The Effects of Wild Ginseng Extract on Psychomotor and Neuromuscular Performance Recovery Following Acute Eccentric Exercise: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Supplementation
2.4. Pre-tests
2.5. Acute Eccentric Exercise
2.6. Psychomotor and Physical Performance Tests
2.7. Blood Analyses
2.8. Perceived Muscle Soreness
2.9. Data Analysis
3. Results
3.1. Basic Characteristics
3.2. Psychomotor and Neuromuscular Performance
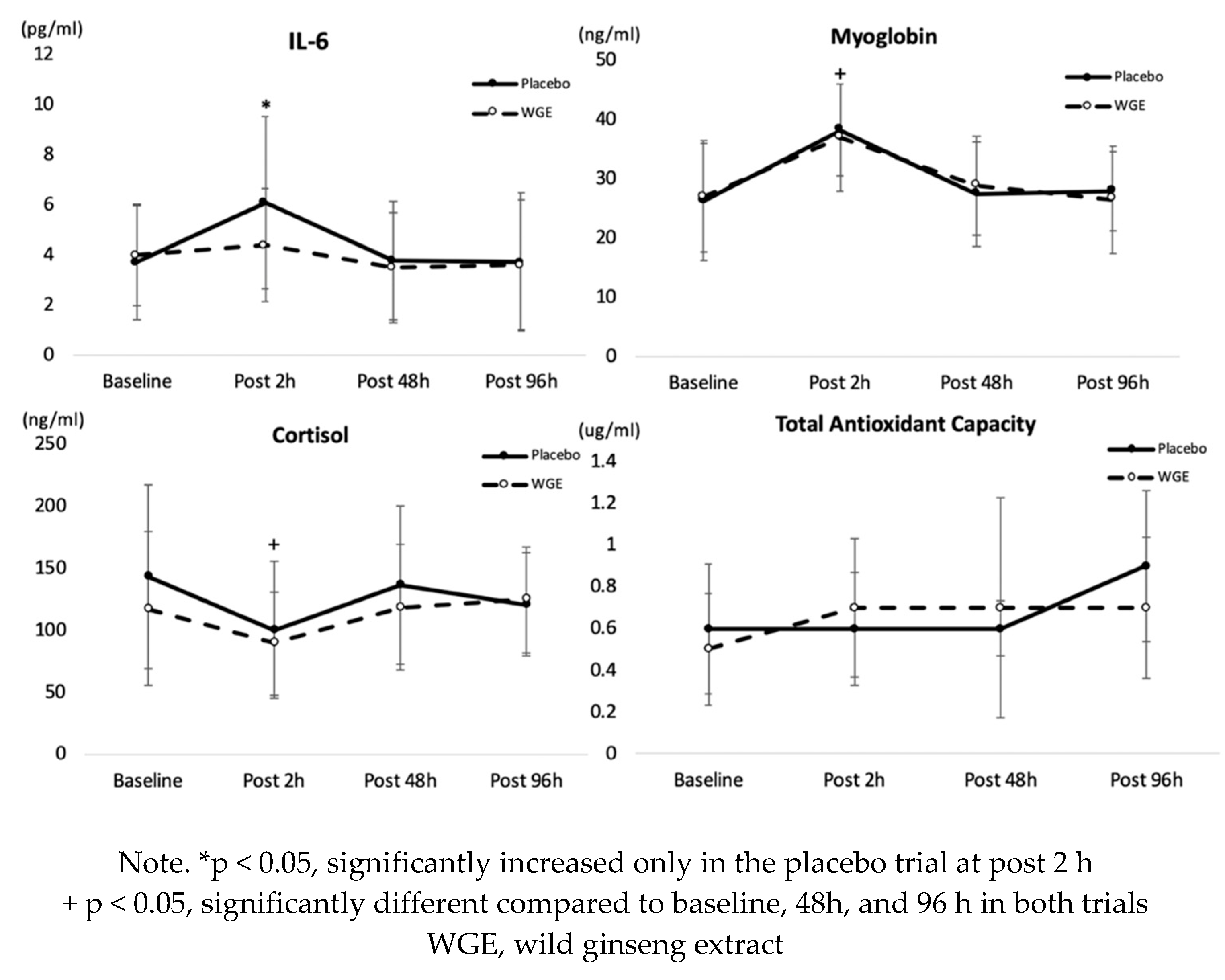
3.3. Blood Analyses
3.4. Perceived Muscle Soreness
4. Discussion
4.1. Psychomotor Performance
4.2. Neuromuscular Performance
4.3. Inflammation Markers of Muscle Damage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provino, R. The role of adaptogens in stress management. Aus. J. Med. Herbal. 2010, 22, 41. [Google Scholar]
- Bach, H.V.; Kim, J.; Myung, S.K.; Cho, Y. Efficacy of Ginseng supplements on fatigue and physical performance: A meta-analysis. J. Kor. Med. Sci. 2016, 31, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Y.G.; Xu, H.; Sun, S.Q.; Wang, Z.T. Differentiation of the root of cultivated ginseng, mountain cultivated ginseng and mountain wild ginseng using FT-IR and two-dimensional correlation IR spectroscopy. J. Mol. Struc. 2008, 883, 228–235. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Park, S.Y.; Paek, K.Y. Quality, safety and efficacy profiling of ginseng adventitious roots produced in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 7309–7317. [Google Scholar] [CrossRef]
- Park, J.S.; Park, E.M.; Kim, D.H.; Jung, K.; Jung, J.S.; Lee, E.J.; Hyun, J.W.; Kang, H.S.; Kim, H.S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng. Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Sohn, E.H.; Yang, Y.J.; Koo, H.J.; Park, D.W.; Kim, Y.J.; Jang, K.H.; Namkoong, K.H.; Kang, S.C. Effects of Korean ginseng and wild simulated cultivation ginseng for muscle strength and endurance. Kor. J. Plant. Res. 2012, 25, 657–663. [Google Scholar] [CrossRef][Green Version]
- Chang, W.H.; Tsai, Y.L.; Huang, C.Y.; Hsieh, C.C.; Chaunchaiyakul, R.; Fang, Y.; Lee, S.D.; Kuo, C.H. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J. Int. Soc. Sports Nutr. 2015, 12, 34. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, L.H.; Zhang, J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta. Pharm. Sin. 2005, 26, 143–149. [Google Scholar] [CrossRef]
- Nah, S.Y.; Kim, D.H.; Rhim, H. Ginsenosides: Are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007, 13, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, H.; Shim, I.; Lee, H.; Hahm, D.H. Wild ginseng attenuates anxiety-and depression-like behaviors during morphine withdrawal. J. Microbiol. Biotechnol. 2011, 21, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, D.I. A descriptive statistical approach to the Korean pharmacopuncture therapy. J. Acupunct. Meridian Stud. 2010, 3, 141–149. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Morgan, W.P. Evaluation of the ergogenic properties of ginseng. Sports Med. 2000, 29, 113–133. [Google Scholar] [CrossRef]
- Ma, G.D.; Chiu, C.H.; Hsu, Y.J.; Hou, C.W.; Chen, Y.M.; Huang, C.C. Changbai Mountain ginseng (Panax ginseng CA Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules 2017, 22, 237. [Google Scholar] [CrossRef]
- Oliynyk, S.; Oh, S. Actoprotective effect of ginseng: Improving mental and physical performance. J. Ginseng Res. 2013, 37, 144. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef]
