Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Guidelines
2.2. Search Strategy
2.3. Study Selection
- -
- All studies designs were investigated
- -
- Patients who were treated with rapid maxillary expansion appliances
- -
- Patients demonstrating a narrow hard palate and/or a high arched hard palate
- -
- Presence of polysomnographic data related to the AHI index before and after RME therapy
- -
- Studies involving children and adolescents < 18 years old with OSAS
- -
- All languages
- -
- Any publication years
- -
- OSAS was considered if the obstructive apnea-hypopnea index (AHI) was ≥2/h
- -
- Studies that didn’t discuss RME as treatment for OSAS were excluded
- -
- Studies that lack to provide quantitative data were excluded
2.4. Data Screening and Extraction
2.5. Outcome Measures
2.6. Statistical Analysis
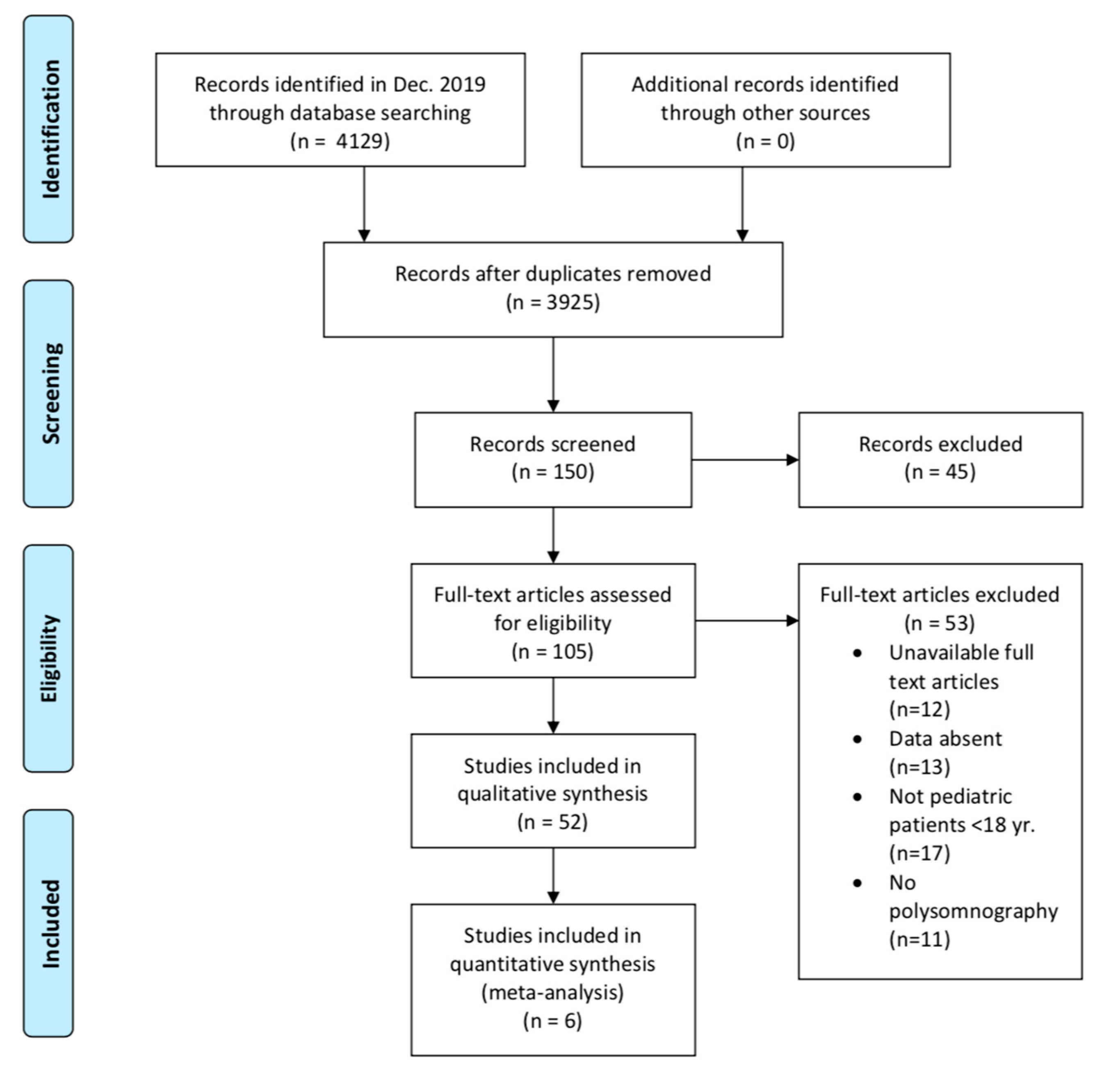
3. Results
Study Selection
- −
- Marino et al. and Fastuca et al. did not report either tonsil sizes or whether any previous surgery was performed. Thus, these studies were excluded from the sub-analysis. Therefore, this sub-analysis was performed on 65 out of 102 children.
