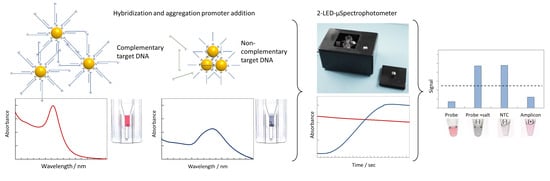
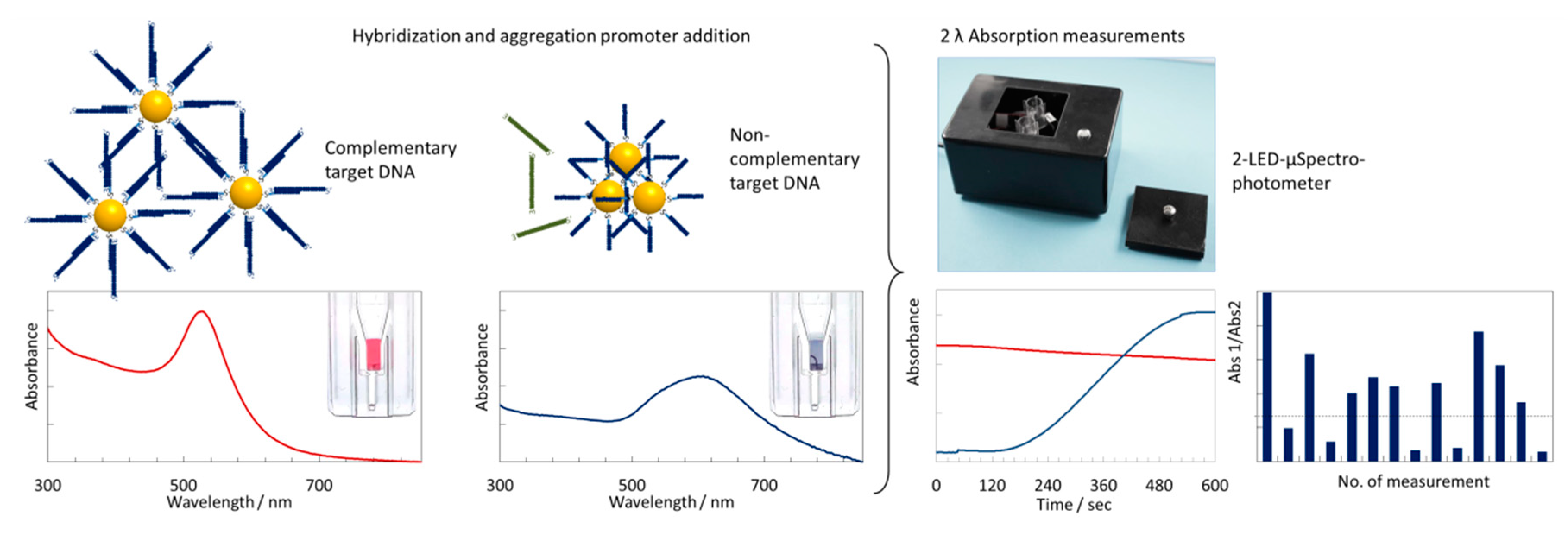
2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays
Abstract
1. Introduction
2. Materials and Methods
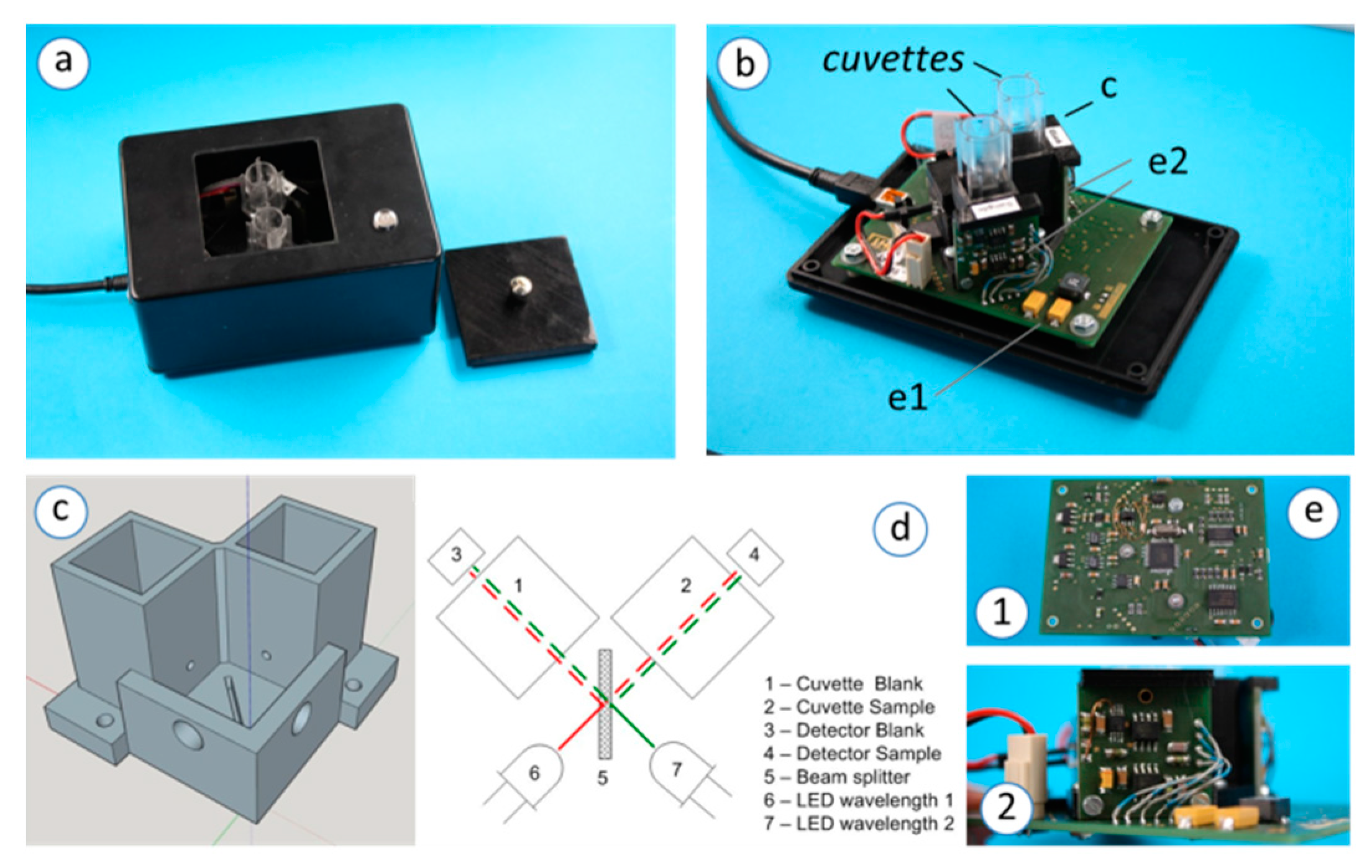
2.1. 2-LED-µSpectrophotometer Set-Up
2.2. Chemicals, Reagents, and Samples
2.2.1. Synthesis of AuNPs
2.2.2. Bacterial Strains and Legionella Isolates
2.2.3. Preparation of AuNPs Probes
2.2.4. LAMP Assay Design and Evaluation
2.2.5. Colorimetric Assay
3. Results
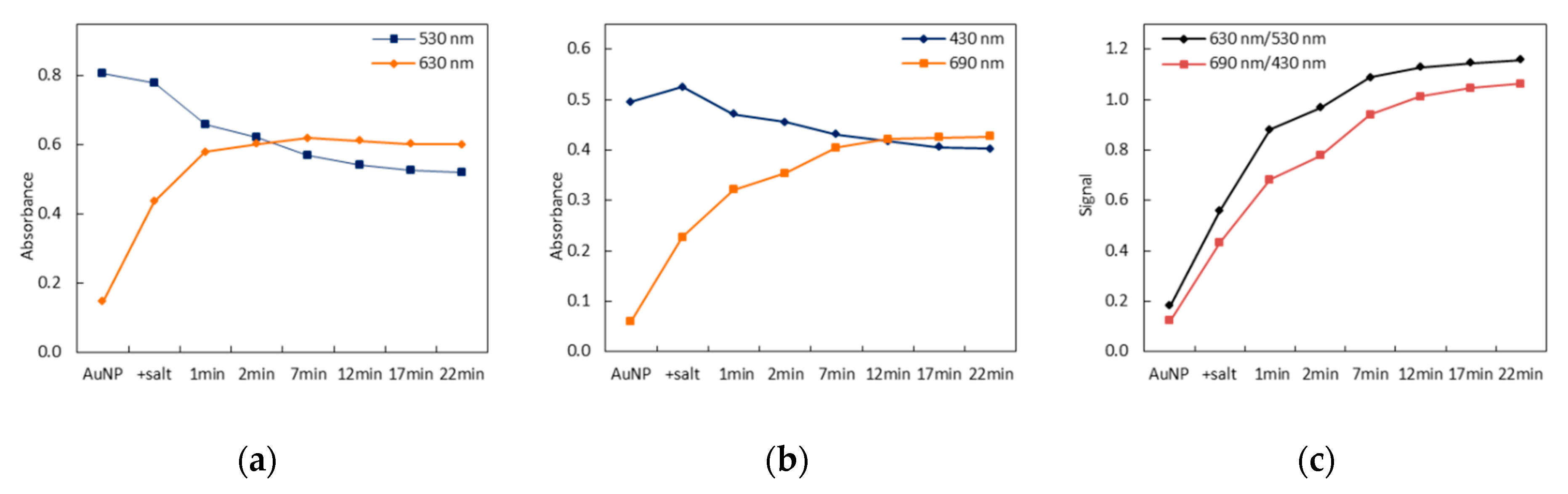
3.1. Principle of the Sensing Strategy
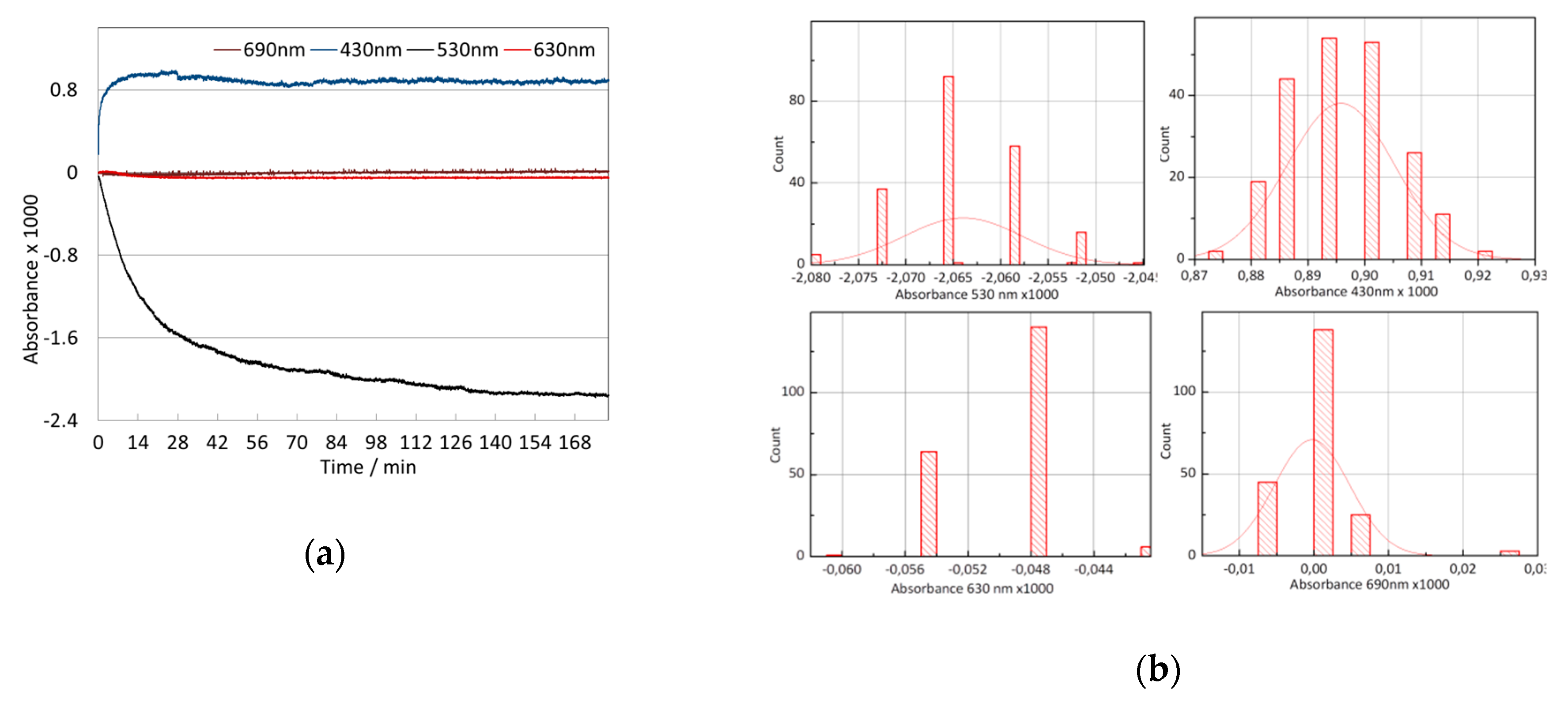
3.2. Signal Stability
3.3. Wavelength Selection
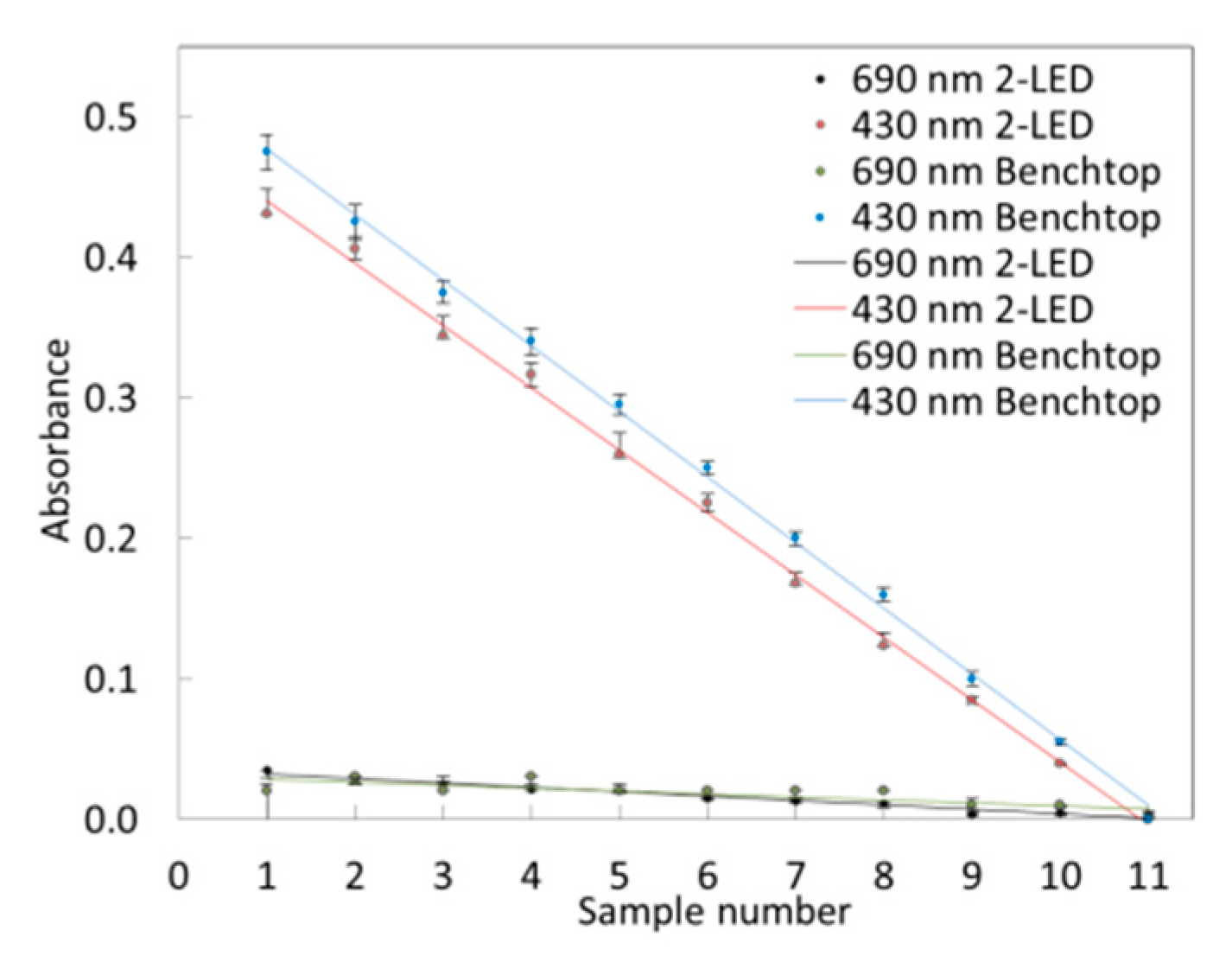
3.4. 2-LED µSpectrophotometer for Colorimetric Measurements
3.4.1. Limit of Detection
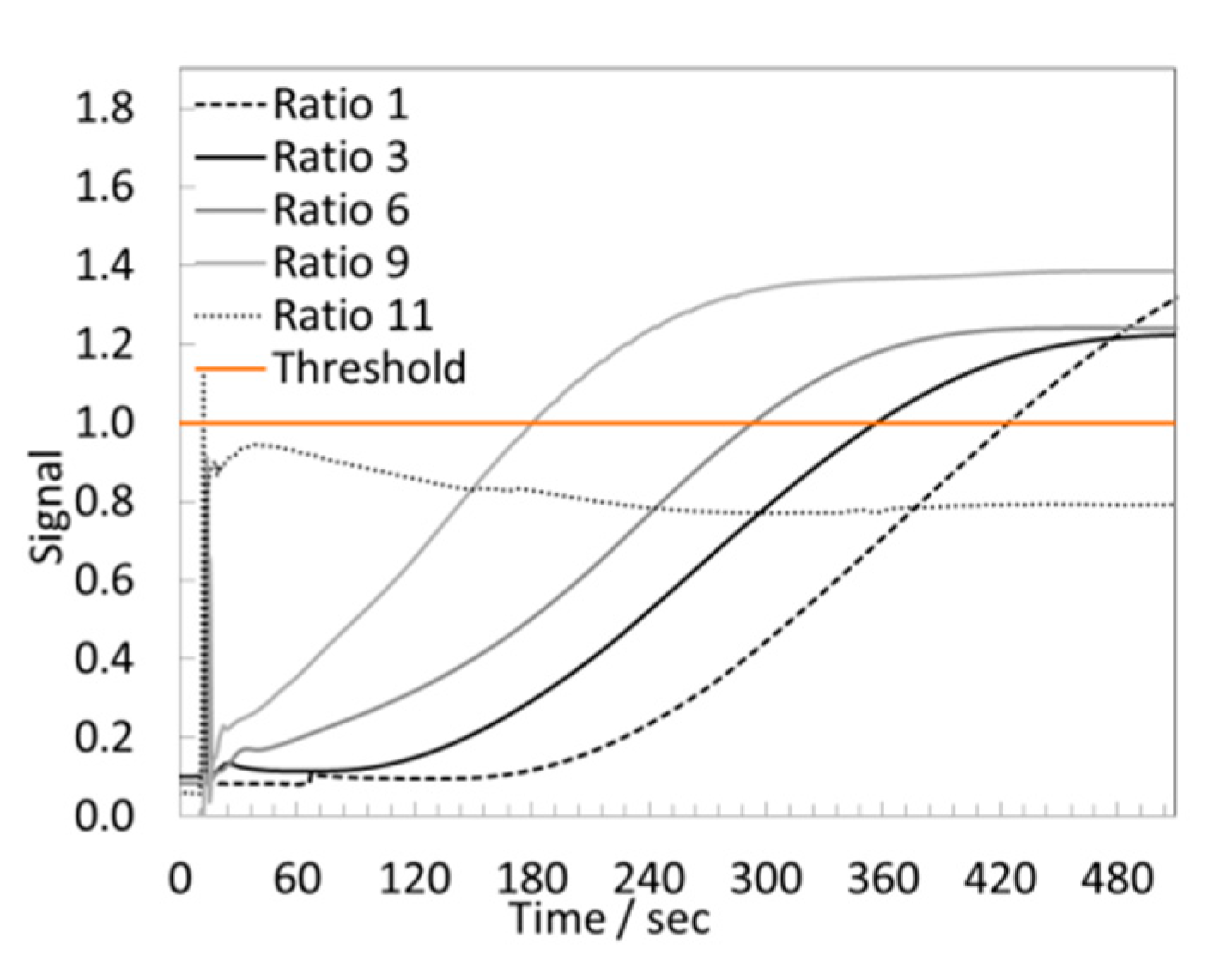
3.4.2. LAMP Assay
3.4.3. Colorimetric Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, X.; He, S.; Qiu, B.; Luo, F.; Guo, L.; Lin, Z. Noble Metal Nanoparticle-Based Multicolor Immunoassays: An Approach toward Visual Quantification of the Analytes with the Naked Eye. ACS Sens. 2019, 4, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomater. Basel Switz. