastoris
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Supplemented COS Concentration
3.2. Comparative Analysis of COS and SO2 Antimicrobial Effects
3.3. Estimating Antimicrobial Effectsof COS on Known Wine Spoilage Microorganisms
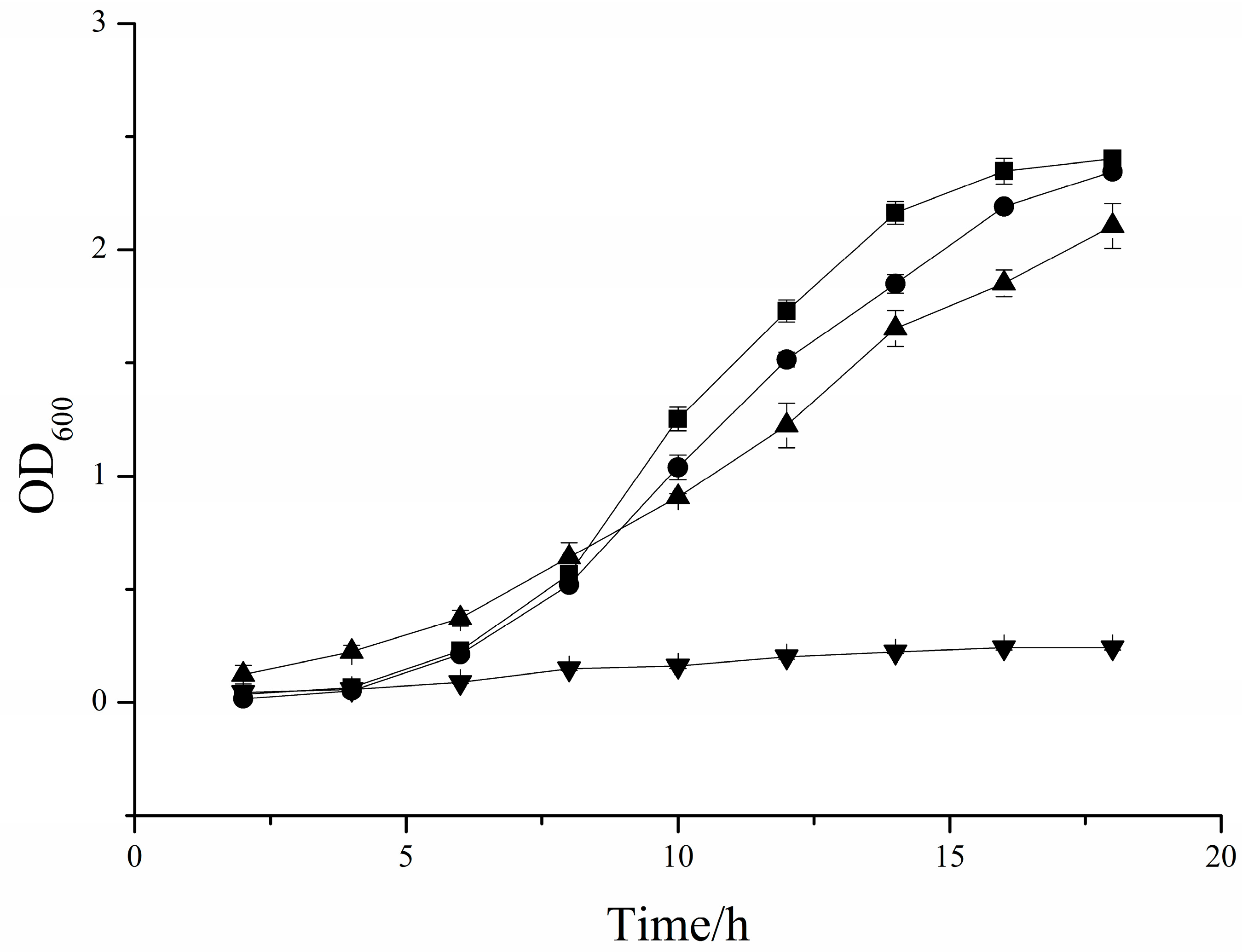
3.4. Effects of COS on Saccharomyces Cerevisiae and PichiaPastoris Cell Growths
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, T.M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons from Nature’s own arsenal-A review. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology: Volume 1. The Microbiology of Wine and Vinifications, 2nd ed.; Wiley: Chichester, UA, USA, 2006. [Google Scholar]
- Barril, C.; Rutledge, D.N.; Scollary, G.R. Ascorbic acid and white wine production: A review of beneficial versus detrimental impacts. Aust. J. Grape Wine Res. 2016, 22, 169–181. [Google Scholar] [CrossRef]
- Tromp, A.; Agenbach, W.A. Sorbic Acid as a Wine Preservative-Its Efficacy and Organoleptic Threshold. S. Afr. J. Enol. Vitic. 1981, 2, 1–5. [Google Scholar] [CrossRef][Green Version]
- Danilewicz, J.C. Role of Tartaric and Malic Acids in Wine Oxidation. J. Agric. Food Chem. 2014, 62, 5149–5155. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.D.; Holdstock, M.G.; Cowey, G.D. Potassium bitartrate crystallisation in wine and its inhibition. Aust. J. Grape Wine Res. 2015, 21, 627–641. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef]
- Rojo-Bezares, B.; Yolanda, S.; Zarazaga, M.; Torres, C.; Ruiz-Larrea, F. Antimicrobial activity of nisin against Oenococcus oeni and other wine bacteria. Int. J. Food Microbiol. 2007, 116, 32–36. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef]
- Badea, G.A.; Oana, A. Glutathione as a possible replacement of sulfur dioxide in winemaking technologies: A review. Sci. Pap. Ser. B Hortic. 2015, 59, 123–140. [Google Scholar]
- Sonni, F.; Chinnici, F.; Natali, N.; Riponi, C. Pre-fermentative replacement of sulphurdioxide by lysozyme andoenological tannins: Effect on the formation and evolution of volatile compounds during the bottle storage of white wines. Food Chem. 2011, 129, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Forns, M.; Ibarz, A. UV-vis irradiation: An alternative to reduce SO2 in white wines. LWT-Food Sci. Technol. 2013, 51, 59–64. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Cappelle, J.; Goncalves, F.J.; Rodrigues, A.; Saraiva, J.A.; Coimbra, M.A. Effect of high pressure treatments on the physicochemical properties of a sulphur dioxide-free red wine. Food Chem. 2013, 141, 2558–2566. [Google Scholar] [CrossRef]