- −
- Kim’s case report subject was treated previously with AT, but the child did not respond to the treatment, done three years before the RME.
- −
- Guilleminault et al. investigated in group 1 the RME after AT in 16 children with narrow maxilla and with a narrow and high hard palate; their AHI improved from 4.9 ± 0.6/h to 0.9 ± 0.3/h.
- −
- Pirelli et al. noted that their patients didn’t undergo any prior surgery (small tonsils).
- −
- Villa et al. excluded those previously treated with AT. They excluded children that had a history of prior OSAS therapies (including tonsillectomy and adenoidectomy). Before the placement of the RME device, all children underwent an otorhinolaryngological examination to grade their tonsillar hypertrophy (enlarged palatal tonsils) following a standardized scale ranging from 0 to 4 (large tonsils). Their study reported children presenting tonsillar hypertrophy as grade 2 (3 out of 10), grade 3 (5 out of 10), and grade 4 (2 out of 10).
- −
- Guilleminault et al. the used RME appliance in 14 children (group 2) that presented a grade 2 or larger tonsils and a narrow or high hard palate and a narrow maxilla, showing an improvement in AHI from 11.1 ± 0.7/h up to 5.4 ± 0.6/h in children with large tonsils.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muzumdar, H.; Arens, R. Physiological effects of obstructive sleep apnea syndrome in childhood. Respirat. Physiol. Neurobol. 2013, 188, 370–382. [Google Scholar] [CrossRef]
- Cielo, C.M.; Marcus, C.L. Obstructive sleep apnoea in children with craniofacial syndromes. Paediatr. Respir. Rev. 2015, 16, 189–196. [Google Scholar] [CrossRef]
- Cha, J.; Zea-Hernandez, J.A.; Sin, S.; Graw-Panzer, K.; Shifteh, K.; Isasi, C.R.; Wagshul, M.E.; Moran, E.E.; Posner, J.; Zimmerman, M.E.; et al. The effects of obstructive sleep apnea syndrome on the dentate gyrus and learning and memory in children. J. Neurosci. 2017, 37, 4280–4288. [Google Scholar] [CrossRef]
- Tsukada, E.; Kitamura, S.; Enomoto, M.; Moriwaki, A.; Kamio, Y.; Asada, T.; Arai, T.; Mishima, K. Prevalence of childhood obstructive sleep apnea syndrome and its role in daytime sleepiness. PLoS ONE 2018, 13, e0204409. [Google Scholar] [CrossRef]
- Gulotta, G.; Iannella, G.; Vicini, C.; Polimeni, A.; Greco, A.; De Vicentiis, M.; Visconti, I.C.; Meccariello, G.; Cammaroto, G.; De Vito, A.; et al. Risk factors for obstructive sleep apnea syndrome in children: State Art. Int. J. Environ. Res. Public Health 2019, 16, 3235. [Google Scholar] [CrossRef]
- Zhang, W.; Si, L.Y. Obstructive sleep apnea syndrome (OSAS) and hypertension: Pathogenic mechanisms and possible therapeutic approaches. Ups. J. Med. Sci. 2012, 117, 370–382. [Google Scholar] [CrossRef]