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Baptista, P.; Pereira, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold nanoparticles for the development of clinical diagnosis methods. Anal. Bioanal. Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078. [Google Scholar] [CrossRef] [PubMed]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.-M.; Bisetty, K. A Review of Gold and Silver Nanoparticle-Based Colorimetric Sensing Assays. Adv. Eng. Mater. 2017, 19, 1700270. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Design of asymmetric DNAzymes for dynamic control of nanoparticle aggregation states in response to chemical stimuli. Org. Biomol. Chem. 2006, 4, 3435–3441. [Google Scholar] [CrossRef]
- Wang, G.; Akiyama, Y.; Shiraishi, S.; Kanayama, N.; Takarada, T.; Maeda, M. Cross-Linking versus Non-Cross-Linking Aggregation of Gold Nanoparticles Induced by DNA Hybridization: A Comparison of the Rapidity of Solution Color Change. Bioconjug. Chem. 2017, 28, 270–277. [Google Scholar] [CrossRef]
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef] [PubMed]
- Csáki, A.; Thiele, M.; Jatschka, J.; Dathe, A.; Zopf, D.; Stranik, O.; Fritzsche, W. Plasmonic nanoparticle synthesis and bioconjugation for bioanalytical sensing. Eng. Life Sci. 2015, 15, 266–275. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Antunes, P.W.P.; Yapuchura, E.R.; Guimarães, M.C.C. Impact of conjugation strategies for targeting of antibodies in gold nanoparticles for ultrasensitive detection of 17β-estradiol. Sci. Rep. 2019, 9, 13859. [Google Scholar] [CrossRef]
- Wang, Z.; Lévy, R.; Fernig, D.G.; Brust, M. Kinase-Catalyzed Modification of Gold Nanoparticles: A New Approach to Colorimetric Kinase Activity Screening. J. Am. Chem. Soc. 2006, 128, 2214–2215. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Poinard, B.; Yeo, E.L.L.; Kah, J.C.Y. An instantaneous colorimetric protein assay based on spontaneous formation of a protein corona on gold nanoparticles. Analyst 2015, 140, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.L.; Field, R.A.; Russell, D.A. Glyconanoparticles for the Colorimetric Detection of Cholera Toxin. Anal. Chem. 2007, 79, 1356–1361. [Google Scholar] [CrossRef]
- Borghei, Y.-S.; Hosseini, M.; Dadmehr, M.; Hosseinkhani, S.; Ganjali, M.R.; Sheikhnejad, R. Visual detection of cancer cells by colorimetric aptasensor based on aggregation of gold nanoparticles induced by DNA hybridization. Anal. Chim. Acta 2016, 904, 92–97. [Google Scholar] [CrossRef]
