Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Preparation of Rosehips Samples
2.3. Soil Agrochemical Analyses
2.4. Carotenoids Determination
2.5. Polyphenols Determination
2.6. Ascorbic Acid Determination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Carotenoids Composition
3.2. Total Polyphenols Content
3.3. Phenolic Acids Composition
3.4. Flavonoids Composition
3.5. Ascorbic Acid Content
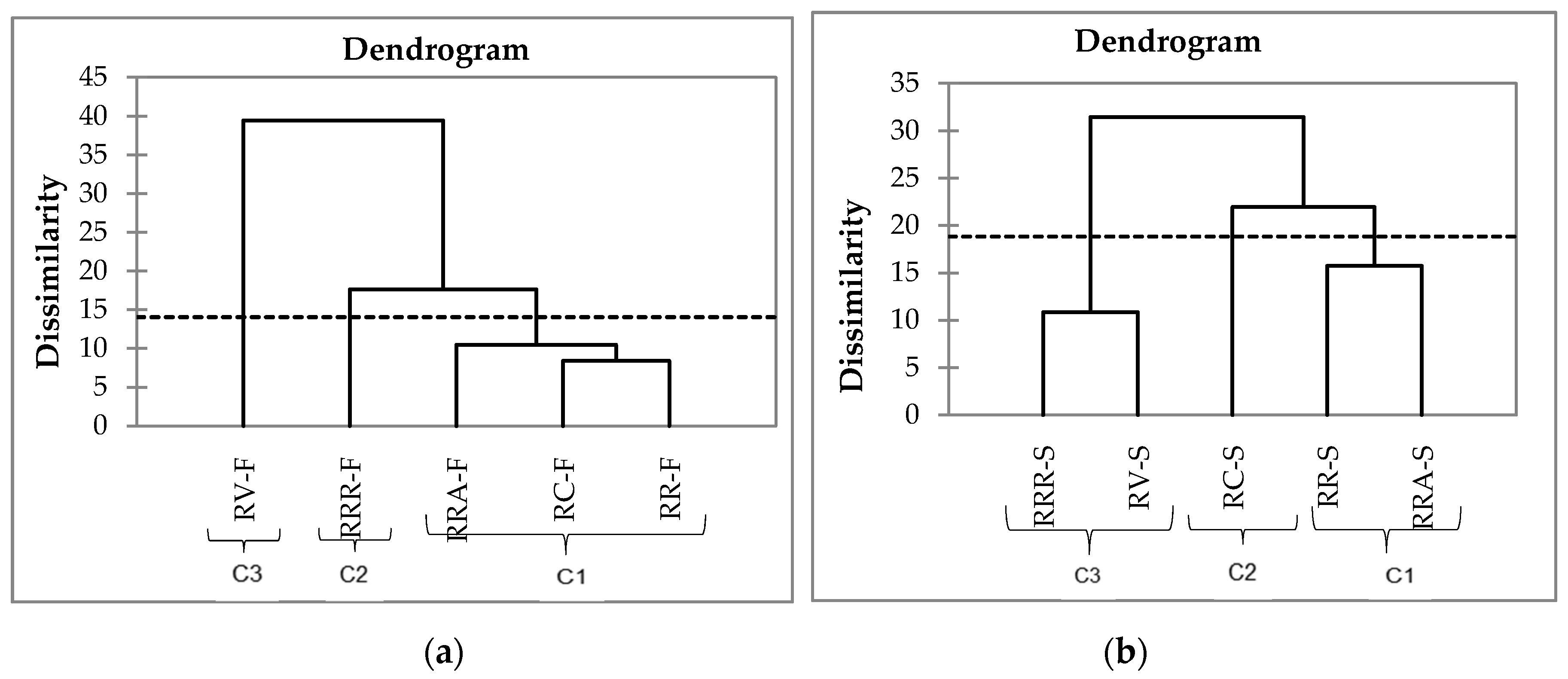
3.6. Hierarchical Cluster Analysis (HCA)
3.7. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Al-Yafeai, A.; Malarskia, A.; Böhma, V. Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chem. 2018, 242, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy erofiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, S.; Baydar, H.; Erbas, S. Variations in chemical compositions of Rosa damascena Mill. and Rosa canina L. fruits. Czech J. Food Sci. 2009, 27, 178–184. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Fan, C.; Pacier, C.; Martirosyan, D.M. Rose hip (Rosa canina L.): A functional food perspective. FFHD 2014, 4, 493–509. [Google Scholar] [CrossRef]
- Olsson, M.; Andersson, S.C.; Werlemark, G.; Uggla, M.; Gustavsson, K.E. Carotenoids and phenolics in rose hips. Acta Hortic. 2005, 690, 249–252. [Google Scholar] [CrossRef]
- Szentmihalyi, K.; Vinkler, P.; Lakatos, B.; Illes, V.; Then, M. Rose hip (Rosa canina L.) oil obtained from waste hip seeds by different extraction methods. Bioresour. Technol. 2002, 82, 195–201. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Huang, X.D.; Wang, H.; Leung, A.K.N.; Chan, C.L.; Fong, D.W.F.; Yub, Z.L. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. hips. J. Ethnopharmacol. 2008, 118, 290–294. [Google Scholar] [CrossRef]
- Chrubasik, C.; Duke, R.K.; Chrubasik, S. The evidence for clinical efficacy of rose hip and seed: A systematic review. Phytother. Res. 2006, 20, 1–3. [Google Scholar] [CrossRef]
- Nadpal, J.D.; Lesjak, M.M.; Šibul, F.S.; Anackov, G.T.; Cetojevic-Simin, D.D.; Mimica-Dukic, N.M.; Ivana, B.N. Comparative study of biological activities and phytochemical composition of two rose hips and their preserves: Rosa canina L. and Rosa arvensis Huds. Food Chem. 2016, 192, 907–914. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Uppadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and antiquorum sensing potential of Moringa olifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Semba, D.R.; Dagnelie, G. Are lutein and zeaxanthin conditionally essential nutrients for eye health? Med. Hypotheses 2003, 61, 465–472. [Google Scholar] [CrossRef]
