Tongue Posture, Tongue Movements, Swallowing, and Cerebral Areas Activation: A Functional Magnetic Resonance Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting and Subjects
2.2. Functional Imaging Acquisition
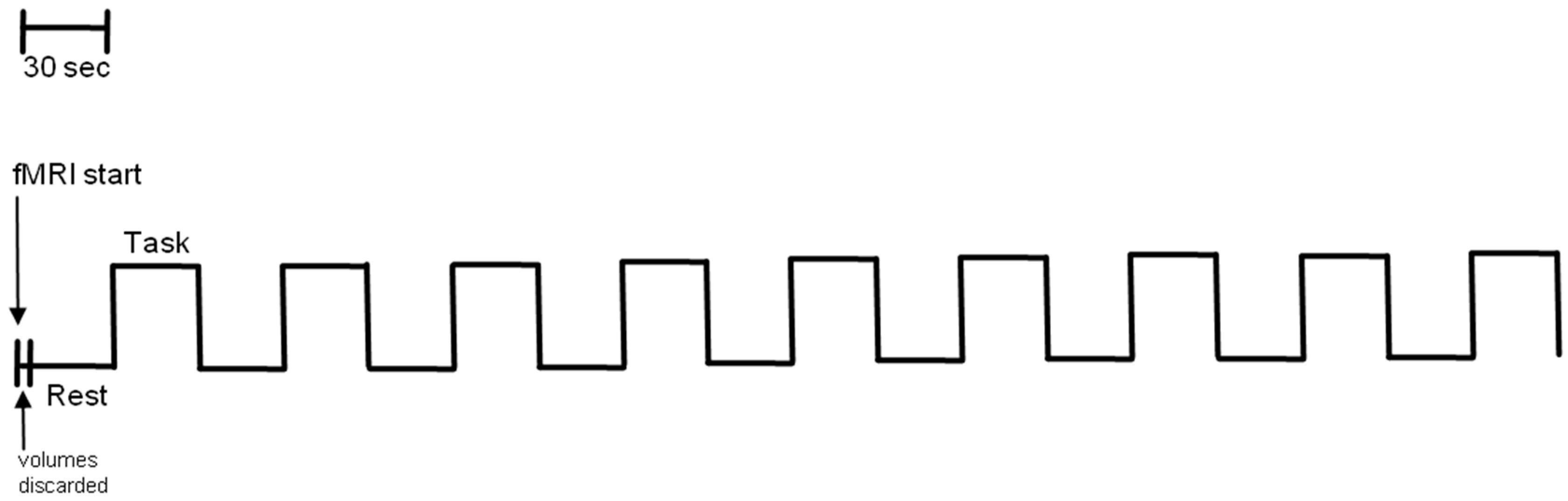
2.3. fMRI Standard Procedure
2.4. fMRI Data Analysis
3. Results
fMRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Precentral gyrus | The precentral gyrus is responsible of the control of voluntary movement. |
| Postcentral gyrus | It is the location of the primary somatosensory cortex, the main sensory receptive area for the sense of touch. |
| Superior parietal lobule | Involved with spatial orientation. |
| Inferior parietal lobule | Involved in the perception of emotions in facial stimuli, and interpretation of sensory information. Moreover, is concerned with language, mathematical operations, and body image, particularly the supramarginal gyrus and the angular gyrus. |
| Medial frontal gyrus | The right middle fontal gyrus (MFG) has been proposed to be a site of convergence of the dorsal and ventral attention networks, by serving as a circuit-breaker to interrupt ongoing endogenous attentional processes in the dorsal network and reorient attention to an exogenous stimulus. |
| Lentiform nucleus | Part of the basal ganglia. The basal ganglia are associated with a variety of functions, including control of voluntary motor movements, procedural learning, habit learning, eye movements, cognition, and emotion. |
| Anterior cingulate | The superior temporal gyrus contains the auditory cortex, which is responsible for processing sounds. Specific sound frequencies map precisely onto the auditory cortex. This auditory (or tonotopic) map is similar to the homunculus map of the primary motor cortex. |
| Superior temporal gyrus | The superior temporal gyrus contains the auditory cortex, which is responsible for processing sounds. Specific sound frequencies map precisely onto the auditory cortex. This auditory (or tonotopic) map is similar to the homunculus map of the primary motor cortex. |
| Insular cortex | The insula controls autonomic functions through the regulation of the sympathetic and parasympathetic systems. It has a role in regulating the immune system. |
| Thalamus | The thalamus relays sensory impulses from receptors in various parts of the body to the cerebral cortex. A sensory impulse travels from the body surface towards the thalamus, which receives it as a sensation. This sensation is then passed onto the cerebral cortex for interpretation as touch, pain, or temperature. |
References