- Santos, M.C.; Cláudia, N.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Kim, S.K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohyd. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Mengíbar, M.; Mateos-Aparicio, I.; Miralles, B.; Heras, Á. Influence of thephysico-chemical characteristics of chito-oligosaccharides (COS) on antioxidant activity. Carbohyd. Polym. 2013, 97, 776–782. [Google Scholar] [CrossRef]
- Singh, P. Effect of chitosans and chitooligosaccharides on the processing and storage quality of foods of animal and aquatic origin: A review. Food Sci. Nutr. 2016, 46, 51–81. [Google Scholar] [CrossRef]
- Yang, F.; Luan, B.; Sun, Z.; Yang, C.; Yu, Z.M.; Li, X.Z. Application of chitooligosaccharides as antioxidants in beer to improve the flavour stability by protecting against beer staling during storage. Biotechnol. Lett. 2017, 39, 305–310. [Google Scholar] [CrossRef]
- Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.-B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J. Appl. Microbiol. 2014, 118, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.S.; Ngo, D.H.; Bach, L.G.; Ngo, D.N.; Kim, S.K. The free radical scavenging and anti-inflammatory activities of gallate-chitooligosaccharides in human lung epithelial A549 cells. Process. Biochem. 2017, 54, 188–194. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg(II) and Pb(II) Ions on Chitosan-Iron(III) from Aqueous Solutions: Single and Binary Systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Lapo, B.; Demey, H.; Carchi, T.; Sastre, A.M. Antimony Removal from Water by a Chitosan-Iron(III) [ChiFer(III)] Biocomposite. Polymers 2019, 11, 351. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Nogueras, M.; Sastre, A.M.; Guibal, E. Boron recovery from seawater with a new low-cost adsorbent material. Chem. Eng. J. 2014, 254, 463–471. [Google Scholar]
- Soleri, R.; Demey, H.; Tria, S.A.; Guiseppi-Elie, A.; IBN Had Hassine, A.; Gonzalez, C.; Bazin, I. Peptide conjugated chitosan foam as a novel approach for capture-purification and rapid detection of hapten—Example of ochratoxin A. Biosens. Bioelectron. 2015, 67, 634–641. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, Ó.S.; Monteiro, M.J.; Pintado, M.E.; Malcata, F.X. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Fonseca, S.C.; Ramos, Ó.S.; Pintado, M.E.; Malcata, F.X. In vitro screening for anti-microbial activity of chitosans and chitooligosaccharides, aiming at potential uses in functional textiles. J. Microbiol. Biotechn. 2010, 20, 311–318. [Google Scholar] [CrossRef]
- Mei, Y.X.; Dai, X.Y.; Yang, W.; Xu, X.W.; Liang, Y.X. Antifungal activity of chitooligosaccharides against the dermatophyte Trichophyton rubrum. Int. J. Biol. Macromol. 2015, 77, 330–335. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hjeljord, L.G.; Aam, B.B.; Sørlie, M.; Tronsmo, A. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur. J. Plant. Pathol. 2015, 141, 147–158. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharm. Sin. 2004, 25, 932–936. [Google Scholar]
