A Mechanism of Gold Nanoparticle Aggregation by Immunoglobulin G Preparation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining GNPs
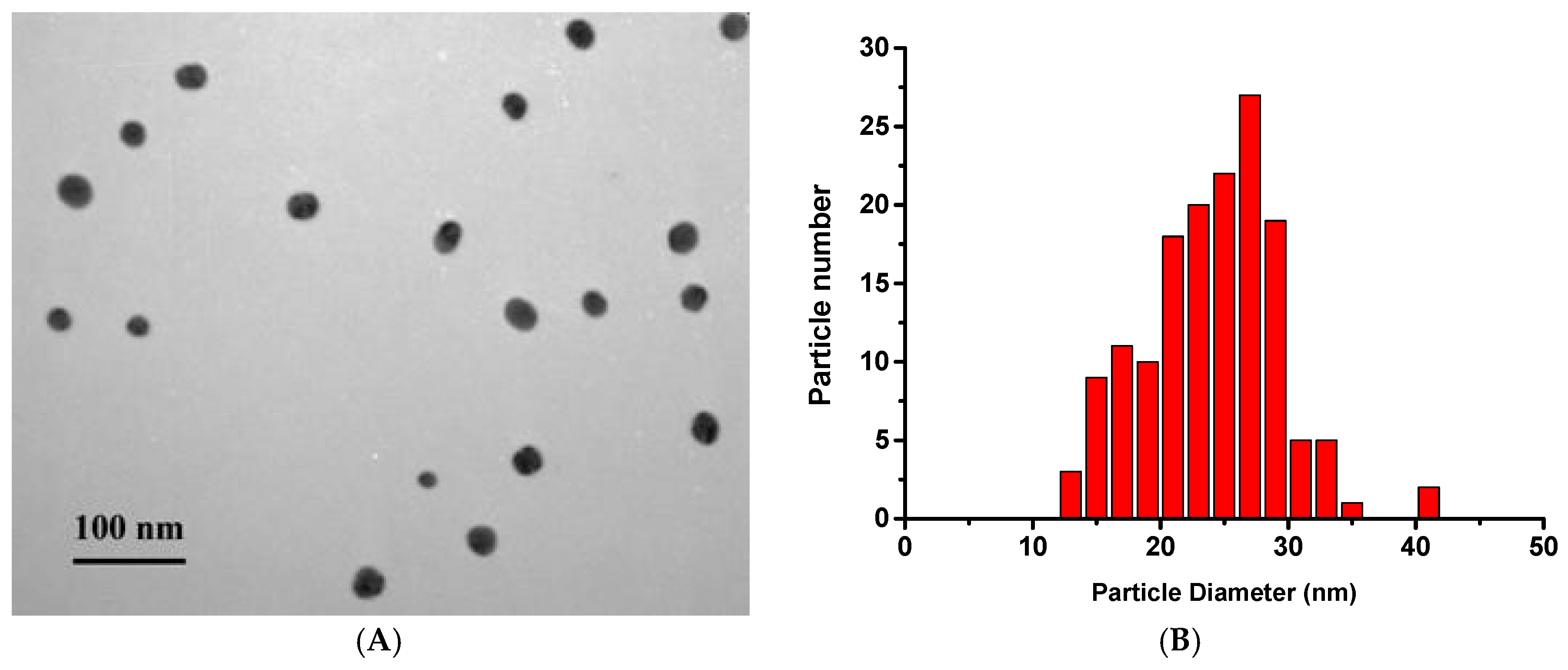
2.2. Determination of GNP Sizes by Transmission Electron Microscopy
2.3. Preparation and Purification of Monoclonal Antibodies
2.4. Obtaining of Flocculation Dependence
2.5. Immobilization of Antibodies on GNPs for Immunochromatography
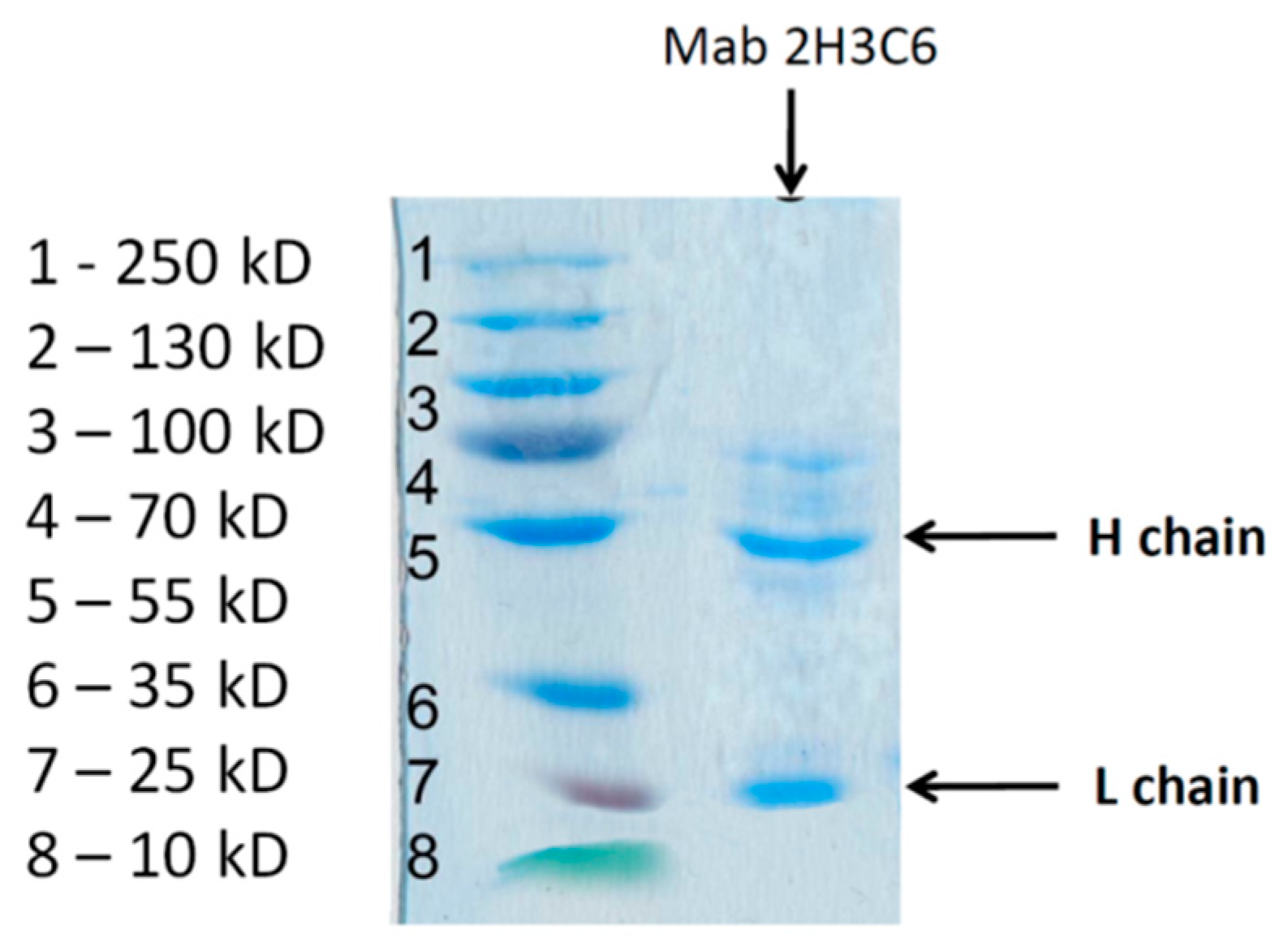
2.6. SDS Electrophoresis in Polyacrylamide Gel
2.7. Characterization of the Proteins Solutions by Asymmetrical Flow Field-Flow Fractionation (AF4)
2.8. Production of Immunochromatographic Test Systems
3. Results and Discussion
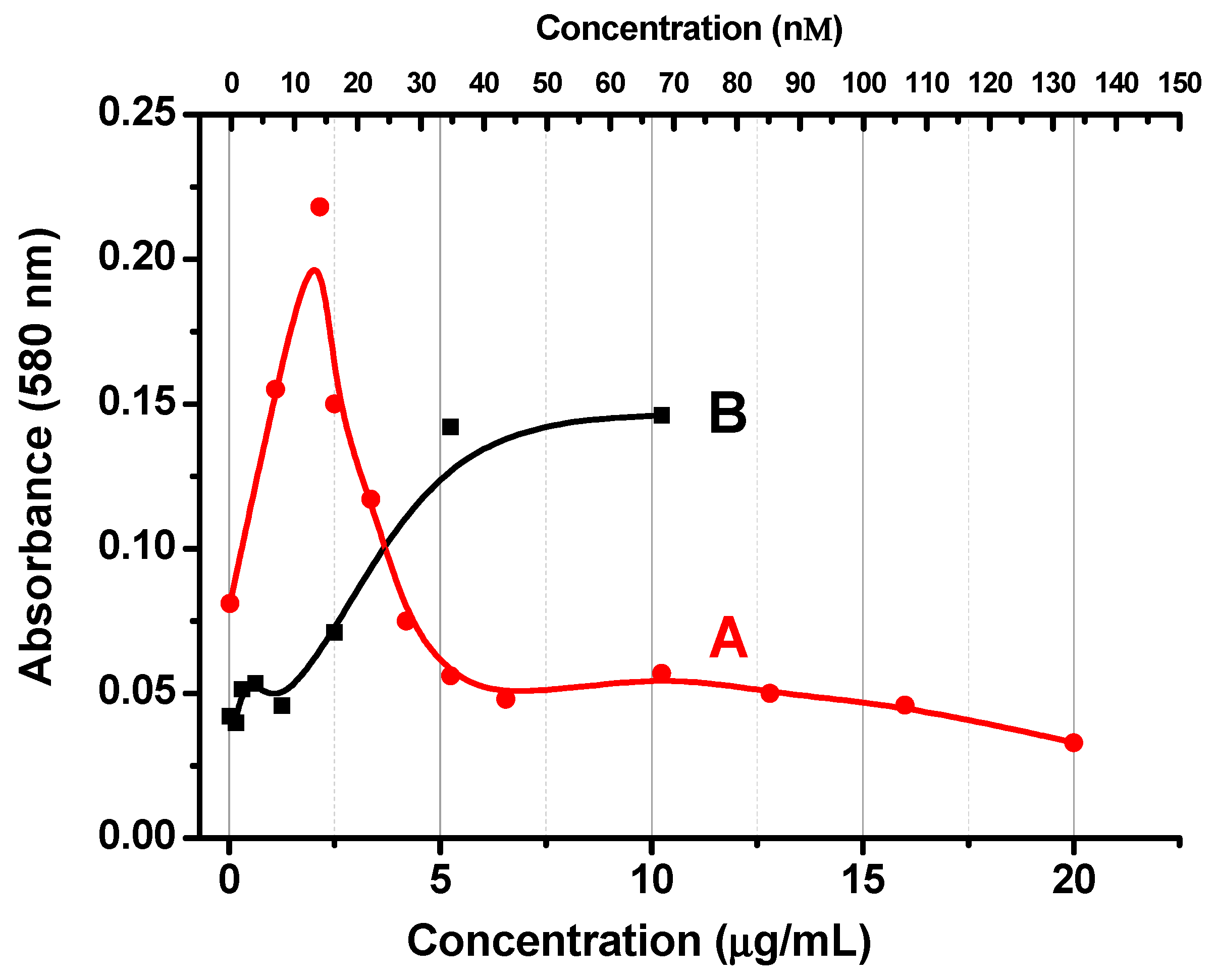
3.1. Flocculation Dependence
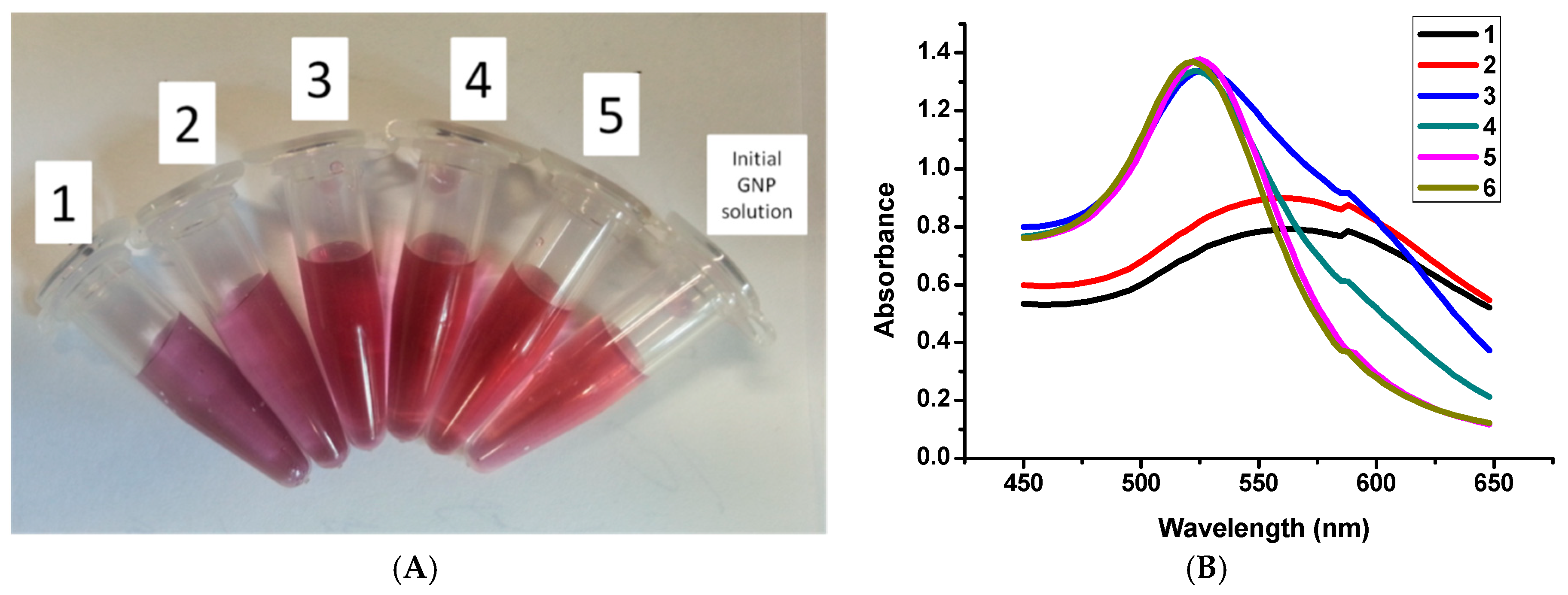
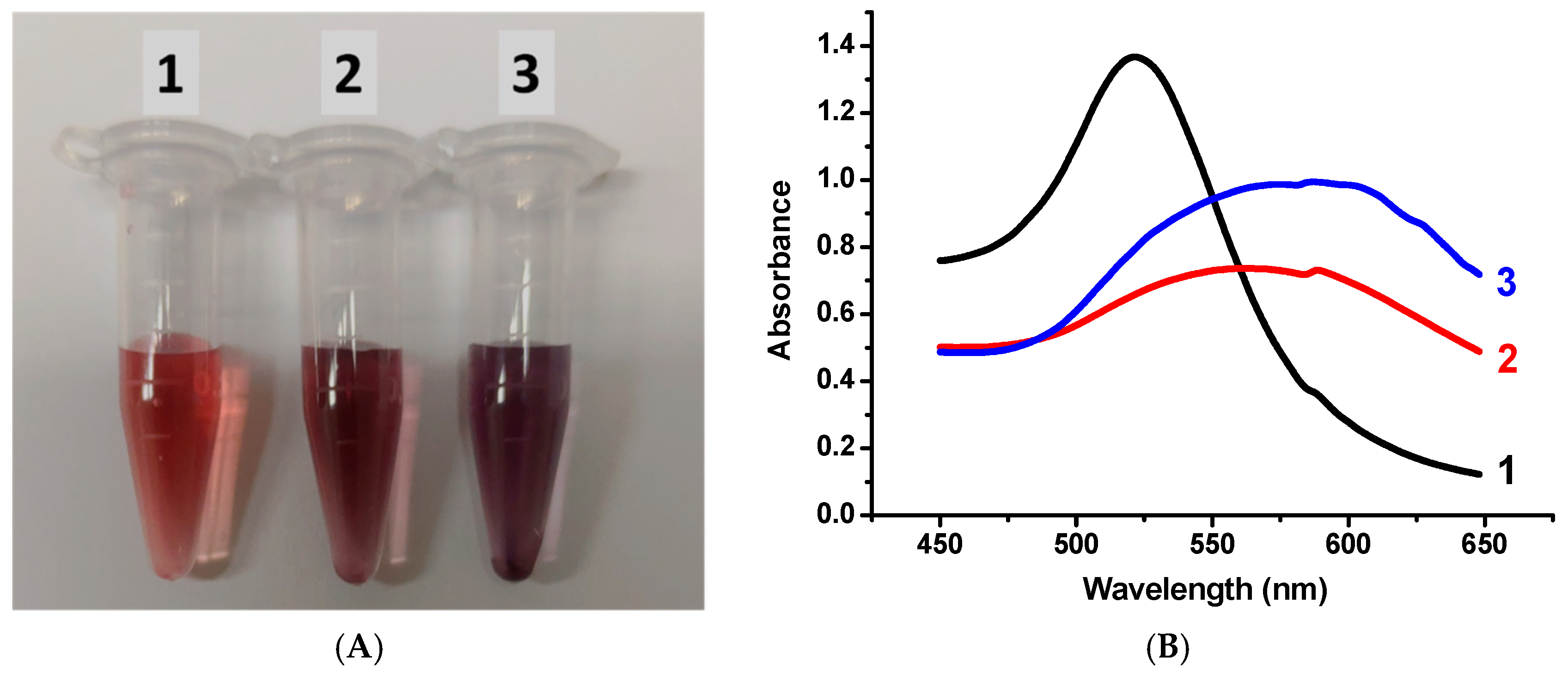
3.2. Centrifugation and Optical Spectroscopy
3.3. SDS Electrophoresis in Polyacrylamide Gel
3.4. Asymmetrical Flow Field-Flow Fractionation
3.5. Aggregation Stability of GNPs with Various Fractions of the 2H3C6 Preparation
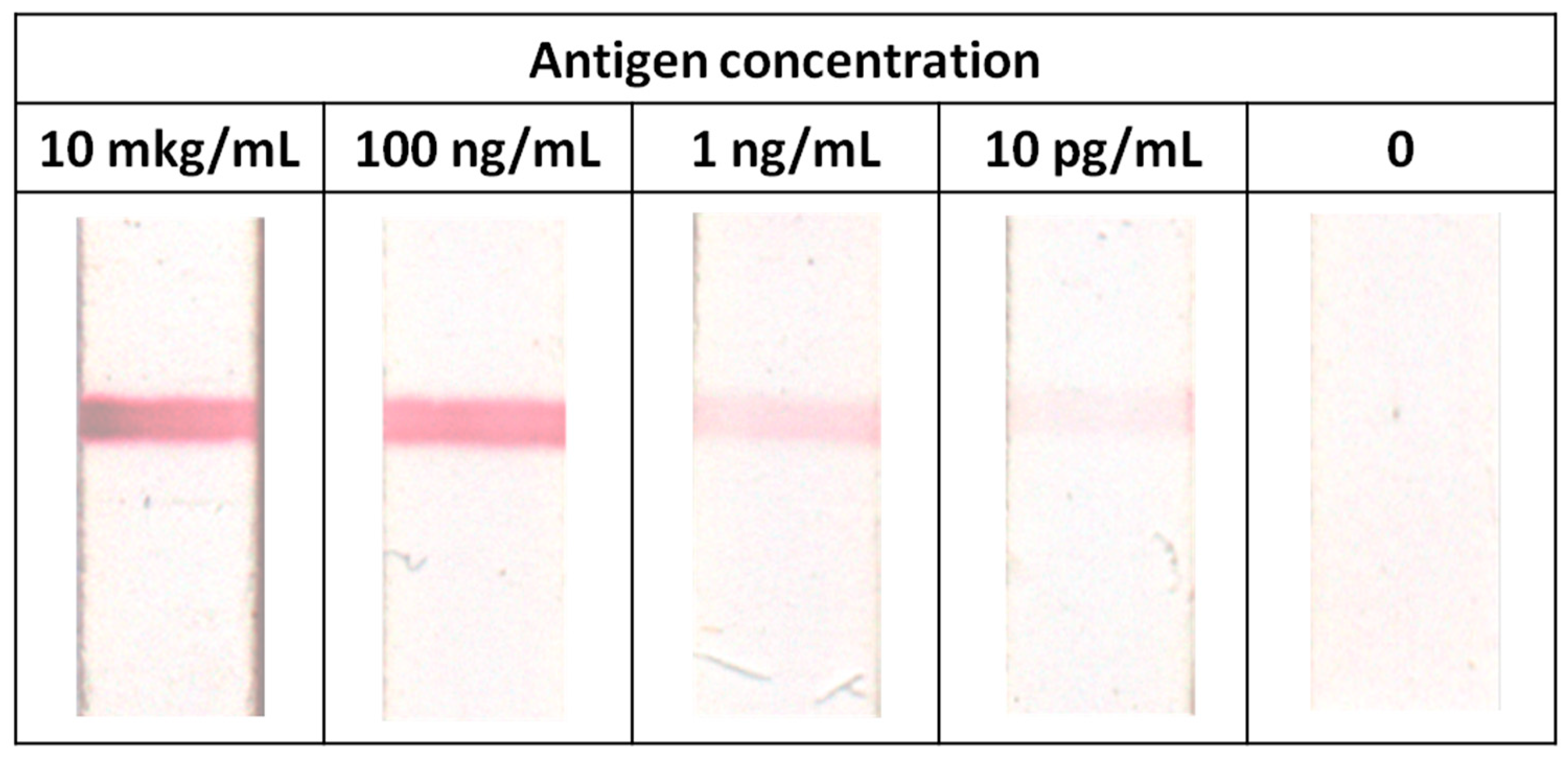
3.6. Immunochromatography