- Hodisan, T.; Socaciu, C.; Ropan, I.; Neamtu, G. Carotenoid composition of Rosa canina fruits determined by thin-layer chromatography and high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1997, 16, 521–528. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef]
- Sellappani, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Mertz, C.; Gancel, A.L.; Gunata, Z.; Alter, P.; Dhuique-Mayer, C.; Vaillant, F.; Perez, M.A.; Ruales, J.; Brat, P. Phenolic compounds, carotenoids and antioxidant activity of three tropical fruits. J. Food Compos. Anal. 2009, 22, 381–387. [Google Scholar] [CrossRef]
- Nowak, R. Comparative study of phenolic acids in pseudofruits of some species of roses. Acta Pol. Pharm. 2006, 63, 281–288. [Google Scholar]
- Fecka, I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem. Anal. 2009, 20, 177–190. [Google Scholar] [CrossRef]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Vitamin C (ascorbic acid) in vitamin preparations and juices. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990; p. 1058. [Google Scholar]
- Andersson, S.C. Carotenoids, Tocochromanols and Chlorophylls in Sea Buckthorn Berries (Hippophae Rhamnoides) and Rose Hips (Rosa sp.). Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2009. [Google Scholar]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, E.M. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 2011, 128, 689–696. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Carotenes and xanthophyll as antioxidants. In Handbook of Antioxidants for Food Preservation, 1st ed.; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 15–39. [Google Scholar]
- Kopsell, D.A.; Kopsell, D.E. Carotenoids in Vegetables: Biosynthesis, occurrence, impacts on human health, and potential for manipulation. In Bioactive Foods in Promoting Health; Ross, R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 645–662. [Google Scholar]
- Shameh, S.; Alirezalu, A.; Hosseini, B.; Maleki, R. Fruit phytochemical composition and color parameters of 21 accessions of five Rosa species grown in North West Iran. J. Sci. Food Agric. 2019, 99, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry tree, blackthorn, and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant activities. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Koczka, N.; Stefanovits-Bányai, E.; Ombódi, A. Total polyphenol content and antioxidant capacity of rosehips of some Rosa species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Ilyasoglu, H. Characterization of Rosehip (Rosa canina L.) seed and seed oil. Int. J. Food Prop. 2014, 17, 1591–1598. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef]
- Czyzowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Winther, K.; Vinther-Hansen, A.S.; Campbelle-Tofte, J. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: In Vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Int. J. Pharm. Sci. Tech. 2011, 6, 12–35. [Google Scholar]
- Adamczak, A.; Buchwald, W.; Zielinski, J.; Mielcarek, S. Flavonoid and organic acid content in rose hips (Rosa L., Sect. Caninae Dc. Em. Christ.). Acta Biol. Crac. Bot. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Galvez, J.; Zarzuelo, A. In Vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Choi, Y.J.; Gong, J.H.; Shin, D.; Kang, M.K.; Kang, Y.H. Astragalin inhibits autophagy-associated airway epithelial fibrosis. Respir. Res. 2015, 16, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Han, J.; Ren, H.; Yang, W.; Zhang, X.; Zheng, Q.; Wang, D. Cardioprotective effects of astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxidative Med. Cell Longev. 2015, 2016, 1–11. [Google Scholar] [CrossRef]
- Tumbas, V.T.; Canadanovic-Brunet, J.M.; Cetojevic-Simin, D.D.; Cetkovic, G.S.; Ethilas, S.M.; Gille, L. Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J. Sci. Food Agric. 2012, 92, 1273–1281. [Google Scholar] [CrossRef]
- Hosni, K.; Chrif, R.; Zahed, N.; Abid, I.; Medfei, W.; Sebei, H.; Brahim, N.B. Fatty acid and phenolic constituents of leaves, flowers and fruits of tunisian dog rose (Rosa canina L.). Riv. Ital. Sostanze Grasse 2010, 87, 117–123. [Google Scholar]
- Türkben, C.; Uyla, V.; Incedayı, B.; Çelikkol, I. Effects of different maturity periods and processes on nutritional components of rose hip (Rosa canina L.). J. Food Agric. Environ. 2010, 8, 26–30. [Google Scholar]


| Rosehip Species | Carotenoid Composition, mg 100 g−1 DW | ||||||
|---|---|---|---|---|---|---|---|
| Total Carotenoid | β-Carotene | α-Carotene | Lutein | Zeaxanthin | Cis-Lycopene | Trans-Lycopene | |
| In rosehips flesh | |||||||
| RRA | 25.29 ± 0.54 b | 16.48 ± 0.42 b | 2.32 ± 0.11 b | 3.87 ± 0.13 b | 0.27 ± 0.02 b | 0.59 ± 0.02 b | 1.76 ± 0.02 b |
| RRR | 16.58 ± 0.58 c | 9.40 ± 0.43 d | 2.24 ± 0.06 b | 2.68 ± 0.17 c | 0.25 ± 0.01 c | 0.49 ± 0.01 d | 1.52 ± 0.05 c |
