Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives
Abstract
1. Introduction
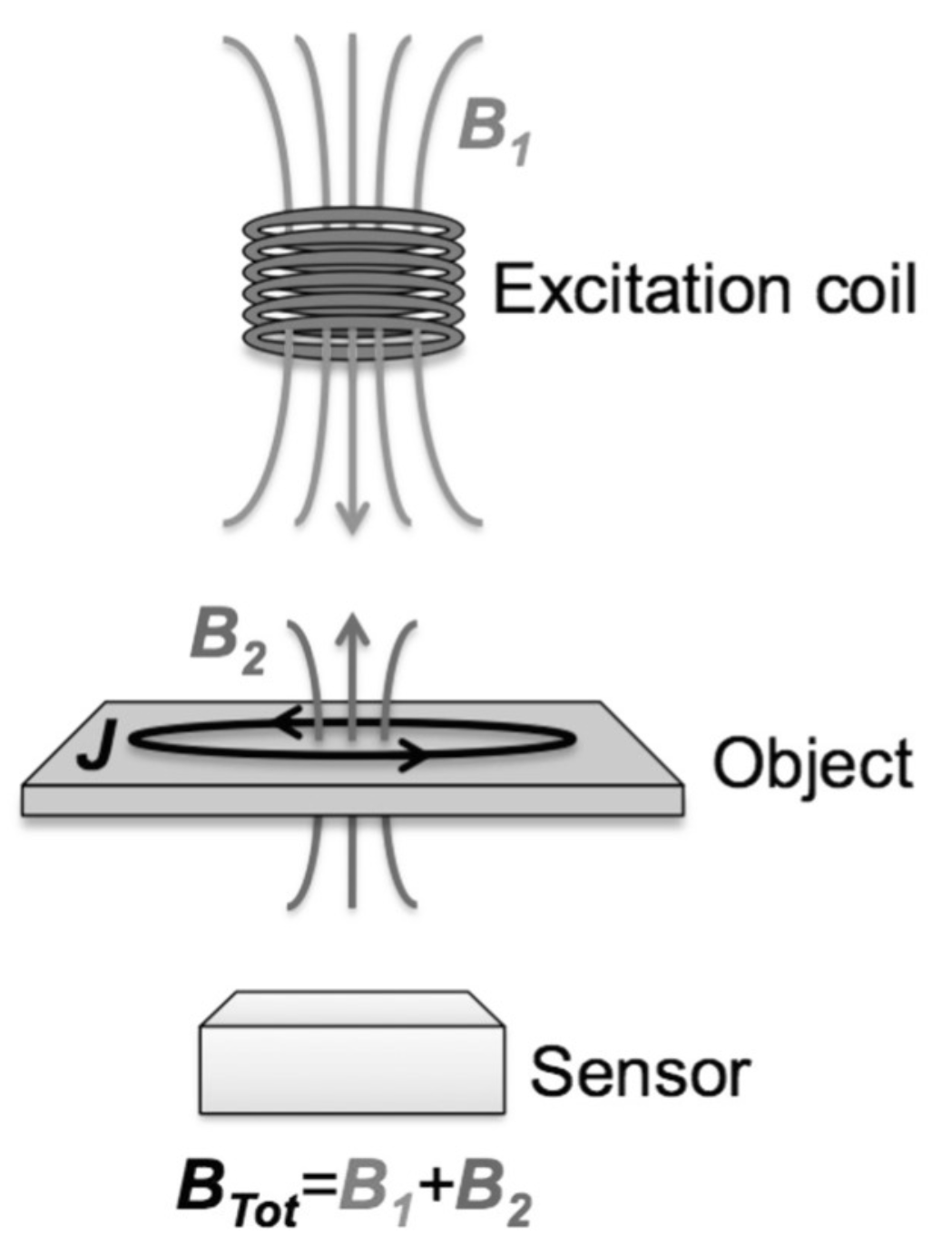
2. Principles of Electromagnetic Induction Imaging
3. Application Areas and Limitations of Inductive Coils
3.1. Security
3.2. Surveillance
3.3. Biomedical Imaging
3.3.1. Head Injuries
3.3.2. Cancer
3.3.3. Atrial Fibrillation
4. Electromagnetic Induction Imaging with Atomic Magnetometers
4.1. Motivations
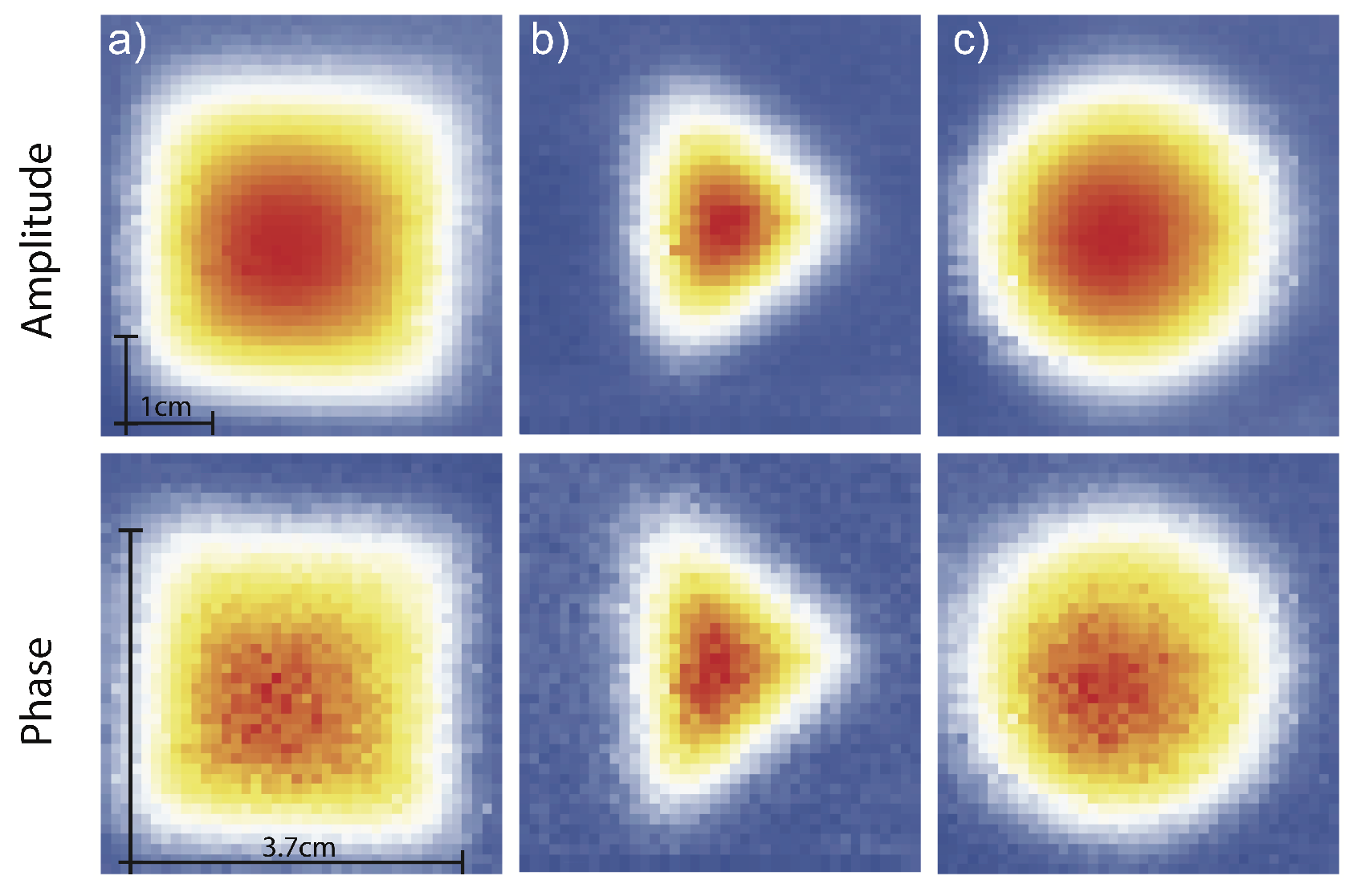
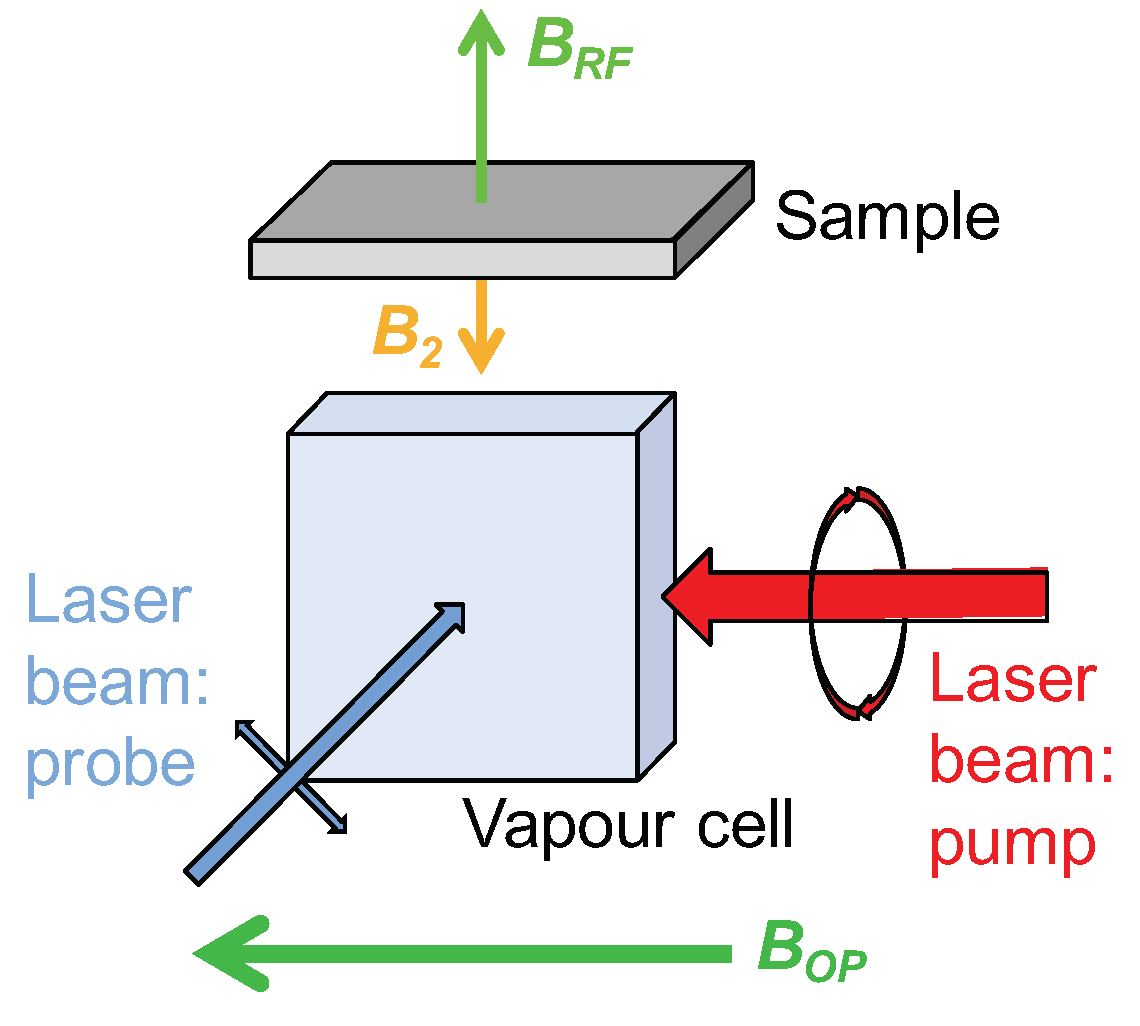
4.2. First Proof-Of-Concept of EMI with Atomic Magnetometers
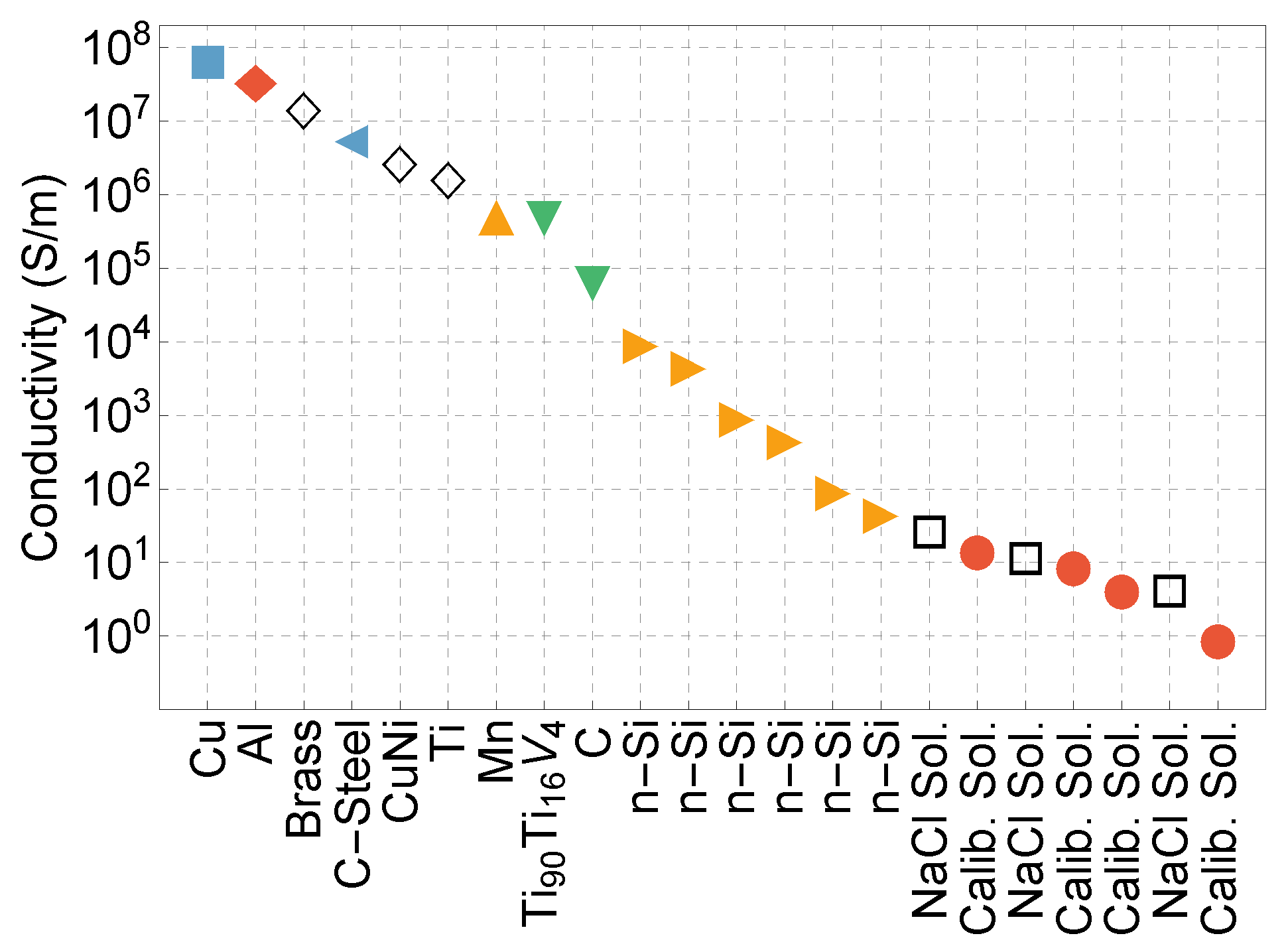
4.3. Development of EMI with AMs and the Quest for Ultimate Sensitivity
4.4. Alghorithms for Image Reconstruction: The Machine Learning Approach
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EMI | Electromagnetic Induction Imaging |
| MIT | Magnetic Induction Tomography |
| AM | atomic magnetometer |
| RF | radio frequency |
References
- Griffiths, H. Magnetic induction tomography. Meas. Sci. Technol. 2001, 12, 1126. [Google Scholar] [CrossRef]
- Korjenevsky, A.; Cherepenin, V.; Sapetsky, S. Magnetic induction tomography: Experimental realization. Physiol. Meas. 2000, 21, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Merwa, R.; Hollaus, K.; Brandstaetter, B.; Scharfetter, H. Numerical solution of the general 3D eddy current problem for magnetic induction tomography (spectroscopy). Physiol. Meas. 2003, 24, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Scharfetter, H.; Casañas, R.C.; Rosell, J. Biological Tissue Characterization by Magnetic Induction Spectroscopy (MIS): Requirements and Limitations. IEEE Trans. Biomed. Eng. 2003, 50, 870–880. [Google Scholar] [CrossRef]
