Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Chemicals
2.3. Dry Matter Content
2.4. HPLC Analysis of Phenolic Acids and Flavonoids
2.5. HPLC Analysis of Carotenoids
2.6. LC-MS/MS Analysis of Mycotoxins
2.7. Statistical Analyses
3. Results and Discussion
3.1. Polyphenols and Carotenoids Content
3.2. Mycotoxin Occurrence
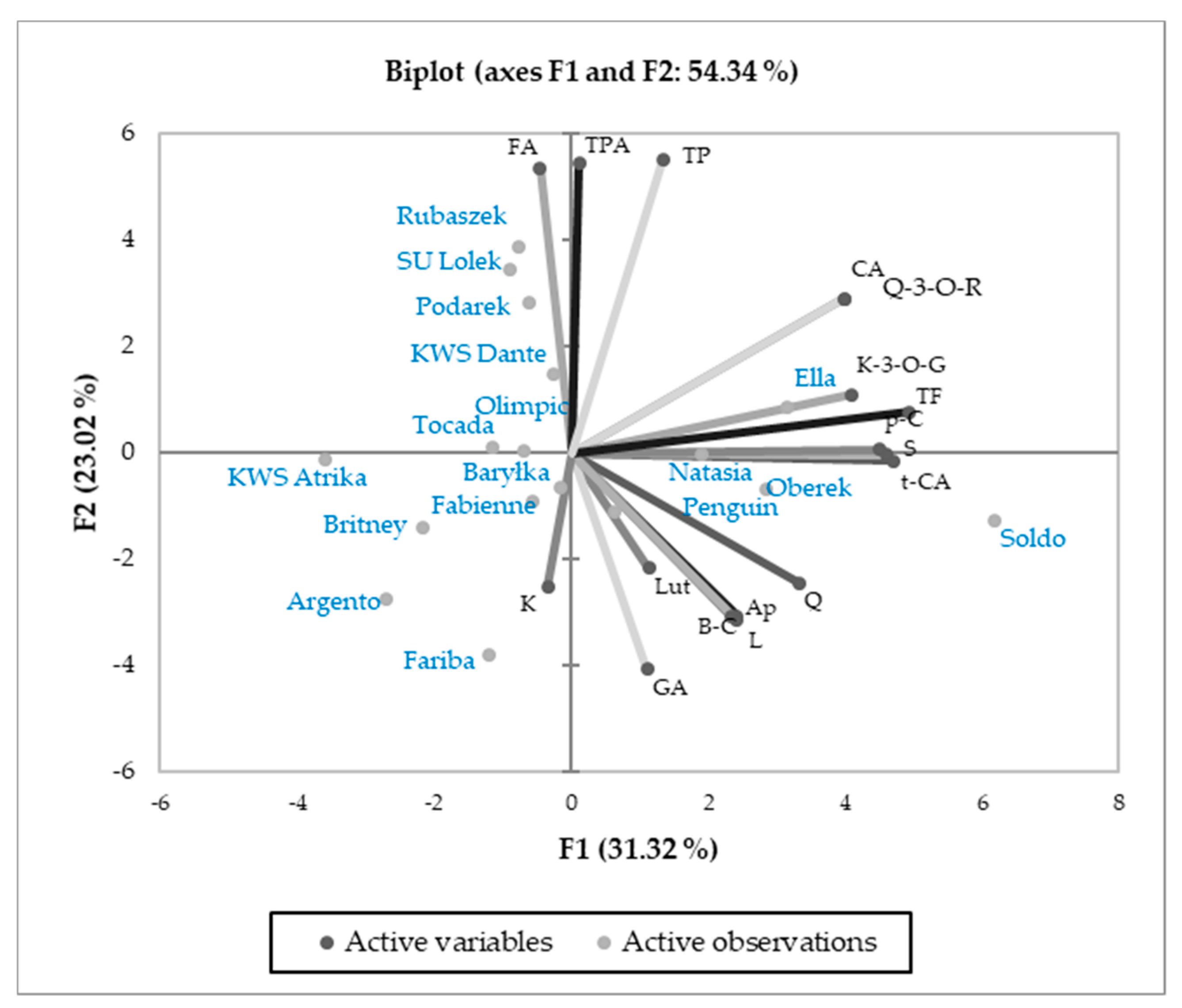
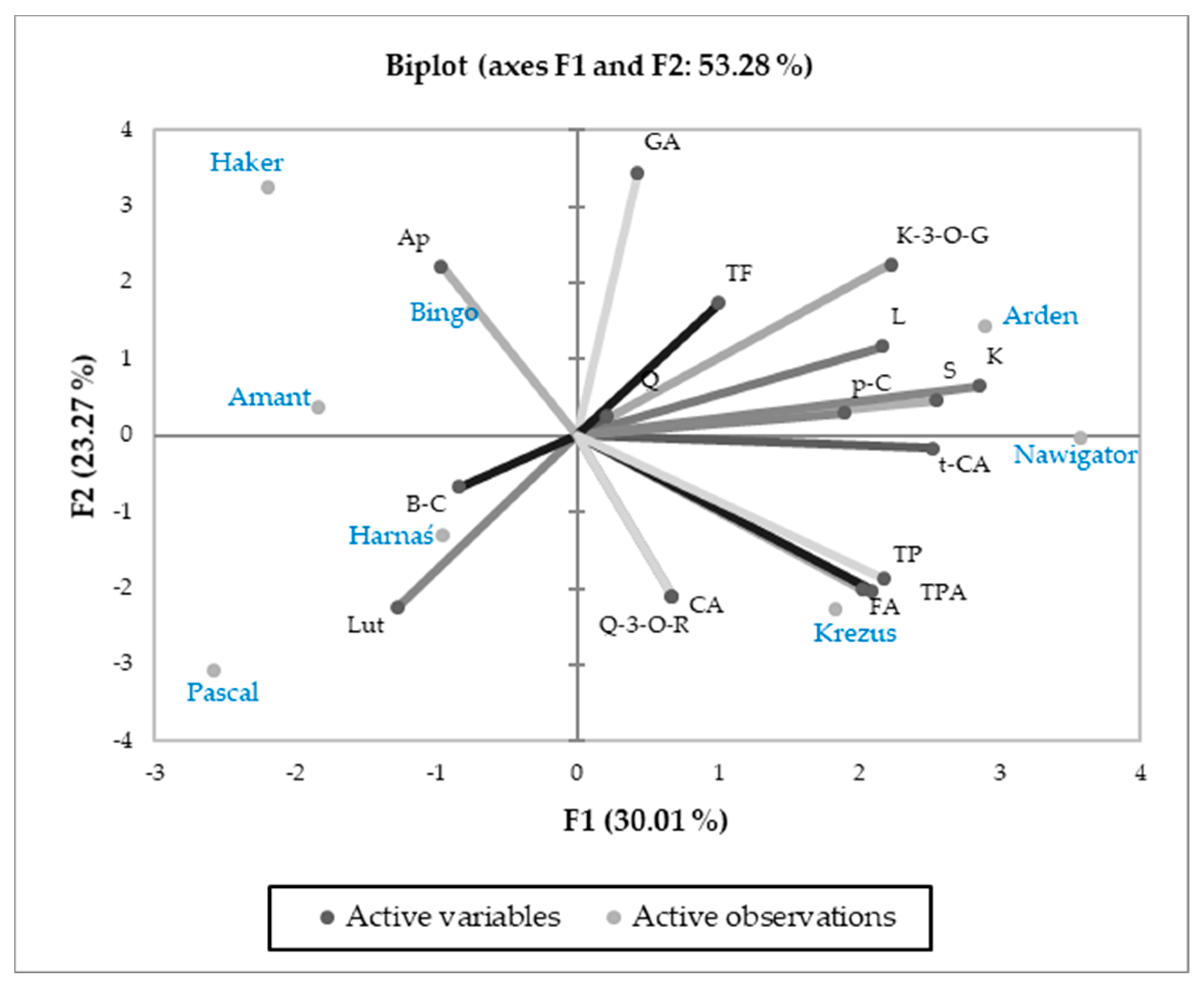
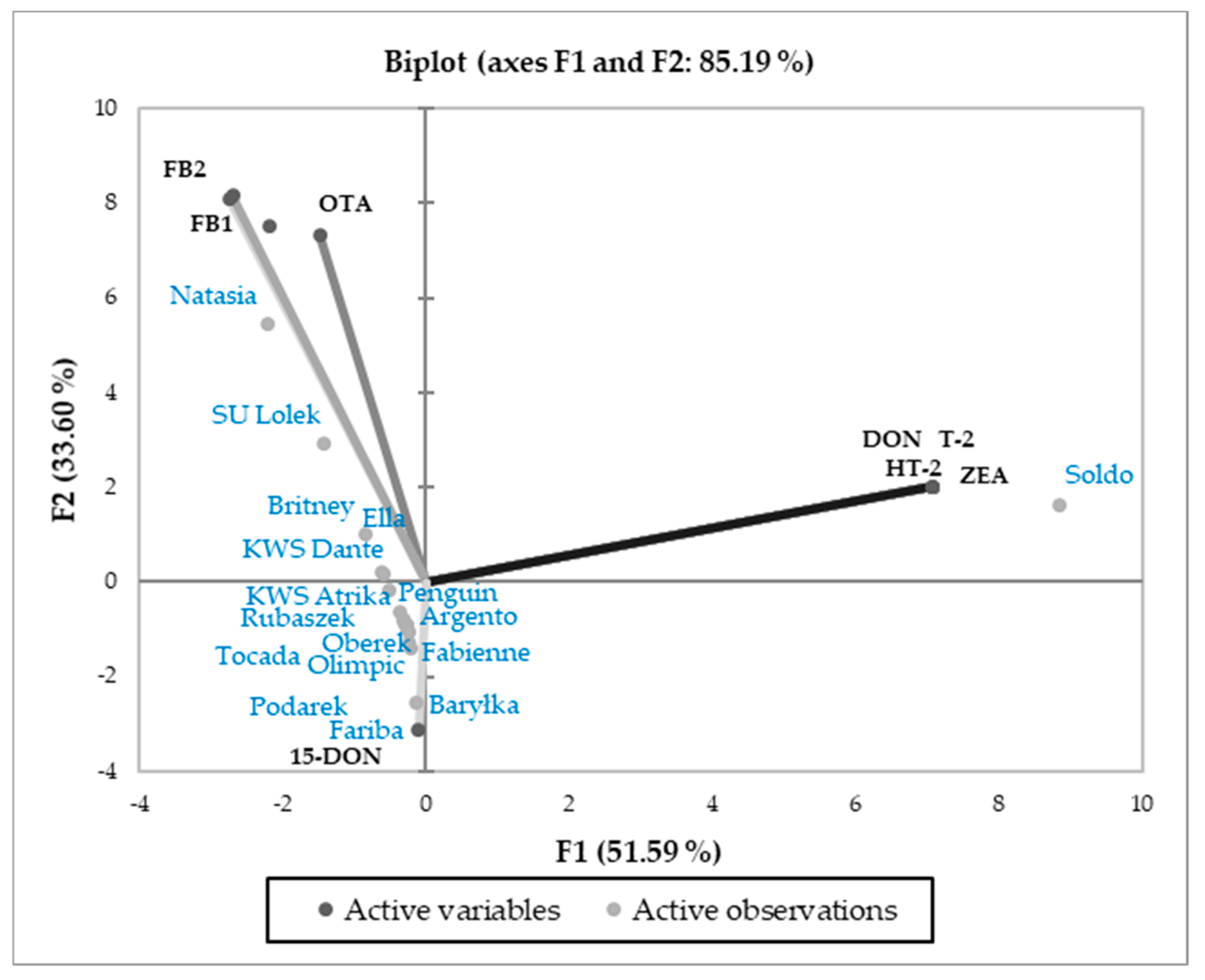
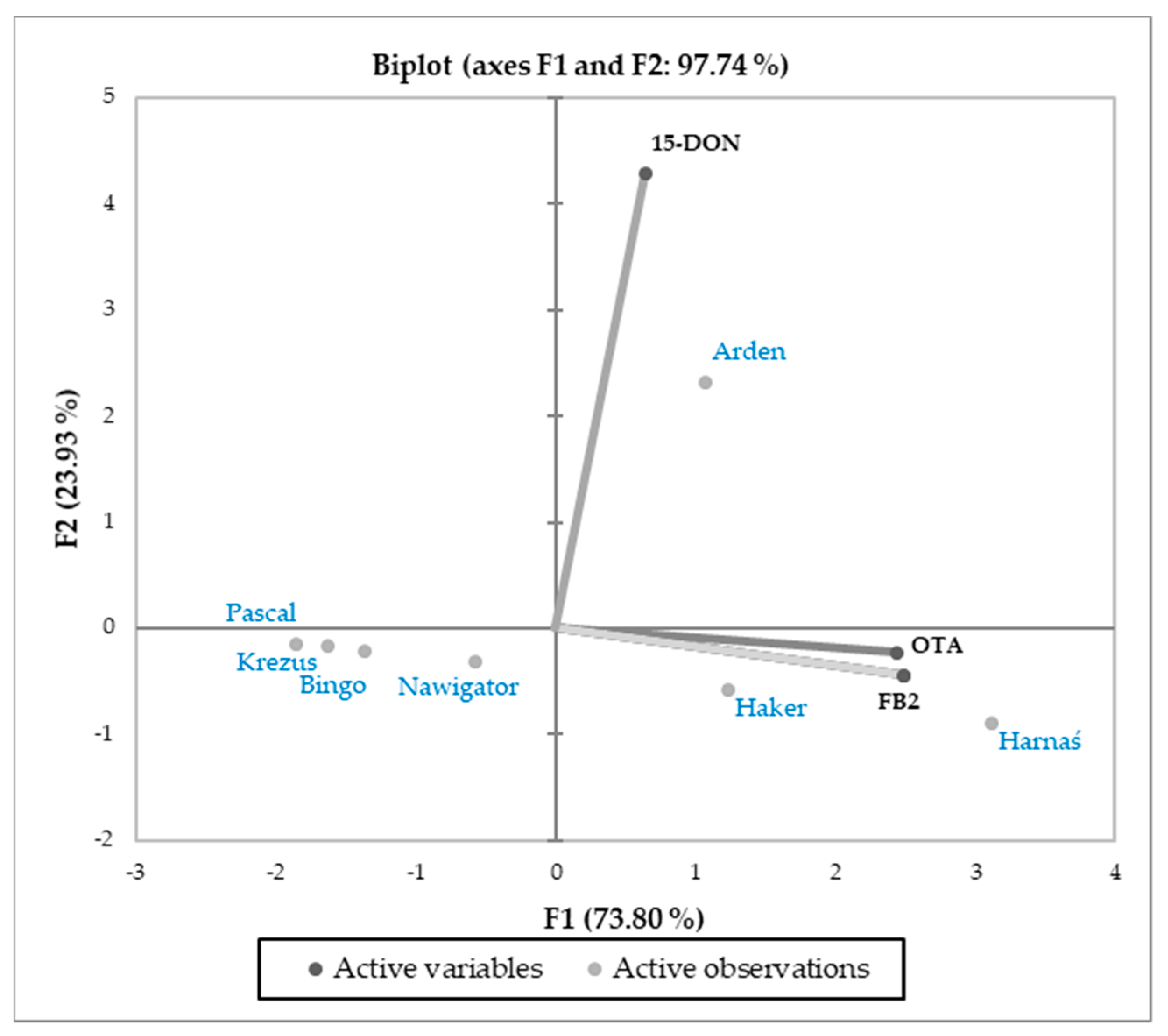
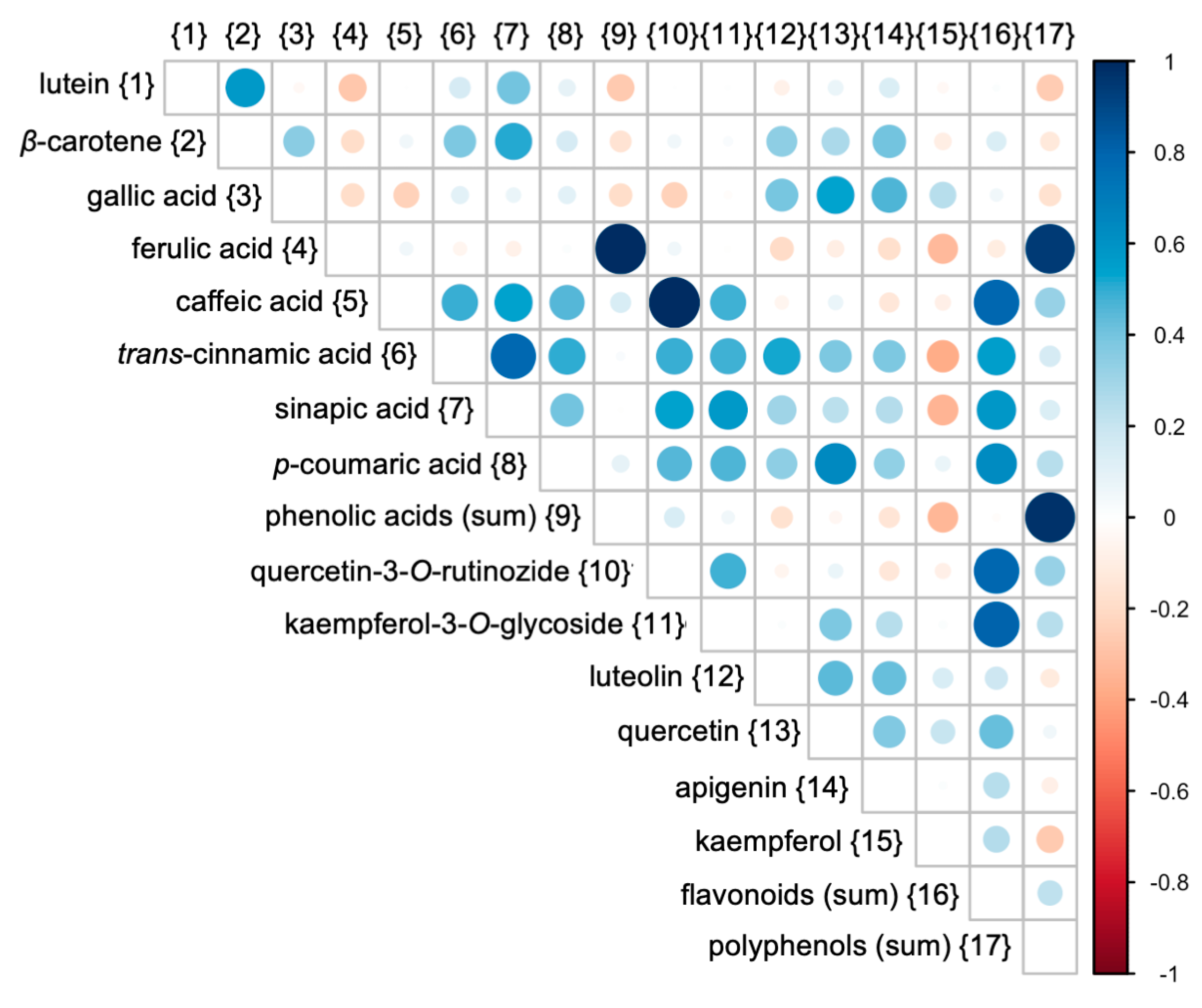
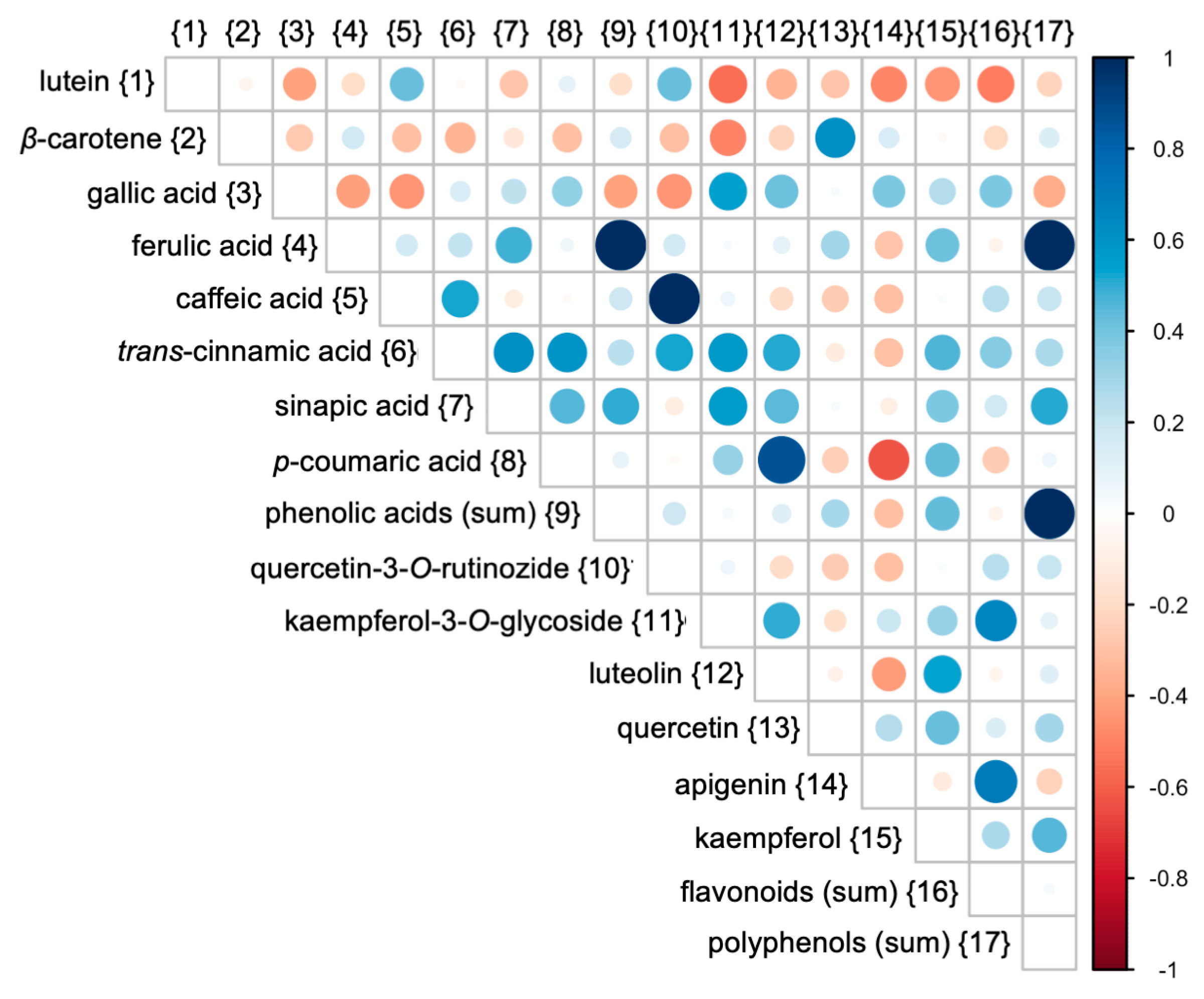
3.3. Relations Between Cultivars and Parameters Tested
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Statistics 2018. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 17 July 2020).
- Gangopadhyay, N.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules 2015, 20, 10884–10909. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.; Rabalski, I.; Messia, M.C.; Abdel-Aal, E.-S.M.; Marconi, E. Effect of processing on phenolic acids composition and radical scavenging capacity of barley pasta. Food Res. Int. 2017, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Hajji, T.; Mansouri, S.; Vecino-Bello, X.; Cruz-Freire, J.M.; Rezgui, S.; Ferchichi, A. Identification and characterization of phenolic compounds extracted from barley husks by LC-MS and antioxidant activity in vitro. J. Cereal Sci. 2018, 81, 83–90. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Amarowicz, R. Natural phenolic compounds protect LDL against oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 677–679. [Google Scholar] [CrossRef]
- Gani, A.; Wani, S.; Masoodi, F.; Hameed, G. Whole-grain cereal bioactive compounds and their health benefits: A review. J. Food Process. Technol. 2012, 3, 146. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Zhai, S.; Xia, X.; He, Z. Carotenoids in staple cereals: Metabolism, regulation, and genetic manipulation. Front. Plant Sci. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Golob, P. Good practices for animal feed and livestock. In On-Farm Mycotoxin Control in Food and Feed Grain; Training Manual 1; Food & Agriculture Organization: Roma, Italy, 2007; ISBN 978-92-5-105852-7. [Google Scholar]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Kontturi, M.; Rämö, S.; Eurola, M.; Kangas, A.; Niskanen, M.; Saastamoinen, M. Contents of trichothecenes in oats during official variety, organic cultivation and nitrogen fertilization trials in Finland. Agric. Food Sci. 2004, 13, 54–67. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Bernhoft, A.; Clasen, P.-E.; Kristoffersen, A.B.; Torp, M. Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Addit. Contam. Part A 2010, 27, 842–852. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional oats. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 1063–1069. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Kolseth, A.-K.; Steinberg, C.; Persson, P. Agricultural factors affecting Fusarium communities in wheat kernels. Int. J. Food Microbiol. 2017, 252, 53–60. [Google Scholar] [CrossRef]
- Sturite, I.; Kruma, Z.; Tomsone, L.; Åssveen, M.; Kronberga, A.; Straumite, E.; Galoburda, R. Effect of organic and conventional farming system on the total phenolic content and antioxidant activity of oats and barley grains. In Proceedings of the 4th Organic Conference: Organics for Tomorrow’s Food Systems, Mikkeli, Finland, 19–21 June 2017; Aakkula, J., Hakala, K., Huhta, H., Iivonen, S., Jurvanen, U., Kreismane, D., Land, A., Lähdesmäki, M., Malingen, M., Mikkola, M., et al., Eds.; Organic Eprints, NJF Report. 2017; 13, pp. 89–91. [Google Scholar]
- Ostrowska-Kołodziejczak, A.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Rissmann, I.; Wiwart, M.; Perkowski, J. Concentration of fungal metabolites, phenolic acids and metals in mixtures of cereals grown in organic and conventional farms. J. Anim. Feed Sci. 2016, 25, 74–81. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, C. Agroecosystem management and nutritional quality of plant foods: The case of organic fruits and vegetables. CRC Crit. Rev. Plant Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Antichi, D.; Silvestri, N.; Ciantelli, G.; Sgherri, C. Organically vs conventionally grown winter wheat: Effects on grain yield, technological quality, and on phenolic composition and antioxidant properties of bran and refined flour. Food Chem. 2015, 175, 445–451. [Google Scholar] [CrossRef] [PubMed]
- The Polish Committee for Standarization. Polish Norm: Fruits and Vegetable Juices—Determination of Dry Matter—Gravimetric Method; PN-EN 12145 2001; The Polish Committee for Standarization: Warsaw, Poland, 2001. [Google Scholar]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. Capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef] [PubMed]
- Spanjer, M.; Rensen, P.; Scholten, J. LC-MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 472–489. [Google Scholar] [CrossRef]