- Andreou, G.; Vlachos, F.; Makanikas, K. Effects of chronic obstructive pulmonary disease and obstructive sleep apnea on cognitive functions: Evidence for a common nature. Sleep Disord. 2014, 2014, 768210. [Google Scholar] [CrossRef]
- Dehlink, E.; Tan, H.L. Update on paediatric obstructive sleep apnoea. J. Thorac. Dis. 2016, 8, 224–235. [Google Scholar] [CrossRef]
- Tapia, I.E.; Marcus, C.L.; McDonough, J.M.; Kim, J.Y.; Cornaglia, M.A.; Xiao, R.; Allen, J.L. Airway resistance in children with obstructive sleep apnea syndrome. Sleep 2016, 39, 793–799. [Google Scholar] [CrossRef]
- Alonso-Álvarez, M.L.; Cordero-Guevara, J.A.; Terán-Santos, J.; González, M.; Jurado-Luque, M.J.; Corral-Peñafiel, J.; Duran-Cantolla, J.; Kheirandish-Gozal, L.; Gozal, D. Obstructive sleep apnea in obese community-dwelling children: The NANOS study. Sleep 2014, 37, 943–949. [Google Scholar] [CrossRef]
- Tapia, I.E.; McDonough, J.M.; Huang, J.; Marcus, C.L.; Gallagher, P.R.; Shults, J.; Davenport, P.W. Respiratory cortical processing to inspiratory resistances during wakefulness in children with the obstructive sleep apnea syndrome. J. Appl. Physiol. 2015, 118, 400–407. [Google Scholar] [CrossRef]
- Joosten, K.F.M.; Larramona, H.; Miano, S.; Van Waardenburg, D.; Kaditis, A.G.; Vandenbussche, N.; Ersu, R. How do we recognize the child with OSAS? Pediatr. Pulmonol. 2016, 52, 260–271. [Google Scholar] [CrossRef]
- Villa, M.P.; Rizzoli, A.; Miano, S.; Malagola, C. Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up. Sleep Breath 2011, 15, 179–184. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Caruso, S.; Quinzi, V.; Marchetti, E.; Marzo, G. Salivary markers and microbial flora in mouth breathing late adolescents. BioMed Res. Int. 2018, 2018, 8687608. [Google Scholar] [CrossRef]
- Quinzi, V.; Rossi, O.; Paglia, L.; Marzo, G.; Caprioglio, A. Paediatric Orthodontics Part 2: Periodontal effects of maxillary expansion. Eur. J. Paediatr. Dent. 2019, 20, 164–166. [Google Scholar]
- Mummolo, S.; Marchetti, E.; Albani, F.; Campanella, V.; Pugliese, F.; Di Martino, S.; Tecco, S.; Marzo, G. Comparison between rapid and slow palatal expansion: Evaluation of selected periodontal indices. Head Face Med. 2014, 10, 30. [Google Scholar] [CrossRef]
- Marino, A.; Ranieri, R.; Chiarotti, F.; Villa, M.P.; Malagola, C. Rapid maxillary expansion in children with Obstructive Sleep Apnoea Syndrome (OSAS). Eur. J. Paediatr. Dent. 2012, 13, 57–63. [Google Scholar]
- Mummolo, S.; Nota, A.; Marchetti, E.; Padricelli, G.; Marzo, G. The 3D tele motion tracking for the orthodontic facial analysis. BioMed Res. Int. 2016, 2016, 4932136. [Google Scholar] [CrossRef]
- Mummolo, S.; Ortu, E.; Necozione, S.; Monaco, A.; Marzo, G. Relationship between mastication and cognitive function in elderly in L’Aquila. Int. J. Clin. Exp. Med. 2014, 7, 1040–1046. [Google Scholar]
- Urquhart, D. Investigation and management of childhood sleep apnoea. Hippokratia 2013, 17, 196–202. [Google Scholar]