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Golchin, K.; Golchin, J.; Ghaderi, S.; Alidadiani, N.; Eslamkhah, S.; Eslamkhah, M.; Davaran, S.; Akbarzadeh, A. Gold nanoparticles applications: From artificial enzyme till drug delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 250–254. [Google Scholar] [CrossRef]
- Verma, M.S.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015, 33, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Anniebell, S.; Gopinath, S.C. Polymer conjugated gold nanoparticles in biomedical applications. Curr. Medic. Chem. 2018, 25, 1433–1445. [Google Scholar] [CrossRef]
- Zeng, S.W.; Yong, K.T.; Roy, I.; Dinh, X.P.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Jeong, H.H.; Choi, E.; Ellis, E.; Lee, T.C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Arruebo, M.; Valladares, M.; González-Fernández, Á. Antibody-conjugated nanoparticles for biomedical applications. J. Nanomater. 2009, 2009, 37. [Google Scholar] [CrossRef]
- Nobs, L.; Buchegger, F.; Gurny, R.; Allémann, E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J. Pharm. Sci. 2004, 93, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Sellers, H.; Ulman, A.; Shnidman, Y.; Eilers, J.E. Structure and binding of alkanethiolates on gold and silver surfaces: Implications for self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 9389–9401. [Google Scholar] [CrossRef]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2009, 4, 365–379. [Google Scholar] [CrossRef]
- Sen, T.; Haldar, K.K.; Patra, A. Au nanoparticle-based surface energy transfer probe for conformational changes of BSA protein. J. Phys. Chem. C 2008, 112, 17945–17951. [Google Scholar] [CrossRef]
- Xiulan, S.; Xiaolian, Z.; Jian, T.; Zhou, J.; Chu, F.S. Preparation of gold-labeled antibody probe and its use in immunochromatography assay for detection of aflatoxin B1. Int. J. Food Microbiol. 2005, 99, 185–194. [Google Scholar] [CrossRef]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef]
- Filbrun, S.L.; Filbrun, A.B.; Lovato, F.L.; Oh, S.H.; Driskell, E.A.; Driskell, J.D. Chemical modification of antibodies enables the formation of stable antibody–gold nanoparticle conjugates for biosensing. Analyst 2017, 142, 4456–4467. [Google Scholar] [CrossRef] [PubMed]
- Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Progress in rapid optical assays for heavy metal ions based on the use of nanoparticles and receptor molecules. Microchim. Acta 2019, 186, 172. [Google Scholar] [CrossRef] [PubMed]
- Iarossi, M.; Schiattarella, C.; Rea, I.; De Stefano, L.; Fittipaldi, R.; Vecchione, A.; Ventura, B.D. Colorimetric immunosensor by aggregation of photochemically functionalized gold nanoparticles. ACS Omega 2018, 3, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Lizoń, A.; Wytrwal-Sarna, M.; Gajewska, M.; Drożdż, R. Silver nanoparticle-based assay for the detection of immunoglobulin free light chains. Materials 2019, 12, 2981. [Google Scholar] [CrossRef]
- Busch, R.T.; Karim, F.; Weis, J.; Sun, Y.; Zhao, C.; Vasquez, E.S. Optimization and structural stability of gold nanoparticle–antibody bioconjugates. ACS Omega 2019, 4, 15269–15279. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L. Gold nanoparticle probes. Coord. Chem. Rev. 2009, 253, 1607–1618. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: New York, NY, USA, 2013; p. 1200. ISBN 9780123822390. [Google Scholar]
- Lai, Y.H.; Koo, S.; Oh, S.H.; Driskell, E.A.; Driskell, J.D. Rapid screening of antibody–antigen binding using dynamic light scattering (DLS) and gold nanoparticles. Anal. Methods 2015, 7, 7249–7255. [Google Scholar] [CrossRef]
- Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Factors influencing the detection limit of the lateral-flow sandwich immunoassay: A case study with potato virus X. Anal. Bioanal. Chem. 2012, 403, 1595–1605. [Google Scholar] [CrossRef]
- Gaza-Bulseco, G.; Liu, H. Fragmentation of a recombinant monoclonal antibody at various pH. Pharm. Res. 2008, 25, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, D.; Alessandri, L.; Piparia, R.; Aikhoje, A.; Chin, A.; Radziejewski, C.; Correia, I. Elevated cleavage of human immunoglobulin gamma molecules containing a lambda light chain mediated by iron and histidine. Anal. Biochem. 2009, 389, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Diemel, R.V.; Ter Hart, H.G.; Derksen, G.J.; Koenderman, A.H.; Aalberse, R.C. Characterization of immunoglobulin G fragments in liquid intravenous