| RR | 18.07 ± 0.93 c | 12.71 ± 1.07 c | 1.26 ± 0.11 c | 2.68 ± 0.15 c | 0.24 ± 0.005 cd | 0.25 ± 0.01 e | 0.93 ± 0.02 d |
| RC | 8.67 ± 0.93 d | 3.95 ± 0.80 e | 0.80 ± 0.12 d | 1.55 ± 0.08 d | 0.23 ± 0.003 d | 0.55 ± 0.03 c | 1.59 ± 0.09 c |
| RV | 49.51 ± 1.04 a | 31.40 ± 0.22 a | 6.11 ± 0.35 a | 6.06 ± 0.56 a | 0.32 ± 0.02 a | 1.44 ± 0.03 a | 4.18 ± 0.04 a |
| In rosehips seeds | |||||||
| RRA | 0.62 ± 0.004 c | - | - | - | 0.21 ± 0.004 c | 0.13 ± 0.001 a | 0.28 ± 0.001 b |
| RRR | 1.19 ± 0.09 a | - | 0.20 ± 0.07 a | 0.31 ± 0.01 b | 0.22 ± 0.006 b | 0.14 ± 0.01 a | 0.32 ± 0.005 a |
| RR | 0.55 ± 0.07 c | - | - | - | 0.23 ± 0.004 a | 0.06 ± 0.07 b | 0.26 ± 0.004 c |
| RC | 0.58 ± 0.003 c | - | - | - | 0.21 ± 0.004 c | 0.13 ± 0.001 a | 0.24 ± 0.001 d |
| RV | 1.07 ± 0.03 b | - | 0.04 ± 0.01 b | 0.40 ± 0.02 a | 0.22 ± 0.007 b | 0.13 ± 0.001 a | 0.28 ± 0.007 b |
| Rosehip Species | Total Phenolic Acids | Phenolic Acids, mg 100 g−1 DW | ||||
|---|---|---|---|---|---|---|
| Gallic | Chlorogenic | Caffeic | p-Coumaric | Ferulic | ||
| In rosehip flesh | ||||||
| RRA | 107.62 ±1.34 c | 15.80 ± 0.23 c | 8.02 ± 0.18 d | 9.24 ± 0.42 c | 55.09 ± 0.96 a | 19.46 ± 0.13 b |
| RC | 121.81 ± 1.91 a | 22.67 ± 0.65 b | 9.80 ± 0.11 cd | 22.08 ± 2.86 ab | 48.22 ± 6.96 a | 19.03 ± 0.41 b |
| RR | 116.83 ± 2.67 b | 36.77 ± 0.04 a | 14.95 ± 0.26 b | 16.86 ± 2.90 b | 25.38 ± 2.26 b | 22.87 ± 0.42 a |
| RRR | 119.61 ± 2.67 b | 36.99 ± 2.37 a | 21.01 ± 2.87 a | 24.54 ± 7.96 a | 25.55 ± 1.10 b | 11.51 ± 0.29 c |
| RV | 89.23 ± 1.65 d | 36.61 ± 1.61 a | 11.08 ± 0.22 c | 8.72 ± 0.40 c | 21.27 ± 2.56 b | 11.55 ± 1.25 b |
| In rosehip seeds | ||||||
| RRA | 177.03 ± 7.05 a | 153.71 ± 6.07 a | 3.67 ± 0.03 b | 0.99 ± 0.09 c | 15.56 ± 0.58 c | 3.08 ± 0.45 c |
| RC | 175.12 ± 5.40 a | 89.69 ± 0.78 c | 45.83 ± 1.97 d | 12.73 ± 0.78 a | 22.04 ± 2.34 b | 4.83 ± 0.57 b |
| RR | 174.17 ± 8.08 a | 97.29 ± 3.75 bc | 2.02 ± 0.03 a | 1.99 ± 0.05 b | 64.72 ± 4.61 a | 8.15 ± 0.26 a |
| RRR | 114.86 ± 1.65 b | 103.94 ± 2.93 b | 3.24 ± 0.07 ab | 0.67 ± 0.10 c | 6.62 ± 0.46 e | 0.39 ± 0.01 d |
| RV | 111.59 ± 10.49 b | 92.48 ± 10.02 c | 6.33 ± 1.12 c | 0.79 ± 0.03 c | 11.57 ± 1.15 d | 0.40 ± 0.01 d |
| Rosehip Species | Total Flavonoids | Flavonoids Composition, mg 100 g − 1 DW | ||||
|---|---|---|---|---|---|---|
| Rutin | Kaempferol-3-O-Glucoside | Luteolin | Quercetin | Quercetin-3-O-Glucoside | ||
| In rosehip flesh | ||||||
| RRA | 37.63 ± 1.01 ab | 12.39 ± 0.83 b | 11.62 ± 0.34 a | 5.81 ± 0.20 b | 6.72 ± 0.29 b | 1.09 ± 0.16 c |