- Wiles, C.M. Theneuroscientificprinciples of swallowing and dysphagia. Brain 1999, 122, 788–789. [Google Scholar] [CrossRef][Green Version]
- Doty, R.W.; Bosma, J.F. An electromyographic analysis of reflex deglutition. J. Neurophysiol. 1956, 19, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J.; Henry, J.L. Neural mechanisms of swallowing: Neurophysiological and neurochemical studies on brain stem neurons in the solitary tract region. Dysphagia 1989, 4, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Rosenbek, J.; Shoemaker, J.K.; Martin, R.E. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer’s disease: A scoping review of the evidence. J. Am. Geriatr. Soc. 2013, 61, 2203–2213. [Google Scholar] [CrossRef]
- Halata, Z.; Baumann, K.I. Sensory nerve endings in the hard palate and papilla incisiva of the rhesus monkey. Anat. Embryol. 1999, 199, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Halata, Z.; Cooper, B.Y.; Baumann, K.I.; Schwegmann, C.; Friedman, R.M. Sensory nerve endings in the hard palate and papilla incisiva of the goat. Exp. Brain Res. 1999, 129, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, J.G.; Deiber, M.P.; Passingham, R.E.; Friston, K.J.; Frackowiak, R.S. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J. Neurophysiol. 1991, 65, 1392–1401. [Google Scholar] [CrossRef]
- Grafton, S.T.; Mazziotta, J.C.; Presty, S.; Friston, K.J.; Frackowiak, R.S.; Phelps, M.E. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J. Neurosci. 1992, 12, 2542–2548. [Google Scholar] [CrossRef]
- Di Vico, R.; Ardigo, L.P.; Salernitano, G.; Chamari, K.; Padulo, J. The acute effect of the tongue position in the mouth on knee isokinetic test performance: A highly surprising pilot study. Muscles Ligaments Tendons J. 2013, 3, 318–323. [Google Scholar] [CrossRef]
- Cuccia, A.; Caradonna, C. The relationship between the stomatognathic system and body posture. Clinics 2009, 64, 61–66. [Google Scholar] [CrossRef]
- Stone, M.; Woo, J.; Lee, J.; Poole, T.; Seagraves, A.; Chung, M.; Kim, E.; Murano, E.Z.; Prince, J.L.; Blemker, S.S. Structure and variability in human tongue muscle anatomy. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Ogawa, S.; Menon, R.S.; Tank, D.W.; Kim, S.G.; Merkle, H.; Ellermann, J.M.; Ugurbil, K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys. J. 1993, 64, 803–812. [Google Scholar] [CrossRef]
- Hamdy, S.; Mikulis, D.J.; Crawley, A.; Xue, S.; Lau, H.; Henry, S.; Diamant, N.E. Cortical activation during human volitional swallowing: An event-related fMRI study. Am. J. Physiol. 1999, 277, G219–G225. [Google Scholar] [CrossRef] [PubMed]