- Griffiths, H.; Gough, W.; Watson, S.; Williams, R.J. Residual capacitive coupling and the measurement of permittivity in magnetic induction tomography. Physiol. Meas. 2007, 28, S301–S311. [Google Scholar] [CrossRef]
- Darrer, B.J.; Watson, J.C.; Bartlett, P.; Renzoni, F. Magnetic Imaging: A New Tool for UK National Nuclear Security. Sci. Rep. 2015, 5, 7944. [Google Scholar] [CrossRef]
- Darrer, B.J.; Watson, J.C.; Bartlett, P.A.; Renzoni, F. Electromagnetic imaging through thick metallic enclosures. AIP Adv. 2015, 5, 087143. [Google Scholar] [CrossRef]
- Guilizzoni, R.; Watson, J.C.; Bartlett, P.; Renzoni, F. Penetrating power of resonant electromagnetic induction imaging. AIP Adv. 2016, 6, 095017. [Google Scholar] [CrossRef]
- Alzeibak, S.; Saunders, N.H. A feasibility study of in vivo electromagnetic imaging. Phys. Med. Biol. 1993, 38, 151–160. [Google Scholar] [CrossRef]
- Merwa, R.; Hollaus, K.; Biró, O.; Scharfetter, H. Detection of brain oedema using magnetic induction tomography: A feasibility study of the likely sensitivity and detectability. Phys. Meas. 2004, 25, 347–354. [Google Scholar] [CrossRef]
- Pan, W.; Yan, Q.; Qin, M.; Jin, G.; Sun, J.; Ning, X.; Wei, Z.; Bin, P.; Gen, L. Detection of Cerebral Hemorrhage in Rabbits by Time-Difference Magnetic Inductive Phase Shift Spectroscopy. PLoS ONE 2015, 10, e0128127. [Google Scholar] [CrossRef] [PubMed]