immunoglobulin products. Transfusion 2005, 45, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Vlasak, J.; Ionescu, R. Fragmentation of monoclonal antibodies. MAbs 2011, 3, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; May, K. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012, 4, 17–23. [Google Scholar] [CrossRef]
- Acres, R.G.; Feyer, V.; Tsud, N.; Cadino, E.; Prince, K.C. Mechanisms of aggregation of cysteine functionalized gold nanoparticles. J. Phys. Chem. C 2014, 118, 10481–10487. [Google Scholar] [CrossRef]
- Doyen, M.; Goole, J.; Bartik, K.; Bruylants, G. Amino acid induced fractal aggregation of gold nanoparticles: Why and how. J. Colloid Interface Sci. 2016, 464, 160–166. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ruzgas, T.; Ramanavicius, A.; Ramanaviciene, A. Antibody fragment immobilization on planar gold and gold nanoparticle modified quartz crystal microbalance with dissipation sensor surfaces for immunosensor applications. Anal. Methods 2014, 6, 2134–2140. [Google Scholar] [CrossRef]
- Stobiecka, M.; Hepel, M. Effect of buried potential barrier in label-less electrochemical immunodetection of glutathione and glutathione-capped gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3524–3530. [Google Scholar] [CrossRef]
- Stobiecka, M.; Chalupa, A.; Dworakowska, B. Piezometric biosensors for anti-apoptotic protein survivin based on buried positive-potential barrier and immobilized monoclonal antibodies. Biosens. Bioelectron. 2016, 84, 37–43. [Google Scholar] [CrossRef]
- Goossens, J.; Sein, H.; Lu, S.; Radwanska, M.; Muyldermans, S.; Sterckx, Y.G.J.; Magez, S. Functionalization of gold nanoparticles with nanobodies through physical adsorption. Anal. Methods 2017, 9, 3430–3440. [Google Scholar] [CrossRef]
- Bradbury, A.; Plückthun, A. Reproducibility: Standardize antibodies used in research. Nature 2015, 518, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Tieman, B.; Moore, J.; Pan, Y.; Muerhoff, A.S. Myeloma-derived light chain paired with a diagnostic monoclonal antibody hinders immunoassay performance. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 113–118. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotnikov, D.V.; Safenkova, I.V.; Zherdev, A.V.; Avdienko, V.G.; Kozlova, I.V.; Babayan, S.S.; Gergert, V.Y.; Dzantiev, B.B. A Mechanism of Gold Nanoparticle Aggregation by Immunoglobulin G Preparation. Appl. Sci. 2020, 10, 475. https://doi.org/10.3390/app10020475
Sotnikov DV, Safenkova IV, Zherdev AV, Avdienko VG, Kozlova IV, Babayan SS, Gergert VY, Dzantiev BB. A Mechanism of Gold Nanoparticle Aggregation by Immunoglobulin G Preparation. Applied Sciences. 2020; 10(2):475. https://doi.org/10.3390/app10020475
Chicago/Turabian StyleSotnikov, Dmitriy V., Irina V. Safenkova, Anatoly V. Zherdev, Vadim G. Avdienko, Irina V. Kozlova, Suren S. Babayan, Vladislav Ya. Gergert, and Boris B. Dzantiev. 2020. "A Mechanism of Gold Nanoparticle Aggregation by Immunoglobulin G Preparation" Applied Sciences 10, no. 2: 475. https://doi.org/10.3390/app10020475
APA StyleSotnikov, D. V., Safenkova, I. V., Zherdev, A. V., Avdienko, V. G., Kozlova, I. V., Babayan, S. S., Gergert, V. Y., & Dzantiev, B. B. (2020). A Mechanism of Gold Nanoparticle Aggregation by Immunoglobulin G Preparation. Applied Sciences, 10(2), 475. https://doi.org/10.3390/app10020475