- Humbert, I.A.; Robbins, J.A. Normal Swallowing and Functional Magnetic Resonance Imaging: A Systematic Review. Dysphagia 2007, 22, 266–275. [Google Scholar] [CrossRef]
- Matthews, P.M.; Jezzard, P. Functional magnetic resonance imaging. J. Neurol. Psychiatry 2004, 75, 6–12. [Google Scholar]
- Bullmore, E.T.; Suckling, J.; Overmeyer, S.; Rabe-Hesket, S.; Taylor, E.; Brammer, M.J. Global, Voxel, and Cluster Test, by Theoryand Pemutation, for a Difference Between TwoGroups of Structural MRI images of the Brain. IEE Trans. Med. Imaging 1999, 18. [Google Scholar] [CrossRef]
- Suzuki, M.; Asada, Y.; Ito, J.; Hayashi, K.; Inoue, H.; Kitano, H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 2003, 18, 71–77. [Google Scholar] [CrossRef]
- Hartnick, C.J.; Rudolph, C.; Willging, J.P.; Holland, S.K. Functional magnetic resonance imaging of the pediatric swallow: Imaging the cortex and the brainstem. Laryngoscope 2001, 111, 1183–1191. [Google Scholar] [CrossRef]
- Birn, R.M.; Bandettini, P.A.; Cox, R.W.; Shaker, R. Event-related fMRI of tasks involving brief motion. Hum. Brain Mapp. 1999, 7, 106–114. [Google Scholar] [CrossRef]
- Birn, R.M.; Bandettini, P.A.; Cox, R.W.; Shaker, R. Improved technique for study of brain activity during swallowing by functional magnetic resonance imaging (FMRI). Gastroenterology 1998, 114, A721. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Crone, R.; Hughes, D.; Tallis, R.C.; Thompson, D.G. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. Lancet 1997, 350, 686–692. [Google Scholar] [CrossRef]
- Hamdy, S.; Rothwell, J.C.; Brooks, D.J.; Bailey, D.; Aziz, Q.; Thompson, D.G. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J. Neurophysiol. 1999, 81, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Mosier, K.; Patel, R.; Liu, W.C.; Kalnin, A.; Maldjian, J.; Baredes, S. Cortical representation of swallowing in normal adults: Functional implications. Laryngoscope 1999, 109, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Sessle, B.J. The role of the cerebral cortex in swallowing. Dysphagia 1993, 8, 195–202. [Google Scholar] [CrossRef]
- Shogry, M.E.; Elster, A.D. Cerebrovascular enhancement in spoiled GRASS (SPGR) images: Comparison with spin-echo technique. J. Comput. Assist. Tomogr. 1992, 16, 48–53. [Google Scholar] [CrossRef]
- Martin, R.; Barr, A.; MacIntosh, B.; Smith, R.; Stevens, T.; Taves, D.; Gati, J.; Menon, R.; Hachinski, V. Cerebral cortical processing of swallowing in older adults. Exp. Brain Res. 2007, 176, 12–22. [Google Scholar] [CrossRef]
- Soros, P.; Inamoto, Y.; Martin, R.E. Functional brain imaging of swallowing: An activation likelihood estimation meta-analysis. Hum. Brain Mapp. 2009, 30, 2426–2439. [Google Scholar] [CrossRef]