- Malfeito-ferreira, M. Wine spoilage yeasts and bacteria. In Encyclopedia of Food Microbiology, 1st ed.; Carl, B., Carl, A.B., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 3, pp. 805–810. [Google Scholar]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
| Wine Spoilage Organism | Colony Count (CFU/mL) | |||
|---|---|---|---|---|
| No COS | 100 mg/L COS | 500 mg/L COS | 1000 mg/L COS | |
| Enterococcus | (1.46 ± 0.59) × 108 | (1.15 ± 0.54) × 108 | (1.81 ± 0.45) × 104 | (5.30 ± 2.10) × 102 |
| Bacillus cereus | (2.39 ± 1.42) × 108 | (6.45 ± 2.12) × 107 | (1.61 ± 0.93) × 103 | (1.99 ± 0.30) × 102 |
| Paenibacillus | (1.71 ± 1.03) × 108 | (6.16 ± 2.58) × 107 | (8.47 ± 3.12) × 103 | (1.73 ± 0.65) × 102 |
| Alkaliphilus | (1.83 ± 0.77) × 107 | (4.24 ± 1.31) × 106 | (4.92 ± 1.63) × 102 | 0 |
| Pantoea sp. | (2.20 ± 1.25) × 108 | (1.10 ± 0.20) × 107 | (1.85 ± 0.51) × 105 | (9.33 ± 6.66) × 102 |
| Lactococcus | (1.77 ± 0.12) × 107 | (1.55 ± 0.31) × 107 | (5.67 ± 2.73) × 102 | 0 |
| Aspergillus flavus | (1.61 ± 0.76) × 108 | (1.93 ± 0.66) × 107 | (1.89 ± 0.74) × 103 | (1.23 ± 0.45) × 102 |
| Aureobasidium pullulans | (2.10 ± 0.41) × 106 | (5.88 ± 1.81) × 105 | (6.43 ± 3.41) × 103 | (7.33 ± 3.66) × 101 |
| Penicillium | (1.52 ± 0.15) × 107 | (8.51 ± 3.17) × 106 | (6.87 ± 2.54) × 104 | (2.55 ± 0.67) × 103 |
| Additive | Concentration (mg/L) | Colony Count (CFU/mL) |
|---|---|---|
| / | / | (2.17 ± 0.32) × 107 |
| COS | 500 | (1.73 ± 0.57) × 104 |
| SO2 | 50 | (1.86 ± 0.21) × 107 |
| 100 | (1.34 ± 0.31) × 104 | |
| 200 | (1.43 ± 0.38) × 103 |
| Wine Spoilage Organisms | Colony Count (CFU/mL) | ||
|---|---|---|---|
| No COS | 500 mg/L COS | 100 mg/L SO2 | |
| Enterococcus | (1.81 ± 0.39) × 103 | (6.60 ± 2.05) × 102 | (1.6 ± 0.47) × 102 |
| Bacillus cereus | (1.49 ± 0.62) × 103 | (5.90 ± 1.93) × 102 | (6.33 ± 0.30) × 102 |
| Paenibacillus | (2.14 ± 1.01) × 103 | (3.20 ± 1.06) × 102 | (5.50 ± 3.69) × 102 |
| Alkaliphilus | (1.94 ± 0.37) × 103 | (1.92 ± 0.74) × 102 | (5.20 ± 2.28) × 101 |
| Pantoea sp. | (1.10 ± 0.20) × 102 | (3.33 ± 1.52) × 101 | (4.96 ± 1.30) × 101 |
| Lactococcus | (1.77 ± 0.52) × 104 | (1.83 ± 0.09) × 102 | (9.03 ± 0.22) × 103 |
| Aspergillus flavus | (1.39 ± 0.16) × 103 | (6.56 ± 2.81) × 102 | (9.96 ± 2.25) × 102 |
| Aureobasidium pullulans | (7.10 ± 3.11) × 103 | (2.10 ± 0.71) × 102 | (2.33 ± 0.45) × 102 |
| Penicillium | (1.63 ± 0.45) × 103 | (2.26 ± 0.59) × 102 | (8.13 ± 0.15) × 102 |
| Colony Count (CFU/mL) | 24 h | 48 h | 72 h | 96 h | 120 h | |
|---|---|---|---|---|---|---|
| Lactobacillus plantarum | / | (1.54 ± 0.52) × 106 | (2.74 ± 0.58) × 107 | 0 | − | − |
| COS | (1.77 ± 0.41) × 105 | (5.08 ± 1.50) × 105 | 0 | − | − | |