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document for the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Luxembourg, 2016; pp. 7–32. [Google Scholar]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Curto, A.F.; Guido, L.F. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants 2015, 4, 563–576. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2013, 52, 662–675. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chem. 2018, 239, 260–267. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Comparative study on antiproliferation properties and cellular antioxidant activities of commonly consumed food legumes against nine human cancer cell lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Bloksma, J.; Northolt, M.; Huber, M.; van der Burgt, G.J.; van de Vijver, L. A new food quality concept based on life processes. In Handbook of Organic Food Safety and Quality; Cooper, J., Niggli, U., Leifert, C., Eds.; Woodhead Publishing Limited: Cambridge, England, 2007; pp. 53–73. ISBN 978-0-8493-9154-5. [Google Scholar]
- Klepacka, J.; Gujska, E.; Michalak, J. Phenolic compounds as cultivar- and variety-distinguishing factors in some plant products. Plant Foods Hum. Nutr. 2011, 66, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Vollmannova, A.; Margitanova, E.; Tóth, T.; Timoracká, M.; Urminska, D.; Bojnanska, T.; Čičová, I. Cultivar influence on total polyphenol and rutin contents and total antioxidant capacity in buckwheat, amaranth, and quinoa seeds. Czech J. Food Sci. 2013, 31, 589–595. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Zuchowski, J. Evaluation of the content of phenolic acids and their antioxidant activity in winter cereal seeds. J. Elem. 2012, 22, 593–605. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013, 139, 663–671. [Google Scholar] [CrossRef]
- Roberts, J.; Dennison, J. The photobiology of lutein and zeaxanthin in the eye. J. Ophthalmol. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Mannaa, M.; Kim, K.D. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Rämö, S.; Hietaniemi, V.; Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R. Molecular quantification and genetic diversity of toxigenic fusarium species in Northern Europe as compared to those in Southern Europe. Microorganisms 2013, 1, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 874–910. [Google Scholar] [CrossRef] [PubMed]
| Months | Temperature (°C) | Long-Term Average Temperature (1871–2000) (°C) | Rainfall (mm) | Long-Term Average Rainfall (1871–2000) (mm) |
|---|---|---|---|---|
| March | 5.2 | 1.6 | 60 | 30 |
| April | 8.6 | 7.7 | 50 | 39 |
| May | 13.0 | 13.4 | 139 | 57 |
| June | 17.3 | 16.7 | 28 | 71 |
| July | 20.1 | 18.3 | 32 | 84 |
| August | 22.4 | 17.3 | 14 | 75 |
| Barley Cultivars | Gallic Acid | Ferulic Acid | Caffeic Acid | t-Cinnamic Acid | Sinapic Acid | P-Coumaric Acid | Sum of Phenolic Acids |