- Martin, R.E.; Goodyear, B.G.; Gati, J.S.; Menon, R.S. Cerebral cortical representation of automatic and volitional swallowing in humans. J. Neurophysiol. 2001, 85, 938–950. [Google Scholar] [CrossRef]
- Dziewas, R.; Sörös, P.; Ishii, R.; Chau, W.; Henningsen, H.; Ringelstein, E.B.; Knecht, S.; Pantev, C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 2003, 20, 135–144. [Google Scholar] [CrossRef]
- Martin, R.E.; MacIntosh, B.J.; Smith, R.C.; Barr, A.M.; Stevens, T.K.; Gati, J.S.; Menon, R.S. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. J. Neurophysiol. 2004, 92, 2428–2443. [Google Scholar] [CrossRef]
- Teismann, I.K.; Dziewas, R.; Steinstraeter, O.; Pantev, C. Time-dependent hemispheric shift of the cortical control of volitional swallowing. Hum. Brain Mapp. 2009, 30, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zald, D.H.; Pardo, J.V. The functional neuroanatomy of voluntary swallowing. Ann. Neurol. 1999, 46, 281–286. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Singh, K.D.; Barlow, J.; Hughes, D.G.; Tallis, R.C.; Thompson, D.G. The cortical topography of human swallowing musculature in health and disease. Nat. Med. 1996, 2, 1217–1224. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocke, M.C.; Berg, C.; Forsum, E.; Hornell, A.; Larsson, C.; Sonestedt, E.; Wirfalt, E.; Akesson, P.; et al. Strengthening the Reporting of Observational Studies in Epidemiology-Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [PubMed]
- Caram-Deelder, C.; Kreuger, A.L.; Rosendaal, F.R.; van der Bom, J.G.; Middelburg, R.A. Middelburg. Continuing use of the terms prospective and retrospective and quality of reporting of observational studies: Time to update the STROBE guideline? Int. J. Epidemiol. 2016, 45, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Field, N.; Cohen, T.; Struelens, M.J.; Palm, D.; Cookson, B.; Glynn, J.R.; Gallo, V.; Ramsay, M.; Sonnenberg, P.; MacCannell, D.; et al. Strengthening the Reporting of Molecular Epidemiology for Infectious Diseases (STROME-ID): An extension of the STROBE statement. Lancet Infect. Dis. 2014, 14, 341–352. [Google Scholar] [CrossRef]
- Saccomanno, S.; Di, A.T.; D’Alatri, L.; Grippaudo, C. Proposal for a myofunctional therapy Protocol in case of altered lingual frenulum. A Pilot study. Eur. J. Paediatr. Dent. 2019, 20, 67–72. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral. Investig. 2020, 17. [Google Scholar] [CrossRef]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of Vitamin d in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tounoux, P. Co-Planar Stereotaxic Atlas of the Human Brain, 1st ed.; Thieme Publishing Group: New York, NY, USA, 1988. [Google Scholar]