| SO2 | (1.98 ± 0.23) × 104 | (5.93 ± 2.23) × 105 | 0 | − | − | |
| Acetobacter aceti | / | (5.83 ± 2.67) × 106 | (9.10 ± 1.73) × 107 | (4.33 ± 1.96) × 108 | (1.60 ± 0.16) × 102 | 0 |
| COS | (1.95 ± 0.18) × 104 | (1.24 ± 0.10) × 105 | (5.57 ± 0.97) × 106 | (6.83 ± 1.76) × 101 | 0 | |
| SO2 | (1.61 ± 0.45) × 105 | (3.13 ± 1.35) × 106 | (2.69 ± 0.45) × 107 | (6.96 ± 1.91) × 101 | 0 | |
| Pediococcus damnosus | / | (1.83 ± 0.23) × 106 | (9.23 ± 3.95) × 106 | (2.85 ± 0.24) × 107 | (7.70 ± 1.25) × 101 | 0 |
| COS | (6.47 ± 3.88) × 104 | (2.23 ± 0.51) × 105 | (7.50 ± 1.27) × 105 | (1.80 ± 0.43) × 101 | 0 | |
| SO2 | (1.16 ± 0.47) × 104 | (2.72 ± 0.62) × 105 | (9.00 ± 1.92) × 105 | (4.23 ± 1.02) × 101 | 0 | |
| Lactobacillus brevis | / | (9.73 ± 1.19) × 105 | (9.10 ± 2.46) × 106 | (1.04 ± 0.59) × 102 | 0 | − |
| COS | (2.75 ± 0.37) × 104 | (1.78 ± 0.58) × 105 | (2.33 ± 0.77) × 101 | 0 | − | |
| SO2 | (5.37 ± 2.94) × 103 | (2.43 ± 0.59) × 105 | (2.93 ± 1.38) × 101 | 0 | − |
| Replacement | Experimental Conditions | Oenological Parameter | Studied Property | |||||
|---|---|---|---|---|---|---|---|---|
| Antimicrobial | Antimicrobial | Antimicrobial | Antimicrobial | Antimicrobial | Antimicrobial | |||
| Addition | Dimethyl dicarbonate [8] | Supplemented concentration of DMDC containing 25, 50 and 100 mg/L; Comparison between added SO2 with different concentrations in wine before bottling. | ||||||
| Bacteriocin [9] | MRS agar for lactic acid bacteria;MLOagar forO. oeni; Mannitol agar plates, 5 g/L yeast extract, 3 g/L peptone, and 15 g/L agar for acetic acid bacteria; MIC, MBC, MIC50, MIC90, MBC50, and MBC90 | |||||||
| Phenolic compounds [10] | Control group, 160 mg/L SO2; Winery-scale trial | O. oeni, L. hilgardii, and P. pentosaceus; Cultures prepared by adding ethanol; IC50, MIC, and MBC; OD600; Electron microscopy | Dipyridyl method;2,2′-azino-bis(3-ethylbenzthiazoline-6- sulphonic) acid (ABTS) method | Major alcohols, esters, and acids; Esters, alcohols, terpenes, C13-norisoprenoids, acids, volatile phenols, lactones, furanic compounds, and vanillin compounds | Tasting at the end of alcoholic fermentation | |||
| Lysozyme [12] | SO2 addition 120 mg/L; No sulphur addition in control tests; Low=SO2-production yeast used; Studied at alcoholic fermentation followed by malolactic fermentation one month later; Must at crushing, wine during and after alcoholic and malolactic fermentation | pH, density, absorbance at 420 nm, total polyphenol index, alcoholic strength, and dry extract | Volatile acids; WLN agar medium to enumerate yeast and MRS supplemented with 20% apple juice to enumerate lactic acid bacteria; IC50; Viable microorganism counts were obtained by the number of CFU per mL | Oxygen radical absorbance capacity (ORAC) | Major alcohols, esters, and acids. | Tasting one week after bottling and after two-month storage; The Expert panel conducted a descriptive analysis of each wine; Triangle difference tests were performed; Panellists were given an option to comment on differences observed in the wines. | ||