- Zolgharni, M.; Griffiths, H.; Ledger, P.D. Frequency-difference MIT imaging of cerebral haemorrhage with a hemispherical coil array: Numerical modelling. Physiol. Meas. 2010, 31, S111–S125. [Google Scholar] [CrossRef] [PubMed]
- Joines, W.T.; Zhang, Y.; Li, C.; Jirtle, R.L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 1994, 21, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: III. parametric models for the dielectric spectrum of tissues. Phys. Med. Biol. 1996, 41, 2271–2293. [Google Scholar] [CrossRef]
- Wang, L.; Al-Jumaily, A.M. Imaging of Lung Structure Using Holographic Electromagnetic Induction. IEEE Access 2017, 5, 20313–20318. [Google Scholar] [CrossRef]
- Longo, D.; Fauci, A.; Kasper, D.; Hauser, S.; Jameson, J.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 18th ed.; McGraw Hill Professional: New York, NY, USA, 2012; Volume 2. [Google Scholar]
- Vaquero, M.; Calvo, D.; Jalife, J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm 2008, 5, 872–879. [Google Scholar] [CrossRef]
- Christ, T.; Rozmaritsa, N.; Engel, A.; Berk, E.; Knaut, M.; Metzner, K.; Canteras, M.; Ravens, U.; Kaumannb, A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 11193–11198. [Google Scholar] [CrossRef]
- Narayan, S.M.; Patel, J.; Mulpuru, S.; Krummen, D.E. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: A video case study. Heart Rhythm 2012, 9, 1436–1439. [Google Scholar] [CrossRef]
- Narayan, S.M.; Krummen, D.E.; Rappel, W.-J. Clinical Mapping Approach To Diagnose Electrical Rotors and Focal Impulse Sources for Human Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2012, 23, 447–454. [Google Scholar] [CrossRef]