- Flynn, F.G. Anatomy of the insula functional and clinical correlates. Aphasiology 1999, 13, 55–78. [Google Scholar] [CrossRef]
- Kern, M.K.; Jaradeh, S.; Arndorfer, R.C.; Shaker, R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G354–G360. [Google Scholar] [CrossRef]
- Affoo, R.H.; Goley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Mortimore, I.L.; Mathur, R.; Douglas, N.J. Effect of posture, route of respiration, and negative pressure on palatal muscle activity in humans. J. Appl. Physiol. 1995, 79, 448–454. [Google Scholar] [CrossRef]
- Martin-Harris, B. Clinical implications of respiratory–swallowing interactions. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 194–199. [Google Scholar] [CrossRef]
- Logeman, J.A. Evaluation and Treatment of Swallowing Disorders. Curr. Opin. Otolaryngol. Head Neck Surg. 1998, 6, 395–400. [Google Scholar] [CrossRef]
- Lasota, K.J.; Ulmer, J.L.; Firszt, J.B.; Biswal, B.B.; Daniels, D.L.; Prost, R.W. Intensity-dependent Activation of the Primary Auditory Cortex in Functional Magnetic Resonance. Imaging J. Comp. Assist. Tomogr. 2003, 27, 213–218. [Google Scholar] [CrossRef]
- Penny, W.D.; Ashburner, J.; Kiebel, S.; Henson, R.; Glaser, D.E.; Phillips, C.; Friston, K. Statistical Parametric Mapping: An annotated bibliography. In Wellcome Department of Cognitive Neurology; University College London: London, UK, 2001; p. 16. [Google Scholar]
- Talairach, J.; Tournoux, P.; Musolino, A.; Missir, O. Stereotaxic exploration in frontal epilepsy. Adv. Neurol. 1992, 57, 651–688. [Google Scholar]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.B.; Heather, J.D.; Frackowiak, R.S. Spatial registration and normalization of images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Frackowiak, R.S.; Turner, R. Characterizing dynamic brain responses with fMRI: A multivariate approach. Neuroimage 1995, 2, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Saccomanno, S.; Quinzi, V.; Sarhan, S.; Laganà, D.; Marzo, G. Perspectives of tele-orthodontics in the COVID-19 emergency and as a future tool in daily practice. Eur. J. Paediatr. Dent. 2020, 21, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sörös, P.; Sokoloff, L.G.; Bose, A.; McIntosh, A.R.; Graham, S.J.; Stuss, D.T. Clustered functional MRI of overt speech production. Neuroimage 2006, 32, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Zevin, J.D.; Yang, J.; Skipper, J.I.; McCandliss, B.D. Domain general change detection accounts for “dishabituation” effects in temporal-parietal regions in functional magnetic resonance imaging studies of speech perception. J. Neurosci. 2010, 30, 1110–1117. [Google Scholar] [CrossRef]