| RC | 35.61 ± 0.70 b | 11.62 ± 0.31 b | 4.40 ± 0.71 d | 7.46 ± 0.12 a | 9.77 ± 0.24 a | 2.35 ± 0.12 b |
| RR | 34.23 ± 0.85 b | 14.94 ± 0.53 b | 7.40 ± 0.46 b | 4.97 ± 0.29 c | 5.74 ± 0.37 c | 1.18 ± 0.19 c |
| RRR | 37.77 ± 3.63 ab | 21.23 ± 3.52 a | 3.84 ± 0.23 d | 2.27 ± 0.10 e | 5.55 ± 0.21 c | 4.47 ± 0.08 a |
| RV | 41.59 ± 6.61 a | 25.54 ± 6.05 a | 6.40 ± 0.30 c | 4.57 ± 0.04 d | 4.77 ± 0.26 d | 0.62 ± 0.21 d |
| In rosehip seeds | ||||||
| RRA | 28.28 ± 3.09 b | 20.21 ±3.21 a | 0.96 ± 0.06 b | 1.83 ± 0.01 c | 1.65 ± 0.04 b | 3.63 ± 0.33 b |
| RC | 32.19 ± 0.88 a | 19.11 ± 0.81 a | 5.29 ± 0.40 a | 1.89 ± 0.02 b | 2.87 ± 0.39 a | 3.02 ± 0.71 b |
| RR | 15.06 ± 0.22 e | 6.86 ± 0.22 d | 1.03 ± 0.06 b | 1.88 ± 0.03 b | 2.11 ± 0.05 b | 3.19 ± 0.06 b |
| RRR | 25.36 ± 1.27 c | 14.75 ± 1.41 b | 0.53 ± 0.01 c | 2.09 ± 0.03 a | 3.08 ± 0.87 a | 4.90 ± 0.83 a |
| RV | 18.46 ± 1.97 d | 10.59 ± 1.99 c | 0.57 ± 0.03 c | 1.76 ± 0.02 d | 2.09 ± 0.05 b | 3.46 ± 0.10 b |
| Compound | TPC | TFC | TPAC | TCC | ACC |
|---|---|---|---|---|---|
| Rosehip flesh | |||||
| TPC | 1 | 0.24 | 0.96 * | −0.79 * | −0.45 * |
| TFC | 0.24 | 1 | −0.50 * | 0.55 * | −0.05 |
| TPAC | 0.96 * | −0.50 * | 1 | −0.86 * | −0.39 |
| TCC | −0.79 * | −0.55 * | −0.86 * | 1 | 0.44 |
| ACC | −0.45 * | −0.05 | −0.39 | 0.44 | 1 |
| Compound | Rosehip seeds | ||||
| TPC | 1 | 0.46 * | 0.98 * | −0.87 * | −0.28 |
| TFC | 0.46 * | 1 | 0.29 | −0.10 | −0.70 * |
| TPAC | 0.98 * | 0.29 | 1 | −0.91 * | −0.16 |
| TCC | −0.87 * | −0.10 | −0.91 * | 1 | 0.11 |
| ACC | −0.28 | −0.70 * | −0.16 | 0.11 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medveckienė, B.; Kulaitienė, J.; Jarienė, E.; Vaitkevičienė, N.; Hallman, E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. https://doi.org/10.3390/app10155337
Medveckienė B, Kulaitienė J, Jarienė E, Vaitkevičienė N, Hallman E. Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Applied Sciences. 2020; 10(15):5337. https://doi.org/10.3390/app10155337
Chicago/Turabian StyleMedveckienė, Brigita, Jurgita Kulaitienė, Elvyra Jarienė, Nijolė Vaitkevičienė, and Ewelina Hallman. 2020. "Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania" Applied Sciences 10, no. 15: 5337. https://doi.org/10.3390/app10155337
APA StyleMedveckienė, B., Kulaitienė, J., Jarienė, E., Vaitkevičienė, N., & Hallman, E. (2020). Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Applied Sciences, 10(15), 5337. https://doi.org/10.3390/app10155337