- Avis, K.T.; Gamble, K.L.; Schwebel, D.C. Obstructive sleep apnea syndrome increases pedestrian injury risk in children. J. Pediatr. 2015, 166, 109–114. [Google Scholar] [CrossRef]
- Hodges, E.; Marcus, C.L.; Kim, J.Y.; Xanthopoulos, M.; Shults, J.; Giordani, B.; Beebe, D.W.; Rosen, C.L.; Chervin, R.D.; Mitchell, R.B.; et al. Depressive symptomatology in school-aged children with obstructive sleep apnea syndrome: Incidence, demographic factors, and changes following a randomized controlled trial of adenotonsillectomy. Sleep 2018, 41. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chada, D.; Perez-Lloret, S.; Videla, A.J.; Cardinali, D.; Bergna, M.A.; Fernández-Acquier, M.; Larrateguy, L.; Zabert, G.E.; Drake, C.L. Sleep disordered breathing and daytime sleepiness are associated with poor academic performance in teenagers. A Study Using the Pediatric Daytime Sleepiness Scale (PDSS). Sleep 2007, 30, 1698–1703. [Google Scholar] [CrossRef]
- Busch, D.R.; Lynch, J.M.; Winters, M.E.; McCarthy, A.L.; Newland, J.J.; Ko, T.; Cornaglia, M.A.; Radcliffe, J.; McDonough, J.M.; Samuel, J.; et al. Cerebral blood flow response to hypercapnia in children with Obstructive Sleep Apnea Syndrome. Sleep 2016, 39, 209–216. [Google Scholar] [CrossRef]
- Gozal, D.; Tan, H.-L.; Kheirandish-Gozal, L. Obstructive sleep apnea in children: A critical update. Nat. Sci. Sleep 2013, 5, 109–123. [Google Scholar] [CrossRef]
- Guilleminault, C.; Huseni, S.; Lo, L. A frequent phenotype for paediatric sleep apnoea: Short lingual frenulum. ERJ Open Res. 2016, 2, 43. [Google Scholar] [CrossRef]
- Villa, M.P.; Evangelisti, M.; Barreto, M.; Cecili, M.; Kaditis, A.G. Short lingual frenulum as a risk factor for sleep-disordered breathing in school-age children. Sleep Med. 2020, 66, 119–122. [Google Scholar] [CrossRef]
- Gipson, K.; Lu, M.; Kinane, T.B. Sleep-disordered breathing in children. Pediatr. Rev. 2019, 40, 3–13. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chin, W.-C.; Guilleminault, C.; Chu, K.-C.; Lin, C.-H.; Li, H.-Y. Inflammatory factors: Nonobese pediatric obstructive sleep apnea and adenotonsillectomy. J. Clin. Med. 2020, 9, 1028. [Google Scholar] [CrossRef]
- McNamara, J.A.; Lione, R.; Franchi, L.; Angelieri, F.; Cevidanes, L.H.S.; Darendeliler, M.A.; Cozza, P. The role of rapid maxillary expansion in the promotion of oral and general health. Prog. Orthod. 2015, 16, 33. [Google Scholar] [CrossRef]
- Fatsuca, R.; Perinetti, G.; Zecca, P.A.; Nucera, R.; Caprioglio, A. Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study. Angle Orthod. 2015, 85, 955–961. [Google Scholar]
- Camacho, M.; Chang, E.T.; Song, S.A.; Abdullatif, J.; Zaghi, S.; Pirelli, P.; Certal, V.; Guilleminault, C. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2017, 127, 1712–1719. [Google Scholar] [CrossRef]