- Penfield, W. The supplementary motor area in the cerebral cortex of man. Arch. Psychiatr. Nervenkr. Z. Gesamte Neurol. Psychiatr. 1950, 185, 670–674. [Google Scholar] [CrossRef]
- Ruben, J.; Schwiemann, J.; Deuchert, M.; Meyer, R.; Krause, T.; Curio, G.; Villringer, K.; Kurth, R.; Villringer, A. Somatotopic organization of human secondary somatosensory cortex. Cereb. Cortex 2001, 11, 463–473. [Google Scholar] [CrossRef]
- Lim, S.H.; Dinner, D.S.; Pillay, P.K.; Lüders, H.; Morris, H.H.; Klem, G.; Wyllie, E.; Awad, I.A. Functional anatomy of the human supplementary sensorimotor area: Results of extraoperative electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 179–193. [Google Scholar] [CrossRef]
- Pillar, G.; Fogel, R.B.; Malhotra, A.; Beauregard, J.; Edwards, J.K.; Shea, S.A.; White, D.P. Genioglossal inspiratory activation: Central respiratory vs. mechanoreceptive influences. Respir. Physiol. 2001, 127, 23–38. [Google Scholar] [CrossRef]
- Kubler, A.; Dixon, V.; Garavan, H. Automaticity and reestablishment of executive control-an fMRI study. J. Cogn. Neurosci. 2006, 18, 1331–1342. [Google Scholar] [CrossRef]
- Morgan, K.K.; Luu, P.; Tucker, D.M. Changes in P3b Latency and Amplitude Reflect Expertise Acquisition in a Football Visuomotor Learning Task. PLoS ONE 2016, 11, e0154021. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Stripling, R. Neural mechanisms for learning actions in context. Brain Res. 2007, 1179, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Saling, L.L.; Phillips, J.G. Automatic behaviour: Efficient not mindless. Brain Res. Bull. 2007, 73, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Grafman, J.; Hallett, M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science 1994, 263, 1287–1289. [Google Scholar] [CrossRef]
- Tzvi, E.; Stoldt, A.; Witt, K.; Krämer, U.M. Striatal-cerebellar networks mediate consolidation in a motor sequence learning task: An fMRI study using dynamic causal modelling. Neuroimage 2015, 122, 52–64. [Google Scholar] [CrossRef]
- Lafleur, M.F.; Jackson, P.L.; Malouin, F.; Richards, C.L.; Evans, A.C.; Doyon, J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage 2002, 16, 142–157. [Google Scholar] [CrossRef]
- Sörös, P.; Lalone, E.; Smith, R.; Stevens, T.; Theurer, J.; Menon, R.S.; Martin, R.E. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience 2008, 153, 1300–1308. [Google Scholar] [CrossRef]
- Shibamoto, I.; Tanaka, T.; Fujishima, I.; Katagiri, N.; Uematsu, H. Cortical activation during solid bolus swallowing. J. Med. Dent. Sci. 2007, 54, 25–30. [Google Scholar]