|---|---|---|---|---|---|---|---|
| Natasia | 2.12 ± 0.01 1 cdefg 2 | 42.87 ± 0.06 a | 8.37 ± 0.01 g | 1.82 ± 0.02 de | 1.51 ± 0.000 ghi | 7.38 ± 0.13 fg | 64.08 ± 0.21 a |
| SU Lolek | 1.68 ± 0.30 a | 164.69 ± 18.70 e | 4.16 ± 0.18 d | 1.26 ± 0.06 cd | 1.42 ± 0.013 cd | 6.89 ± 0.45 efg | 180.10 ± 18.29 d |
| Britney | 1.92 ± 0.12 abcd | 53.52 ± 7.85 ab | 1.85 ± 0.50 c | 2.10 ± 0.13 e | 1.41 ± 0.007 bc | 3.8 ± 0.45 a | 64.60 ± 7.92 a |
| Ella | 1.96 ± 0.09 abcde | 79.30 ± 16.52 abcd | 5.34 ± 1.16 ef | 2.05 ± 0.15 e | 1.52 ± 0.002 hi | 8.71 ± 2.38 gh | 98.87 ± 15.55 abc |
| KWS Dante | 1.87 ± 0.08 abc | 106.61 ± 64.51 d | 4.45 ± 0.16 d | 1.76 ± 0.31 de | 1.43 ± 0.009 d | 8.65 ± 0.81 gh | 124.77 ± 65.26 c |
| KWS Atrika | 1.83 ± 0.07 ab | 70.13 ± 14.02 abcd | 1.39 ± 0.03 c | 0.96 ± 0.10 abc | 1.46 ± 0.004 e | 3.87 ± 0.23 a | 79.64 ± 14.05 ab |
| Olimpic | 1.99 ± 0.11 bcdef | 62.87 ± 4.75 abc | 4.29 ± 0.36 d | 1.78 ± 0.47 de | 1.51 ± 0.004 ghi | 5.00 ± 0.49 abcd | 77.45 ± 4.97 ab |
| Rubaszek | 2.18 ± 0.13 defg | 156.34 ± 11.41 e | 4.59 ± 0.14 de | 2.07 ± 0.26 e | 1.43 ± 0.001 d | 5.33 ± 0.31 abcde | 171.94 ± 11.08 d |
| Oberek | 2.16 ± 0.15 defg | 66.19 ± 10.00 abc | 5.71 ± 0.07 f | 4.20 ± 0.82 f | 1.53 ± 0.003 i | 7.49 ± 0.43 fg | 87.28 ± 11.32 abc |
| Baryłka | 2.12 ± 0.13 cdefg | 61.62 ± 9.67 abc | 4.32 ± 0.18 d | 1.37 ± 0.01 cd | 1.5 ± 0.0190 fgh | 4.99 ± 1.59 abcd | 75.91 ± 10.94 ab |
| Tocada | 1.99 ± 0.03 bcdef | 66.96 ± 2.16 abc | 4.31 ± 0.05 d | 1.15 ± 0.34 bc | 1.49 ± 0.007 fg | 4.44 ± 0.19 ab | 80.33 ± 2.40 ab |
| Podarek | 2.14 ± 0.01 cdefg | 170.84 ± 0.32 e | 4.07 ± 0.21 d | 1.36 ± 0.02 cd | 1.50 ± 0.027 fgh | 4.68 ± 0.04 abc | 184.6 ± 0.15 d |
| Soldo | 2.36 ± 0.04 g | 81.63 ± 2.65 bcd | 5.83 ± 0.18 f | 5.46 ± 0.16 g | 1.73 ± 0.006 j | 9.39 ± 0.23 h | 106.4 ± 3.26 bc |
| Penguin | 2.24 ± 0.02 efg | 68.21 ± 13.24 abc | 4.06 ± 0.03 d | 2.32 ± 0.08 e | 1.48 ± 0.003 ef | 6.79 ± 1.50 def | 85.1 ± 14.84 ab |
| Fabienne | 2.25 ± 0.07 fg | 97.92 ± 3.56 cd | 1.19 ± 0.54 bc | 2.05 ± 0.11 e | 1.52 ± 0.003 hi | 5.45 ± 0.31 abcde | 110.38 ± 4.45 bc |
| Fariba | 2.73 ± 0.21 h | 61.25 ± 5.28 abc | 0.57 ± 0.16 ab | 0.59 ± 0.06 ab | 1.39 ± 0.001 ab | 6.28 ± 0.09 cdef | 72.80 ± 5.67 ab |
| Argento | 2.35 ± 0.28 g | 62.19 ± 0.43 abc | 0.01 ± 0.02 a | 0.52 ± 0.01 a | 1.37 ± 0.015 a | 5.84 ± 0.67 bcdef | 72.29 ± 1.35 ab |
| Mean | 2.11 ± 0.26 | 86.65 ± 41.30 | 3.79 ± 2.14 | 1.93 ± 1.23 | 1.48 ± 0.080 | 6.18 ± 1.80 | 102.15 ± 41.67 |
| Oat Cultivars | Gallic Acid | Ferulic Acid | Caffeic Acid | t-Cinnamic Acid | Sinapic Acid | p-Coumaric Acid | Sum of Phenolic Acids |