- Marmugi, L.; Renzoni, F. Optical Magnetic Induction Tomography of the Heart. Sci. Rep. 2016, 6, 23962. [Google Scholar] [CrossRef]
- Deans, C.; Marmugi, L.; Hussain, S.; Renzoni, F. Optical atomic magnetometry for magnetic induction tomography of the heart. Proc. SPIE 2016, 9900, 99000F. [Google Scholar]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef]
- Wickenbrock, A.; Tricot, F.; Renzoni, F. Magnetic induction measurements using an all-optical 87Rb atomic magnetometer. Appl. Phys. Lett. 2013, 103, 243503. [Google Scholar] [CrossRef]
- Savukov, I.M.; Seltzer, S.J.; Romalis, M.V. Detection of NMR Signals With a Radio-Frequency Atomic Magnetometer. J. Magn. Reson. 2007, 185, 214–220. [Google Scholar] [CrossRef]
- Wickenbrock, A.; Jurgilas, S.; Dow, A.; Marmugi, L.; Renzoni, F. Magnetic induction tomography using an all-optical 87Rb atomic magnetometer. Opt. Lett. 2014, 39, 6367. [Google Scholar] [CrossRef]
- Schwindt, P.D.D.; Hollberg, L.; Kitching, J. Self-oscillating rubidium magnetometer using nonlinear magneto-optical rotation. Rev. Sci. Instrum. 2005, 76, 126103. [Google Scholar] [CrossRef]
- Belfi, J.; Bevilacqua, G.; Biancalana, V.; Cartaleva, S.; Dancheva, Y.; Khanbekyan, K.; Moi, L. Dual channel self-oscillating optical magnetometer. J. Opt. Soc. Am. B 2009, 26, 910–916. [Google Scholar] [CrossRef]
- Deans, C.; Marmugi, L.; Hussain, S.; Renzoni, F. Electromagnetic Induction Imaging with a Radio-Frequency Atomic Magnetometer. Appl. Phys. Lett. 2016, 108, 103503. [Google Scholar] [CrossRef]