- Dehghani, Z.; Hosseini, M.; Mohammadnejad, J.; Bakhshi, B.; Rezayan, A.H. Colorimetric aptasensor for Campylobacter jejuni cells by exploiting the peroxidase like activity of Au@Pd nanoparticles. Microchim. Acta 2018, 185, 448. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Metal-induced aggregation of valine capped gold nanoparticles: An efficient and rapid approach for colorimetric detection of Pb2+ ions. Sci. Rep. 2017, 7, 9278. [Google Scholar] [CrossRef]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Ankireddy, S.R.; Kim, J. Chapter 23 - Status and Recent Developments in Analytical Methods for the Detection of Foodborne Microorganisms. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 323–334. ISBN 978-0-12-816328-3. [Google Scholar]
- Seetang-Nun, Y.; Jaroenram, W.; Sriurairatana, S.; Suebsing, R.; Kiatpathomchai, W. Visual detection of white spot syndrome virus using DNA-functionalized gold nanoparticles as probes combined with loop-mediated isothermal amplification. Mol. Cell. Probes 2013, 27, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dharanivasan, G.; Jesse, D.M.I.; Rajamuthuramalingam, T.; Rajendran, G.; Shanthi, S.; Kathiravan, K. Scanometric Detection of Tomato Leaf Curl New Delhi Viral DNA Using Mono- and Bifunctional AuNP-Conjugated Oligonucleotide Probes. ACS Omega 2019, 4, 10094–10107. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Pedrosa, P.; Carlos, F.F.; Mancio-Silva, L.; Grosso, A.R.; Fortunato, E.; Mota, M.M.; Baptista, P.V. One nanoprobe, two pathogens: gold nanoprobes multiplexing for point-of-care. J. Nanobiotechnology 2015, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.R.; White, J.S.; Nakhleh, M.B. Development of a Low-Cost Four-Color LED Photometer. J. Chem. Educ. 1996, 73, 1052. [Google Scholar] [CrossRef]
- Fonseca, A.; Raimundo, I.M. A simple method for water discrimination based on an light emitting diode (LED) photometer. Anal. Chim. Acta 2007, 596, 66–72. [Google Scholar] [CrossRef]
- Stephenson, D. A Portable Diode Array Spectrophotometer. Appl. Spectrosc. 2016, 70, 874–878. [Google Scholar] [CrossRef]
- Albert, D.R.; Todt, M.A.; Davis, H.F. A Low-Cost Quantitative Absorption Spectrophotometer. J. Chem. Educ. 2012, 89, 1432–1435. [Google Scholar] [CrossRef]
- Wittbrodt, B.T.; Squires, D.A.; Walbeck, J.; Campbell, E.; Campbell, W.H.; Pearce, J.M. Open-Source Photometric System for Enzymatic Nitrate Quantification. PLOS ONE 2015, 10, e0134989. [Google Scholar] [CrossRef]
- Zhao, C.; Zhong, G.; Kim, D.-E.; Liu, J.; Liu, X. A portable lab-on-a-chip system for gold-nanoparticle-based colorimetric detection of metal ions in water. Biomicrofluidics 2014, 8, 052107. [Google Scholar] [CrossRef]