|---|---|---|---|---|---|---|---|
| Harnaś | 1.34 ± 0.01 1 ab 2 | 136.38 ± 16.25 d | 0.44 ± 0.03 ab | 0.43 ± 0.02 a | 1.36 ± 0.001 a | 4.06 ± 0.23 a | 144.01 ± 16.48 d |
| Haker | 1.49 ± 0.03 c | 16.15 ± 5.91 a | 0.38 ± 0.01 ab | 0.48 ± 0.03 a | 1.37 ± 0.001 a | 3.74 ± 0.33 a | 23.61 ± 6.30 a |
| Arden | 1.49 ± 0.07 c | 125.05 ± 4.69 cd | 0.34 ± 0.02 ab | 0.68 ± 0.01 b | 1.40 ± 0.006 b | 7.59 ± 0.22 c | 136.55 ± 4.87 cd |
| Bingo | 1.44 ± 0.02 bc | 20.19 ± 0.63 a | 0.31 ± 0.06 a | 0.67 ± 0.08 b | 1.35 ± 0.001 a | 7.07 ± 0.09 c | 31.03 ± 0.77 a |
| Nawigator | 1.41 ± 0.06 abc | 142.69 ± 21.24 d | 0.53 ± 0.05 b | 1.01 ± 0.02 c | 1.48 ± 0.037 c | 7.35 ± 0.21 c | 154.48 ± 21.15 d |
| Krezus | 1.34 ± 0.10 ab | 105.02 ± 3.47 bc | 3.19 ± 0.24 e | 1.07 ± 0.15 c | 1.37 ± 0.012 ab | 6.02 ± 0.85 b | 118.01 ± 2.60 bc |
| Pascal | 1.29 ± 0.06 a | 100.86 ± 9.26 b | 0.95 ± 0.01 d | 0.34 ± 0.02 a | 1.35 ± 0.002 a | 5.25 ± 0.31 b | 110.04 ± 9.66 b |
| Amant | 1.46 ± 0.05 bc | 13.88 ± 0.95 a | 0.74 ± 0.03 c | 0.69 ± 0.08 b | 1.37 ± 0.001 a | 7.19 ± 0.55 c | 25.32 ± 1.65 a |
| Mean | 1.41 ± 0.08 | 82.43 ± 54.90 | 0.93 ± 0.86 | 0.67 ± 0.26 | 1.38 ± 0.040 | 6.03 ± 1.51 | 92.88 ± 55.22 |
| Barley Cultivars | Quercetin-3-O-Rutinoside | Kaempferol-3-O-Glucoside | Luteolin | Quercetin | Apigenin | Kaempferol | Sum of Flavonoids |
|---|---|---|---|---|---|---|---|
| Natasia | 27.08 ± 0.03 1 g 2 | 5.54 ± 0.01 fg | 1.87 ± 0.00 a | 2.37 ± 0.03 abc | 6.16 ± 0.02 ab | 9.04 ± 0.23 f | 52.07 ± 0.15 j |
| SU Lolek | 14.46 ± 0.55 d | 3.62 ± 0.45 de | 2.21 ± 0.18 ab | 2.15 ± 0.15 a | 6.14 ± 0.82 ab | 5.57 ± 0.27 d | 34.15 ± 1.52 fgh |
| Britney | 7.53 ± 1.50 c | 3.91 ± 0.02 e | 2.32 ± 0.30 ab | 2.38 ± 0.12 abc | 5.77 ± 0.06 a | 6.03 ± 0.15 d | 27.94 ± 1.99 bc |
| Ella | 17.99 ± 3.48 ef | 19.43 ± 0.41 j | 1.75 ± 0.06 a | 3.63 ± 0.21 fgh | 7.48 ± 1.37 bc | 7.21 ± 0.24 e | 57.50 ± 4.40 k |
| KWS Dante | 15.34 ± 0.48 d | 2.28 ± 0.22 cd | 1.88 ± 0.03 a | 2.89 ± 0.29 bcd | 5.80 ± 0.20 ab | 2.07 ± 0.10 a | 30.26 ± 1.31 cd |
| KWS Atrika | 6.15 ± 0.08 c | 0.92 ± 0.32 ab | 1.81 ± 0.08 a | 2.01 ± 0.01 a | 4.71 ± 0.21 a | 1.88 ± 0.04 a | 17.48 ± 0.75 a |
| Olimpic | 14.86 ± 1.07 d | 5.48 ± 0.12 fg | 2.02 ± 0.06 ab | 2.09 ± 0.09 a | 5.52 ± 0.32 a | 1.82 ± 0.00 a | 31.78 ± 1.30 def |
| Rubaszek | 15.75 ± 0.42 de | 7.48 ± 0.27 h | 1.97 ± 0.06 a | 2.64 ± 0.20 abcd | 5.78 ± 0.30 a | 2.11 ± 0.10 a | 35.72 ± 1.34 h |
| Oberek | 19.12 ± 0.22 f | 6.57 ± 0.36 gh | 3.74 ± 0.10 c | 3.29 ± 0.44 def | 8.02 ± 0.33 c | 2.75 ± 0.09 b | 43.47 ± 0.38 i |