- Kandel, E. Principles of Neural Science, Fifth Edition; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Mosier, K.M.; Liu, W.C.; Maldjian, J.A.; Shah, R.; Modi, B. Lateralization of cortical function in swallowing: A functional MR imaging study. AJNR Am. J. Neuroradiol. 1999, 20, 1520–1526. [Google Scholar]
- Schell, G.R.; Strick, P.L. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J. Neurosci. 1984, 4, 539–560. [Google Scholar] [CrossRef]
- Wiesendanger, R.; Wiesendanger, M. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (Macaca fascicularis). Exp. Brain Res. 1985, 59, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Sörös, P.; Al-Otaibi, F.; Wong, S.W.; Shoemaker, J.K.; Mirsattari, S.M.; Hachinski, V.; Martin, R.E. Stuttered swallowing: Electric stimulation of the right insula interferes with water swallowing. A case report. BMC Neurol. 2011, 11, 20. [Google Scholar]
- Kern, M.K.; Birn, R.M.; Jaradeh, S.; Jesmanowicz, A.; Cox, R.W.; Hyde, J.S.; Shaker, R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology 1998, 115, 1353–1362. [Google Scholar] [CrossRef]
- Kern, M.; Birn, R.; Jaradeh, S.; Jesmanowicz, A.; Cox, R.; Hyde, J.; Shaker, R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G531–G538. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, E.; Moreau, A.; Honoré, J.; Doual, A. Does dysfunctional swallowing influence posture? Orthod. Fr. 2008, 79, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Saccomanno, S.; Antonini, G.; D’Alatri, L.; D’Angelantonio, M.; Fiorita, A.; Deli, R. Causal relationship between malocclusion and oral muscles dysfunction: A model of approach. Eur. J. Paediatr. Dent. 2012, 13, 321–323. [Google Scholar]
- Saccomanno, S.; Antonini, G.; D’Alatri, L.; D’Angeloantonio, M.; Fiorita, A.; Deli, R. Case report of patients treated with an orthodontic and myofunctional protocol. Eur. J. Pediatric Dent. 2014, 15 (Suppl. 2), 184–186. [Google Scholar]
- Proffit, W.R.; McGione, R.E.; Barrett, M.J. Lip and tongue pressures related to dental arch and oral cavity size in Australian aborigines. J. Dent. Res. 1975, 54, 1161–1172. [Google Scholar] [CrossRef]
- Fujita, Y.; Maki, K. Association of feeding behavior with jaw bone metabolism and tongue pressure. JPN Dent. Sci. Rev. 2018, 54, 174–182. [Google Scholar] [CrossRef]
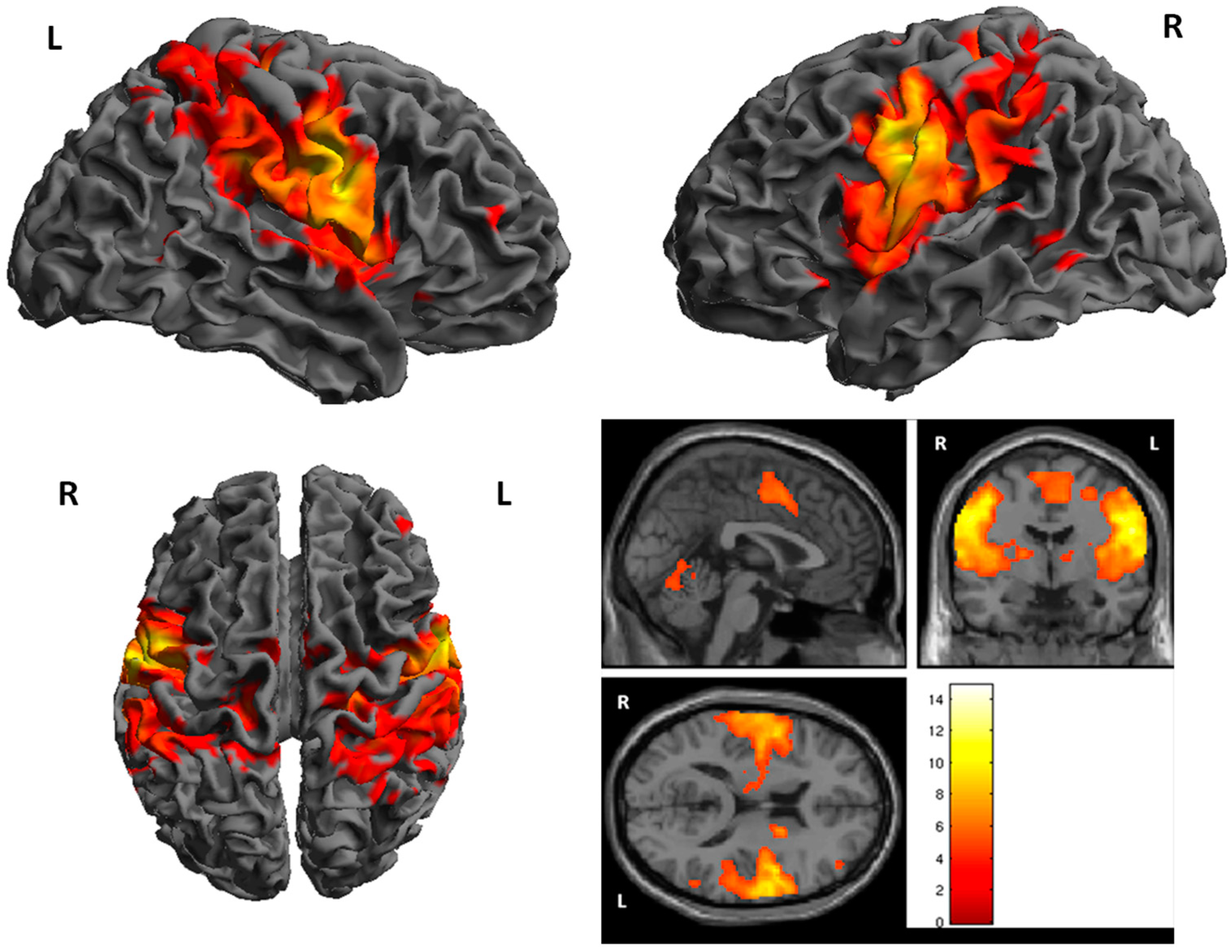
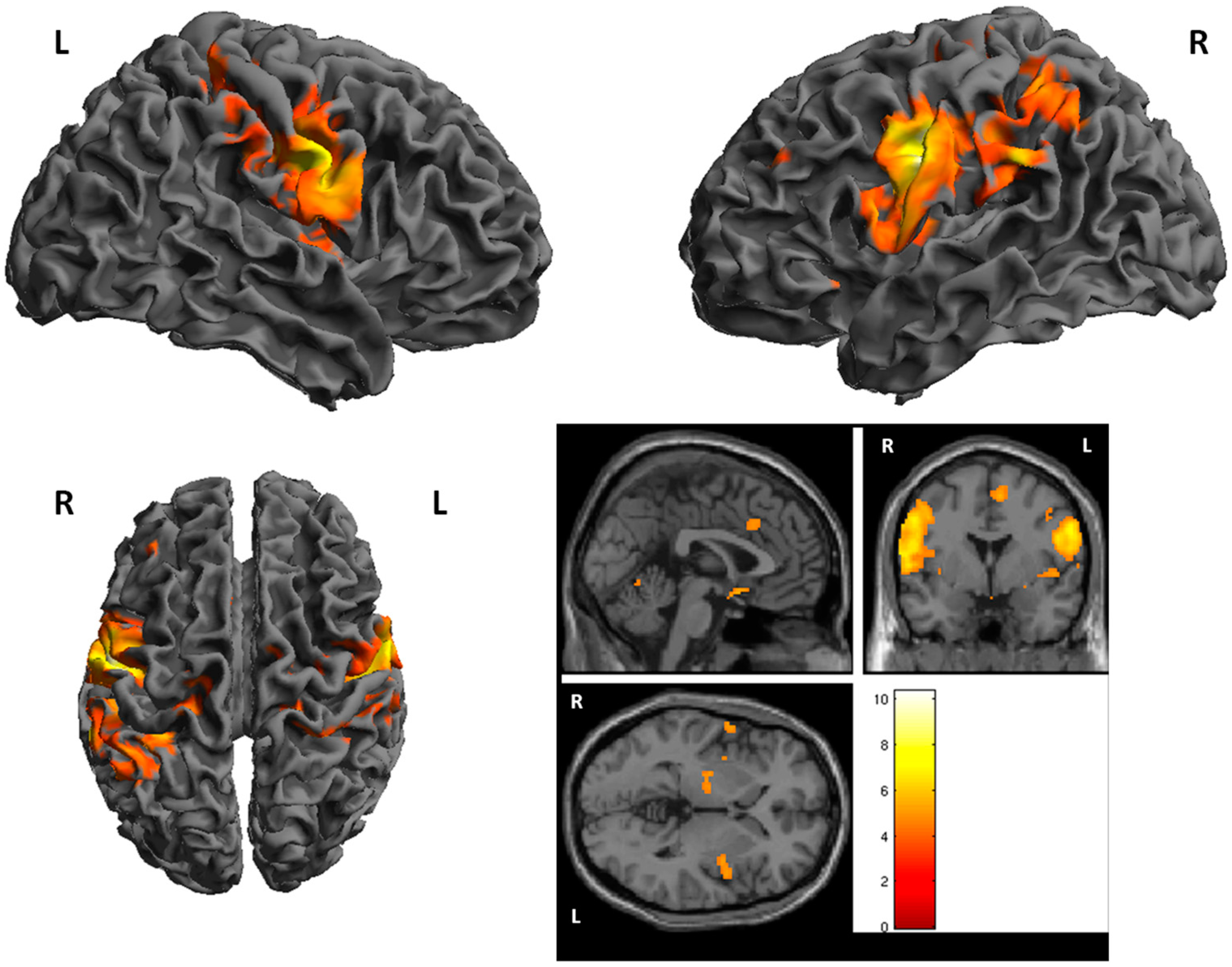
| Cluster | Cluster Size | Brodman Area | MNI Coordinates | Z Score | Structure | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 1 | 7743 | 1, 6 | 48 | −8 | 34 | 7.30 | R. PrecentralGyrus |