- Vasquez, G.; Hernández, Y.; Coello, Y. Portable low-cost instrumentation for monitoring Rayleigh scattering from chemical sensors based on metallic nanoparticles. Sci. Rep. 2018, 8, 14903. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, M.L.; Parlindungan, D.; Sundaryono, A.; Farid, M.; Rahmidar, L.; Maidartati, M.; Amir, H. Development of Low-Cost Spectrophotometry Laboratory Practice Based on the Digital Image for Analytical Chemistry Subject. In Proceedings of the 3rd Asian Education Symposium (AES 2018), Bandung, Indonesia, 25–26 September 2018; Atlantis Press: Paris, France, 2019. [Google Scholar]
- Nuryantini, A.Y.; Mahen, E.C.S.; Sawitri, A.; Nuryadin, B.W. Do it yourself: optical spectrometer for physics undergraduate instruction in nanomaterial characterization. Eur. J. Phys. 2017, 38, 055501. [Google Scholar] [CrossRef]
- Verma, M.S.; Tsuji, J.M.; Hall, B.; Chen, P.Z.; Forrest, J.; Jones, L.; Gu, F.X. Towards point-of-care detection of polymicrobial infections: Rapid colorimetric response using a portable spectrophotometer. Sens. Bio-Sens. Res. 2016, 10, 15–19. [Google Scholar] [CrossRef]