| Baryłka | 14.93 ± 0.55 d | 6.46 ± 1.16 gh | 2.24 ± 1.08 ab | 2.65 ± 0.74 abcd | 5.65 ± 0.92 a | 4.43 ± 0.82 c | 36.36 ± 0.21 h |
| Tocada | 14.90 ± 0.15 d | 4.76 ± 0.92 ef | 2.08 ± 0.07 ab | 2.23 ± 0.19 a | 5.56 ± 0.05 a | 5.70 ± 0.18 d | 35.23 ± 0.74 gh |
| Podarek | 14.19 ± 0.62 d | 3.84 ± 1.62 e | 1.97 ± 0.12 a | 2.58 ± 0.27 abc | 5.26 ± 0.01 a | 3.09 ± 0.57 b | 30.92 ± 2.66 cde |
| Soldo | 19.46 ± 0.55 f | 11.67 ± 0.32 i | 4.28 ± 0.55 c | 3.69 ± 0.13 gh | 8.52 ± 0.03 cd | 2.99 ± 0.01 b | 50.60 ± 0.41 j |
| Penguin | 14.17 ± 0.08 d | 3.96 ± 1.08 e | 2.01 ± 0.20 ab | 3.01 ± 0.70 cde | 9.02 ± 2.53 cd | 1.96 ± 0.04 a | 34.12 ± 3.00 fgh |
| Fabienne | 5.54 ± 1.62 bc | 6.49 ± 0.07 gh | 1.85 ± 0.41 a | 2.27 ± 0.12 ab | 10.08 ± 0.76 d | 1.82 ± 0.13 a | 28.05 ± 0.13 bc |
| Fariba | 3.69 ± 0.48 ab | 2.14 ± 0.32 bc | 2.69 ± 0.07 b | 4.14 ± 0.11 h | 7.50 ± 0.02 bc | 9.01 ± 0.12 f | 29.17 ± 1.07 bcd |
| Argento | 2.02 ± 0.05 a | 0.72 ± 0.05 a | 3.75 ± 0.10 c | 2.56 ± 0.04 abc | 8.11 ± 0.60 c | 9.13 ± 0.03 f | 26.28 ± 0.67 b |
| Means | 13.36 ± 6.41 | 5.60 ± 4.40 | 2.38 ± 0.80 | 2.74 ± 0.65 | 6.77 ± 1.62 | 4.51 ± 2.72 | 35.36 ± 10.16 |
| Oat Cultivars | Quercetin-3-O-Rutinoside | Kaempferol-3-O-Glucoside | Luteolin | Quercetin | Apigenin | Kaempferol | Sum of Flavonoids |
|---|---|---|---|---|---|---|---|
| Harnaś | 3.30 ± 0.10 1 ab 2 | 1.09 ± 0.10 ab | 2.44 ± 0.24 ab | 3.38 ± 0.32 a | 11.93 ± 6.51 b | 9.96 ± 0.80 ab | 32.11 ± 5.14 b |
| Haker | 3.13 ± 0.03 ab | 4.06 ± 1.15 de | 2.38 ± 0.18 a | 2.97 ± 0.41 a | 18.42 ± 1.61 c | 9.36 ± 1.21 ab | 40.32 ± 2.22 d |
| Arden | 3.00 ± 0.05 ab | 4.66 ± 0.09 e | 4.95 ± 0.41 c | 2.95 ± 0.05 a | 7.94 ± 0.18 ab | 10.50 ± 0.33 b | 34.00 ± 1.11 bc |
| Bingo | 2.91 ± 0.17 a | 3.04 ± 0.02 cd | 4.84 ± 0.02 c | 2.79 ± 0.14 a | 6.75 ± 0.14 ab | 9.70 ± 0.13 ab | 30.02 ± 0.04 b |
| Nawigator | 3.57 ± 0.14 b | 4.12 ± 1.01 de | 4.36 ± 0.37 c | 2.92 ± 0.21 a | 7.31 ± 0.38 ab | 10.22 ± 0.45 ab | 32.49 ± 0.50 b |
| Krezus | 11.54 ± 0.72 d | 3.73 ± 0.37 de | 3.30 ± 0.79 b | 2.78 ± 0.38 a | 6.44 ± 0.47 ab | 9.99 ± 0.01 ab | 37.78 ± 0.56 cd |
| Pascal | 4.83 ± 0.03 c | 0.45 ± 0.02 a | 2.61 ± 0.25 ab | 2.73 ± 0.42 a | 4.93 ± 1.18 a | 8.93 ± 0.69 a | 24.48 ± 1.73 a |
| Amant | 4.21 ± 0.08 c | 1.92 ± 0.19 bc | 3.26 ± 0.15 b | 2.73 ± 0.26 a | 7.29 ± 0.41 ab | 9.55 ± 0.48 ab | 28.95 ± 1.57 ab |
| Means | 4.56 ± 2.80 | 2.88 ± 1.55 | 3.52 ± 1.06 | 2.91 ± 0.30 | 8.88 ± 4.56 | 9.78 ± 0.67 | 32.52 ± 5.08 |
| Cultivar | Total Polyphenols (mg 100 g−1) | Lutein (μg kg−1) | β-Carotene (μg kg−1) |
|---|---|---|---|
| Barley | |||
| Natasia | 116.15 ± 0.35 1 abc 2 | 834.44 ± 15.02 fgh | 1.16 ± 0.05 b |