| COS | The optimized concentration is 500mg/L; The compared additive is 100mg/L SO2. | -CFUs in Enterococcus, Bacillus cereus, Paenibacillus, Alkaliphilus, Pantoea sp., Lactococcus, Aspergillus flavus, Aureobasidium pullulans, Penicillium; CFUsin Lactobacillus plantarum, Acetobacter aceti, Pediococcus; damnosus, Lactobacillus brevis; Growth curves of Saccharomyces cerevisiae and Pichia pastoris | ||||||
| Physical methods | Pulsed electric fields [14] | SO2 addition 40 mg/L; No sulphur addition in control tests; 12 months of ageing after bottling | pH, total acidity, volatile acidity, sugars, and ethanol; -Colour intensity, total anthocyanins, and total polyphenol index; CIELab coordinates; | Dekkera anomala, D. bruxellensis, L. hilgardii, and Lactobacillus plantarum; Response variable (S) in experimental designs | Along the time of storage, the wines showed different evolutionS of total phenolics and antioxidant activity values; Folin–Ciocalteu method | Alcohols, esters, and acids. | Color intensity, anthocyanin content, and total polyphenol index | Tasting nine months after bottling |
| Ultraviolet [13] | SO2 addition 50 mg/L; Fresh and frozen must; Produced wine. | NIR multiparametric analysis; Tartaric acid, alcoholic degree, and volatile acidity; Absorbance spectrum | Density | Matrix color influenced the antioxidant analysis. | ||||
| High hydrostatic pressure [15] | Growth of D. bruxellensis using a selective/differential medium; Analysis of 4-ethylphenol by GC EMS | Anthocyanins and proanthocianins by HPLC-DAD-TQD | Analysis of volatile compounds by GCFID | |||||
| Ultrasound [14] | Laboratory scale | pH, total acidity, volatile acidity, sugar, and ethanol | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Zhang, Y.; Sun, Z.; Li, X. Chitooligosaccharide as A Possible Replacement for Sulfur Dioxide in Winemaking. Appl. Sci. 2020, 10, 578. https://doi.org/10.3390/app10020578
Hao Z, Zhang Y, Sun Z, Li X. Chitooligosaccharide as A Possible Replacement for Sulfur Dioxide in Winemaking. Applied Sciences. 2020; 10(2):578. https://doi.org/10.3390/app10020578
Chicago/Turabian StyleHao, Zhenming, Yanrong Zhang, Zhen Sun, and Xianzhen Li. 2020. "Chitooligosaccharide as A Possible Replacement for Sulfur Dioxide in Winemaking" Applied Sciences 10, no. 2: 578. https://doi.org/10.3390/app10020578
APA StyleHao, Z., Zhang, Y., Sun, Z., & Li, X. (2020). Chitooligosaccharide as A Possible Replacement for Sulfur Dioxide in Winemaking. Applied Sciences, 10(2), 578. https://doi.org/10.3390/app10020578