- Wickenbrock, A.; Leefer, N.; Blanchard, J.W.; Budker, D. Eddy current imaging with an atomic radio-frequency magnetometer. Appl. Phys. Lett. 2016, 108, 183507. [Google Scholar] [CrossRef]
- Savukov, I.M.; Seltzer, S.J.; Romalis, M.V.; Sauer, K.L. Tunable atomic magnetometer for detection of radio-frequency magnetic fields. Phys. Rev. Lett. 2005, 95, 063004. [Google Scholar] [CrossRef]
- Marmugi, L.; Hussain, S.; Deans, C.; Renzoni, F. Magnetic induction imaging with optical atomic magnetometers: Towards applications to screening and surveillance. Proc. SPIE 2015, 9652, 965209. [Google Scholar]
- Deans, C.; Marmugi, L.; Renzoni, F. Through-barrier electromagnetic imaging with an atomic magnetometer. Opt. Express 2017, 25, 17911. [Google Scholar] [CrossRef] [PubMed]
- Deans, C.; Marmugi, L.; Renzoni, F. Active Underwater Detection with an Array of Atomic Magnetometers. Appl. Opt. 2018, 57, 2346. [Google Scholar] [CrossRef]
- Marmugi, L.; Deans, C.; Renzoni, F. Electromagnetic induction imaging with atomic magnetometers: Unlocking the low-conductivity regime. Appl. Phys. Lett. 2019, 115, 083503. [Google Scholar] [CrossRef]
- Jensen, K.; Zugenmaier, M.; Arnbak, J.; Stærkind, H.; Balabas, M.V.; Polzik, E.S. Detection of low-conductivity objects using eddy current measurements with an optical magnetometer. Phys. Rev. Res. 2019, 1, 033087. [Google Scholar] [CrossRef]
- Deans, C.; Marmugi, L.; Renzoni, F. Sub-Sm−1 electromagnetic induction imaging with an unshielded atomic magnetometer. Appl. Phys. Lett. 2020, 116, 133501. [Google Scholar] [CrossRef]