- Ferro, R.; Besostri, A.; Olivieri, A.; Quinzi, V.; Scibetta, D. Prevalence of cross-bite in a sample of Italian preschoolers. Eur. J. Paediatr. Dent. 2016, 17, 307–309. [Google Scholar]
- Pirelli, P.; Saponara, M.; Guilleminault, C. Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: A 12-year follow-up. Sleep Med. 2015, 16, 933–935. [Google Scholar] [CrossRef]
- Guilleminault, C.; Monteyrol, P.-J.; Huynh, N.T.; Pirelli, P.; Quo, S.; Li, K. Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study. Sleep Breath 2011, 15, 173–177. [Google Scholar] [CrossRef]
- Kim, M. Orthodontic treatment with rapid maxillary expansion for treating a boy with severe obstructive sleep apnea. Sleep Med. Res. 2014, 5, 33–36. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White paper. Am. J. Orthod. Dentofac. Orthop. 2019, 156, 13–28. [Google Scholar] [CrossRef]
- Torretta, S.; Rosazza, C.; Pace, M.E.; Iofrida, E.; Marchisio, P. Impact of adenotonsillectomy on pediatric quality of life: Review of the literature. Ital. J. Pediatr. 2017, 43, 107. [Google Scholar] [CrossRef]
- Quinzi, V.; Scibetta, E.T.; Marchetti, E.; Mummolo, S.; Giannì, A.B.; Romano, M.; Beltramini, G.; Marzo, G. Analyze my face. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 149–158. [Google Scholar]
- Modesti-Vedolin, G.; Chies, C.; Chaves-Fagondes, S.; Piza-Pelizzer, E.; Grossi, M.L. Efficacy of a mandibular advancement intraoral appliance (MOA) for the treatment of obstructive sleep apnea syndrome (OSAS) in pediatric patients: A pilot-study. Med. Oral Patol. Oral Cirug. Bucal. 2018, 23, e656–e663. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.L.; Sutherland, K.; A Cistulli, P. Mandibular advancement splints for the treatment of obstructive sleep apnea. Exp. Rev. Respir. Med. 2019, 14, 81–88. [Google Scholar] [CrossRef]
- Ng, J.H.; Yow, M. Oral appliances in the management of obstructive sleep apnea. Sleep Med. Clin. 2019, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Fastuca, R.; Zecca, P.A.; Caprioglio, A. Role of mandibular displacement and airway size in improving breathing after rapid maxillary expansion. Prog. Orthod. 2014, 15, 40. [Google Scholar] [CrossRef]
- Guimarães, K.C.; Drager, L.F.; Genta, P.R.; Marcondes, B.F.; Lorenzi-Filho, G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2009, 179, 962–966. [Google Scholar] [CrossRef]
- Baz, H.; Elshafey, M.; Elmorsy, S.; Abu-Samra, M. The role of oral myofunctional therapy in managing patients with mild to moderate obstructive sleep apnea. PAN Arab. J. Rhinol. 2012, 2, 17–22. [Google Scholar]
- Diaféria, G.; Badke, L.; Santos-Silva, R.; Bommarito, S.; Tufik, S.; Bittencourt, L. Effect of speech therapy as adjunct treatment to continuous positive airway pressure on the quality of life of patients with obstructive sleep apnea. Sleep Med. 2013, 14, 628–635. [Google Scholar] [CrossRef]