| 2 | 6165 | 1, 4, 6 | −56 | −8 | 34 | 7.00 | L. PrecentralGyrus |
| 3 | 448 | 18 | −60 | −22 | 6.11 | R. Cerebellum | |
| 4 | 407 | −16 | −62 | −18 | 5.54 | L. Cerebellum | |
| 5 | 275 | 3 | −16 | −28 | 62 | 5.49 | L. PostcentralGyrus |
| 6 | 200 | 7 | 36 | −60 | 64 | 5.38 | R. Superior Perietal Lobule |
| 7 | 232 | −10 | −14 | 6 | 5.35 | L.Thalamus | |
| 8 | 33 | 3, 6 | −34 | 8 | 40 | 5.33 | L. Middle Frontal Gyrus |
| 9 | 24 | 12 | −14 | 0 | 4.69 | R. Thalamus | |
| 10 | 29 | 1, 6 | −8 | −4 | 64 | 4.66 | L. Medial Frontal Gyrus |
| 11 | 17 | 39 | 56 | −56 | 12 | 4.60 | R. Superior Temporal Gyrus |
| 12 | 19 | 40 | 56 | −52 | 52 | 4.50 | R. Inferior Parietal Lobule |
| Cluster | Cluster Size | Brodman Area | MNI Coordinates | Z Score | Structure | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 1 | 1637 | 1, 6 | −46 | −10 | 36 | 6.25 | L. PrecentralGyrus |
| 2 | 231 | −16 | −60 | −22 | 5.67 | L. Cerebellum | |
| 3 | 180 | 26 | −62 | −22 | 5.56 | R. Cerebellum | |
| 4 | 1013 | 1, 6 | 52 | −8 | 26 | 5.43 | R. PrecentralGyrus |
| 5 | 86 | 40 | −64 | −38 | 30 | 5.42 | L. Inferior Parietal Lobule |
| 6 | 62 | 22 | −4 | −10 | 5.31 | R. Lentiform Nucleus | |
| 7 | 261 | 3, 40 | −34 | −40 | 42 | 5.20 | L. Inferior Parietal Lobule |
| 8 | 64 | 5 | 46 | −38 | 64 | 5.00 | R. PostcentralGyrus |
| 9 | 19 | 40 | 54 | −26 | 30 | 4.55 | R. InferiorParietalLobule |
| 10 | 14 | 1, 25 | 4 | 6 | −14 | 4.50 | R. AnteriorCingulate |
| 11 | 16 | 13 | −48 | 10 | −8 | 4.50 | L. Insula |
| 12 | 18 | 40 | 30 | −40 | 62 | 5.45 | R. InferiorParietal Lobule |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scoppa, F.; Saccomanno, S.; Bianco, G.; Pirino, A. Tongue Posture, Tongue Movements, Swallowing, and Cerebral Areas Activation: A Functional Magnetic Resonance Imaging Study. Appl. Sci. 2020, 10, 6027. https://doi.org/10.3390/app10176027
Scoppa F, Saccomanno S, Bianco G, Pirino A. Tongue Posture, Tongue Movements, Swallowing, and Cerebral Areas Activation: A Functional Magnetic Resonance Imaging Study. Applied Sciences. 2020; 10(17):6027. https://doi.org/10.3390/app10176027
Chicago/Turabian StyleScoppa, Fabio, Sabina Saccomanno, Gianluca Bianco, and Alessio Pirino. 2020. "Tongue Posture, Tongue Movements, Swallowing, and Cerebral Areas Activation: A Functional Magnetic Resonance Imaging Study" Applied Sciences 10, no. 17: 6027. https://doi.org/10.3390/app10176027
APA StyleScoppa, F., Saccomanno, S., Bianco, G., & Pirino, A. (2020). Tongue Posture, Tongue Movements, Swallowing, and Cerebral Areas Activation: A Functional Magnetic Resonance Imaging Study. Applied Sciences, 10(17), 6027. https://doi.org/10.3390/app10176027