- Bevington, P.; Gartman, R.; Chalupczak, W. Enhanced material defect imaging with a radio-frequency atomic magnetometer. J. Appl. Phys. 2019, 125, 094503. [Google Scholar] [CrossRef]
- Lee, S.-K.; Sauer, K.L.; Seltzer, S.J.; Alem, O.; Romalis, M.V. Subfemtotesla radio-frequency atomic magnetometer for detection of nuclear quadrupole resonance. Appl. Phys. Lett. 2006, 89, 214106. [Google Scholar] [CrossRef]
- Deans, C.; Griffin, L.D.; Marmugi, L.; Renzoni, F. Machine learning based localization and classification with atomic magnetometers. Phys. Rev. Lett. 2018, 120, 033204. [Google Scholar] [CrossRef]
- Bevington, P.; Gartman, R.; Chalupczak, W.; Deans, C.; Marmugi, L.; Renzoni, F. Non-Destructive Structural Imaging of Steelwork with Atomic Magnetometers. Appl. Phys. Lett. 2018, 113, 063503. [Google Scholar] [CrossRef]
- Bevington, P.; Gartman, R.; Chalupczak, W. Imaging of material defects with a radio-frequency atomic magnetometer. Rev. Sc. Instr. 2019, 90, 013103. [Google Scholar] [CrossRef] [PubMed]
- Bevington, P.; Gartman, R.; Chalupczak, W. Alkali-metal spin maser for non-destructive tests. Appl. Phys. Lett. 2019, 115, 173502. [Google Scholar] [CrossRef]
- Bevington, P.; Gartman, R.; Chalupczak, W. Magnetic induction tomography of structural defects with alkali–metal spin maser. Appl. Opt. 2020, 59, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Bevington, P.; Gartman, R.; Botelho, D.J.; Crawford, R.; Packer, M.; Fromhold, T.M.; Chalupczak, W. Object surveillance with radio-frequency atomic magnetometers. Rev. Sci. Instrum. 