- Diaféria, G.; Santos-Silva, R.; Truksinas, E.; Haddad, F.L.M.; Santos, R.; Bommarito, S.; Gregório, L.C.; Tufik, S.; Bittencourt, L.R.A. Myofunctional therapy improves adherence to continuous positive airway pressure treatment. Sleep Breath 2017, 21, 387–395. [Google Scholar] [CrossRef]
- Ieto, V.; Kayamori, F.; Montes, M.I.; Hirata, R.P.; Gregorio, M.G.; Alencar, A.M.; Drager, L.F.; Genta, P.R.; Lorenzi-Filho, G. Effects of oropharyngeal exercises on snoring. Chest 2015, 148, 683–691. [Google Scholar] [CrossRef]
- Villa, M.P.; Brasili, L.; Ferretti, A.; Vitelli, O.; Rabasco, J.; Mazzotta, A.R.; Pietropaoli, N.; Martella, S. Oropharyngeal exercises to reduce symptoms of OSA after AT. Sleep Breath 2015, 19, 281–289. [Google Scholar] [CrossRef]
- Verma, R.K.; Johnson, J.R.J.; Goyal, M.; Banumathy, N.; Goswami, U.; Panda, N.K. Oropharyngeal exercises in the treatment of obstructive sleep apnoea: Our experience. Sleep Breath 2016, 20, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Sharshar, R.S.; Elkolaly, R.M.; Serageldin, S.M. Upper airway muscle exercises outcome in patients with obstructive sleep apnea syndrome. Chest 2017, 66, 121–125. [Google Scholar] [CrossRef]
| Author | Investigator | Title | Source | Findings of the Study |
|---|---|---|---|---|
| Villa et al. 2011 | [13] | Efficacy of rapid maxillary expansion in children with obstructive sleep apnea syndrome: 36 months of follow-up | Sleep and Breathing | After RME treatment, the AHI decreased, and the clinical symptoms had resolved. |
| Marino et al. 2012 | [17] | Rapid maxillary expansion in children with Obstructive Sleep Apnoea Syndrome (OSAS) | European Journal of Pediatric Dentistry | The nasopharyngeal airway measurements showed a significant increase after treatment with RME. |
| Fastuca et al. 2015 | [31] | Airway compartments volume and oxygen saturation changes after rapid maxillary expansion: A longitudinal correlation study | Angle Orthodontist | Oxygen saturation was increased, and the apnea/hypopnea index was improved. |
| Pirelli et al. 2015 | [34] | Rapid maxillary expansion (RME) for pediatric obstructive sleep apnea: a 12-year follow-up | Sleep Medicine | PSG showed an improvement in the AHI value and oxygen saturation nadir. Moreover, a total resolution of clinical complaints was reported. |
| Guilleminault et al. 2011 | [35] | Adeno-tonsillectomy and rapid maxillary distraction in pre-pubertal children, a pilot study | Sleep Breath | Children presented an improvement of both clinical symptoms and PSG findings. Nevertheless, none of the children presented normal results after treatment 1 (only RME or Adenotonsillectomy). In fact, both treatments are needed to obtain normal results. |