- de Morais, C.D.; Carvalho, J.C.; Sant'Anna, C.; Eugênio, M.; Gasparotto, L.H.; Lima, K.M. A low-cost microcontrolled photometer with one color recognition sensor for selective detection of Pb2+ using gold nanoparticles. Anal. Methods 2015, 7, 7917–7922. [Google Scholar] [CrossRef]
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154. [Google Scholar] [CrossRef]
- Shirato, K. Detecting amplicons of loop-mediated isothermal amplification. Microbiol. Immunol. 2019, 63, 407–412. [Google Scholar] [CrossRef]
- Nuthong, B.; Wilailuckana, C.; Tavichakorntrakool, R.; Boonsiri, P.; Daduang, S.; Bunyaraksyotin, G.; Suphan, O.; Daduang, J. One step for Legionella pneumophila detection in environmental samples by DNA-gold nanoparticle probe. J. Appl. Microbiol. 2018, 125, 1534–1540. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Reuter, C.; Slesiona, N.; Hentschel, S.; Aehlig, O.; Breitenstein, A.; Csáki, A.; Henkel, T.; Fritzsche, W. Loop-mediated amplification as promising on-site detection approach for Legionella pneumophila and Legionella spp. Appl. Microbiol. Biotechnol. 2020, 104, 405–415. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Instantaneous and Quantitative Functionalization of Gold Nanoparticles with Thiolated DNA Using a pH-Assisted and Surfactant-Free Route. J. Am. Chem. Soc. 2012, 134, 7266–7269. [Google Scholar] [CrossRef]