2020, 91, 055002. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Jadeja, K.; Sula, S.; Venturelli, M.; Deans, C.; Marmugi, L.; Renzoni, F. A cold atom radio-frequency magnetometer. Appl. Phys. Lett. 2019, 114, 073505. [Google Scholar] [CrossRef]
- Chatzidrosos, G.; Wickenbrock, A.; Bougas, L.; Zheng, H.; Tretiak, O.; Yang, Y.; Budker, D. Eddy-current imaging with nitrogen-vacancy centers in diamond. Phys. Rev. Appl. 2019, 11, 014060. [Google Scholar] [CrossRef]
- Giovannetti, V.; Lloyd, S.; Maccone, L. Quantum-Enhanced Measurements: Beating the Standard Quantum Limit. Science 2004, 306, 1330. [Google Scholar] [CrossRef]
- Ciurana, F.M.; Colangelo, G.; Slodička, L.; Sewell, R.J.; Mitchell, M.W. Entanglement-Enhanced Radio-Frequency Field Detection and Waveform Sensing. Phys. Rev. Lett. 2017, 119, 043603. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmugi, L.; Renzoni, F. Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives. Appl. Sci. 2020, 10, 6370. https://doi.org/10.3390/app10186370
Marmugi L, Renzoni F. Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives. Applied Sciences. 2020; 10(18):6370. https://doi.org/10.3390/app10186370
Chicago/Turabian StyleMarmugi, Luca, and Ferruccio Renzoni. 2020. "Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives" Applied Sciences 10, no. 18: 6370. https://doi.org/10.3390/app10186370
APA StyleMarmugi, L., & Renzoni, F. (2020). Electromagnetic Induction Imaging with Atomic Magnetometers: Progress and Perspectives. Applied Sciences, 10(18), 6370. https://doi.org/10.3390/app10186370