| Kim 2014 | [36] | Orthodontic Treatment with Rapid Maxillary Expansion for Treating a Boy with Severe Obstructive Sleep Apnea | Sleep Medicine | Banded RME was used to correct the quality of sleep and improve the narrow maxillary arch. |
| Search Method No. of Abstracts without Overlap | |
|---|---|
| Wiley Online | 1200 |
| PubMed | 611 |
| Cochrane Controlled Clinical Trials Register | 8 |
| Springer Link | 1435 |
| Science Direct | 855 |
| Reference selected articles | 20 |
| Total | 4129 |
| Study, Design | Study Site | Outcomes Analyzed |
|---|---|---|
| Pirelli et al. 2015, PCS | Italy | AHI, LSAT |
| Fastuca et al. 2015, PCS | Italy | AHI, LSAT |
| Kim 2014, RCR | Korea | AHI, LSAT, RDI |
| Marino et al. 2012, RCS | Italy | AHI, CEPH |
| Guilleminault et al. 2011, PRT | France-Italy | AHI, LSAT |
| Villa et al. 2011, PCS | Italy | AHI, MSAT |
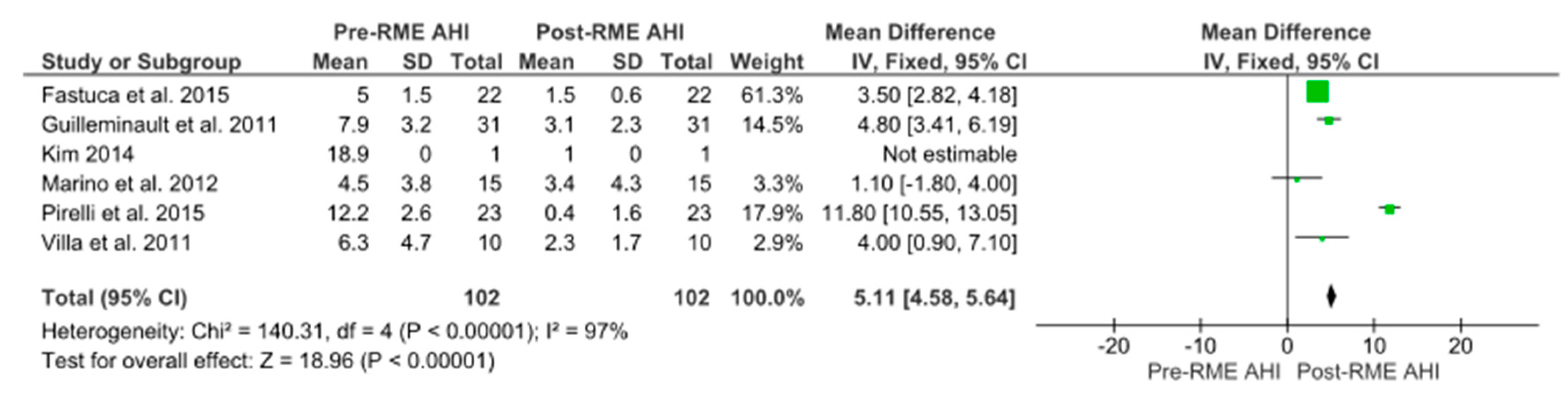
| Study | No. | Age, Year * | BMI, kg/m2 | F/U | Pre-RME AHI | Post-RME AHI | AHI% Change |
|---|---|---|---|---|---|---|---|
| Pirelli et al. 2015 | 23 | 8.6 | 22.7 ± 1.3 | 12.3 ± 1.5 year | 12.2 ± 2.6 | 0.4 ± 1.6 | −97.7% |
| Fastuca et al. 2015 | 22 | 8.3 ± 0.9 | NR | 1 year | 5.0 ± 1.5 | 1.5 ± 0.6 | −70% |
| Kim 2014 | 1 | 11 | 22.4 | 2 year 5 month | 18.9 | 1 | −94.7% |
| Marino et al. 2012 | 15 | 5.9 ± 1.6 | NR | 1.6 ± 0.6 year | 4.5 ± 3.8 | 3.4 ± 4.3 | −24.4% |
| Guilleminault et al. 2011 | 31 | 6.5 ± 1.1 | NR | 3 month | 7.9 ± 3.2 | 3.1 ± 2.3 | −60.7% |
| Villa et al. 2011 | 10 | 6.6 ± 2.1 | 16.7 ± 3.6 | 2 year 11 month | 6.3 ± 4.7 | 2.3 ± 1.7 | −63.4% |
| TOTAL | 102 | 6.7 ± 1.3 | 19.4 ± 2.5 | ≤3 year | 7.5 ± 3.2 | 2.5 ± 2.6 | −66.1% |
| Tonsil Status | Pre-RME AHI | Post-RME AHI | AHI% Change |
|---|---|---|---|
| No tonsils, n = 17 | 4.9 ± 0.6 | 0.9 ± 0.3 | −82.4% |
| Small tonsils, n = 23 | 12.2 ± 2.6 | 0.4 ± 1.6 | −97.7% |
| Large tonsils, n = 24 | 7.1 ± 4 | 3.3 ± 1.3 | −56.4% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinzi, V.; Saccomanno, S.; Manenti, R.J.; Giancaspro, S.; Coceani Paskay, L.; Marzo, G. Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis. Appl. Sci. 2020, 10, 6485. https://doi.org/10.3390/app10186485
Quinzi V, Saccomanno S, Manenti RJ, Giancaspro S, Coceani Paskay L, Marzo G. Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis. Applied Sciences. 2020; 10(18):6485. https://doi.org/10.3390/app10186485
Chicago/Turabian StyleQuinzi, Vincenzo, Sabina Saccomanno, Rebecca Jewel Manenti, Silvia Giancaspro, Licia Coceani Paskay, and Giuseppe Marzo. 2020. "Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis" Applied Sciences 10, no. 18: 6485. https://doi.org/10.3390/app10186485
APA StyleQuinzi, V., Saccomanno, S., Manenti, R. J., Giancaspro, S., Coceani Paskay, L., & Marzo, G. (2020). Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis. Applied Sciences, 10(18), 6485. https://doi.org/10.3390/app10186485