| SU Lolek | 214.25 ± 16.77 e | 810.30 ± 20.20 ef | 1.06 ± 0.12 b |
| Britney | 92.53 ± 9.90 a | 843.85 ± 32.48 gh | 1.23 ± 0.13 b |
| Ella | 156.37 ± 19.95 d | 793.22 ± 2.95 de | 0.96 ± 0.34 ab |
| KWS Dante | 155.04 ± 16.57 cd | 808.20 ± 10.14 def | 1.16 ± 0.09 b |
| KWS Atrika | 97.12 ± 13.31 a | 860.52 ± 4.54 h | 0.92 ± 0.21 ab |
| Olimpic | 109.24 ± 6.27 ab | 798.93 ± 5.43 de | 1.20 ± 0.04 b |
| Rubaszek | 207.67 ± 9.74 e | 596.69 ± 6.60 a | 0.91 ± 0.02 a |
| Oberek | 130.75 ± 11.71 abcd | 737.59 ± 6.63 c | 0.89 ± 0.10 b |
| Baryłka | 112.27 ± 11.15 ab | 777.69 ± 4.54 d | 1.48 ± 0.01 c |
| Tocada | 115.56 ± 1.66 abc | 810.62 ± 23.80 ef | 1.31 ± 0.13 b |
| Podarek | 215.52 ± 2.51 e | 812.37 ± 10.90 efg | 1.20 ±0.02 b |
| Soldo | 157.00 ± 3.67 d | 897.34 ± 28.41 i | 1.72 ± 0.24 c |
| Penguin | 119.22 ± 11.84 abcd | 838.15 ± 20.26 fgh | 1.56 ± 0.15 b |
| Fabienne | 138.43 ± 4.58 bcd | 858.44 ± 18.11 h | 1.35 ± 0.05 b |
| Fariba | 101.97 ± 6.74 ab | 852.84 ± 10.05 h | 1.50 ± 0.14 b |
| Argento | 98.57 ± 0.68 a | 671.03 ± 1.74 b | 1.22 ± 0.07 b |
| Mean | 137.51 ± 42.73 | 800.13 ± 73.36 | 1.23 ± 0.31 |
| Oat | |||
| Harnaś | 176.12 ± 11.35 cd | 688.95 ± 9.95 a | 1.53 ± 0.11 b |
| Haker | 63.93 ± 8.51 a | 664.29 ± 4.08 a | 1.61 ± 0.40 a |
| Arden | 170.55 ± 5.98 cd | 652.98 ± 1.19 a | 0.89 ± 0.02 ab |
| Bingo | 61.05 ± 0.73 a | 686.54 ± 15.04 a | 1.32 ± 0.39 ab |
| Nawigator | 186.97 ± 21.65 d | 700.71 ± 12.63 a | 1.19 ± 0.14 ab |
| Krezus | 155.79 ± 2.04 bc | 828.98 ± 35.03 b | 1.07 ± 0.11 ab |
| Pascal | 134.51 ± 11.39 b | 908.79 ± 33.32 bc | 0.82 ± 0.57 ab |
| Amant | 54.27 ± 3.23 a | 930.30 ± 1.58 c | 1.40 ± 0.04 ab |
| Mean | 125.40 ± 55.15 | 757.69 ± 83.51 | 1.23 ± 0.38 |
| OTA 1 | FB1 2 | FB2 3 | FB1 + FB2 | ZEA 4 | HT-2 5 | T-2 6 | HT2 + T2 | DON 7 | 15-AcDON 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum levels for mycotoxins in cereal grains 9 | 5.0 | - 10 | - | - | 100.0 | - | - | 200.0 | 1250.0 | - |
| Barley cultivars | ||||||||||
| Natasia | 3.48 | 141.37 | 645.34 | 786.71 | - | - | - | - | - | - |
| SU Lolek | 2.90 | 83.59 | 393.13 | 476.72 | - | - | - | - | - | - |
| Britney | 2.68 | - | 289.52 | 289.52 | - | - | - | - | - | - |
| Ella | 2.04 | - | 197.50 | 197.50 | - | - | - | - | - | - |
| KWS Dante | 2.16 | - | 181.32 | 181.32 | - | - | - | - | - | - |
| KWS Atrika | 1.84 | - | 148.48 | 148.48 | - | - | - | - | - | - |
| Olimpic | - | - | 89.03 | 89.03 | - | - | - | - | - | - |
| Rubaszek | 1.78 | - | 72.39 | 72.39 | - | - | - | - | - | - |
| Oberek | 1.61 | - | 60.55 | 60.55 | - | - | - | - | - | - |
| Baryłka | 1.62 | - | 52.85 | 52.85 | - | - | - | - | - | - |