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Fu, L.T.; Wong, J.K.F.; Chau, L.Y.; Yip, S.P.; Lee, T.M.H. Precipitation of PEG/Carboxyl-Modified Gold Nanoparticles with Magnesium Pyrophosphate: A New Platform for Real-Time Monitoring of Loop-Mediated Isothermal Amplification. ACS Appl. Mater. Interfaces 2017, 9, 10472–10480. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuter, C.; Urban, M.; Arnold, M.; Stranik, O.; Csáki, A.; Fritzsche, W. 2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays. Appl. Sci. 2020, 10, 2658. https://doi.org/10.3390/app10082658
Reuter C, Urban M, Arnold M, Stranik O, Csáki A, Fritzsche W. 2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays. Applied Sciences. 2020; 10(8):2658. https://doi.org/10.3390/app10082658
Chicago/Turabian StyleReuter, Cornelia, Matthias Urban, Manuel Arnold, Ondrej Stranik, Andrea Csáki, and Wolfgang Fritzsche. 2020. "2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays" Applied Sciences 10, no. 8: 2658. https://doi.org/10.3390/app10082658
APA StyleReuter, C., Urban, M., Arnold, M., Stranik, O., Csáki, A., & Fritzsche, W. (2020). 2-LED-µSpectrophotometer for Rapid On-Site Detection of Pathogens Using Noble-Metal Nanoparticle-Based Colorimetric Assays. Applied Sciences, 10(8), 2658. https://doi.org/10.3390/app10082658