| Tocada | 1.53 | - | 40.98 | 40.98 | - | - | - | - | - | - |
| Podarek | 1.50 | - | 45.43 | 45.43 | - | - | - | - | - | - |
| Soldo | 1.53 | - | 33.74 | 33.74 | 8.00 | 3.42 | 3.55 | 6.97 | 58.71 | - |
| Penguin | 1.53 | - | 30.61 | 30.61 | - | - | - | - | - | - |
| Fabienne | - | 28.95 | 33.57 | 62.52 | - | - | - | - | - | - |
| Fariba | - | - | 26.34 | 26.34 | - | - | - | - | - | 6.1 |
| Argento | 1.47 | - | 23.13 | 23.13 | - | - | - | - | - | - |
| Means | 1.63 | 14.94 | 139.05 | 153.99 | 0.47 | 0.20 | 0.21 | 0.41 | 3.45 | 0.36 |
| OTA 1 | FB1 2 | FB2 3 | FB1 + FB2 | ZEA 4 | HT-2 5 | T-2 6 | HT2 + T2 | DON 7 | 15-AcDON 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Maximum levels for mycotoxins in cereal grains 9 | 5.0 | - 10 | - | - | 100.0 | - | - | 200.0 | 1250.0 | - |
| Oat cultivars | ||||||||||
| Harnaś | 2.02 | - | 69.72 | 69.72 | - | - | - | - | - | - |
| Haker | 1.93 | - | 52.07 | 52.07 | - | - | - | - | - | - |
| Arden | 1.80 | - | 49.48 | 49.48 | - | - | - | - | - | 2.28 |
| Bingo | 1.51 | - | 36.07 | 36.07 | - | - | - | - | - | - |
| Nawigator | 1.71 | - | 38.90 | 38.90 | - | - | - | - | - | - |
| Krezus | 1.42 | - | 33.27 | 33.27 | - | - | - | - | - | - |
| Pascal | 1.49 | - | 33.75 | 33.75 | - | - | - | - | - | - |
| Amant | - | - | - | - | - | - | - | - | - | - |
| Means | 1.49 | 0.00 | 39.16 | 39.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, R.; Średnicka-Tober, D.; Leszczyńska, D.; Nowacka, A.; Hallmann, E.; Barański, M.; Kopczyńska, K.; Gnusowski, B. Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management. Appl. Sci. 2020, 10, 6369. https://doi.org/10.3390/app10186369
Kazimierczak R, Średnicka-Tober D, Leszczyńska D, Nowacka A, Hallmann E, Barański M, Kopczyńska K, Gnusowski B. Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management. Applied Sciences. 2020; 10(18):6369. https://doi.org/10.3390/app10186369
Chicago/Turabian StyleKazimierczak, Renata, Dominika Średnicka-Tober, Danuta Leszczyńska, Anna Nowacka, Ewelina Hallmann, Marcin Barański, Klaudia Kopczyńska, and Bogusław Gnusowski. 2020. "Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management" Applied Sciences 10, no. 18: 6369. https://doi.org/10.3390/app10186369
APA StyleKazimierczak, R., Średnicka-Tober, D., Leszczyńska, D., Nowacka, A., Hallmann, E., Barański, M., Kopczyńska, K., & Gnusowski, B. (2020). Evaluation of Phenolic Compounds and Carotenoids Content and Mycotoxins Occurrence in Grains of Seventeen Barley and Eight Oat Cultivars Grown under Organic Management. Applied Sciences, 10(18), 6369. https://doi.org/10.3390/app10186369