- Matthews, C.E.; Ockene, I.S.; Freedson, P.S.; Rosal, M.C.; Merriam, P.A.; Hebert, J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med. Sci. Sports Exerc. 2002, 34, 1242–1248. [Google Scholar] [CrossRef]
- Sayers, S.P.; Clarkson, P.M. Short-term immobilization after eccentric exercise. Part II: Creatine kinase and myoglobin. Med. Sci. Sports Exerc. 2003, 35, 762–768. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, antioxidant and physical exercise. Mol. Cell Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.A.; Maxwell, L. Delayed onset muscle soreness. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Nottle, C.; Nosaka, K. Changes in power assessed by the Wingate Anaerobic Test following downhill running. J. Strength Cond. Res. 2007, 21, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Skurvydas, A.; Brazaitis, M.; Kamandulis, S.; Sipaviciene, S. Muscle damaging exercise affects isometric force fluctuation as well as intraindividual variability of cognitive function. J. Motor Behav. 2010, 42, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.T.; Podolka, T.D.; Chuang, W.J. Panax notoginseng supplementation enhances physical performance during endurance exercise. J. Strength Cond. Res. 2005, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Yang, Y.J.; Lee, J.H.; Yoon, Y.S. Effect of high-dose ginsenoside complex (UG0712) supplementation on physical performance of healthy adults during a 12-week supervised exercise program: A randomized placebo-controlled clinical trial. J. Ginseng Res. 2018, 42, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.S.; Chan, K.H.; Hsu, M.C.; Liu, J.F. Supplementation with soybean peptides, taurine, Pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J. Med. Food 2011, 14, 219–225. [Google Scholar] [CrossRef]
- Pumpa, K.L.; Fallon, K.E.; Bensoussan, A.; Papalia, S. The effects of Panax notoginseng on delayed onset muscle soreness and muscle damage in well-trained males: A double blind randomised controlled trial. Complement. Ther. Med. 2013, 21, 131–140. [Google Scholar] [CrossRef]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 2004, 91, 161–168. [Google Scholar] [CrossRef]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. 1961. Nutrition 1993, 9, 480–491. [Google Scholar]
- Peake, J.M.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; Mackinnon, L.; Coombes, J.S. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef]
- Kirby, T.J.; Triplett, N.T.; Haines, T.L.; Skinner, J.W.; Fairbrother, K.R.; McBride, J.M. Effect of leucine supplementation on indices of muscle damage following drop jumps and resistance exercise. Amino Acids 2012, 42, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. The positive cognitive impact of aerobic fitness is associated with peripheral inflammatory and brain-derived neurotrophic biomarkers in young adults. Physiol. Behav. 2017, 179, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.C.; Lee, N.H.; Lee, S. Jumping exercise restores stretching-induced power loss in healthy adults. Mont. J. Sports Sc. Med. 2018, 7, 55–62. [Google Scholar] [CrossRef]
- Abizanda, P.; Navarro, J.L.; García-Tomás, M.I.; López-Jiménez, E.; Martínez-Sánchez, E.; Paterna, G. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community-dwelling older persons. Arch. Gerontol. Geriatr. 2012, 54, 21–27. [Google Scholar] [CrossRef]
- Arnold, C.M.; Warkentin, K.D.; Chilibeck, P.D.; Magnus, C.R. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J. Strength Cond. Res. 2010, 24, 815–824. [Google Scholar] [CrossRef]
- Martin, J.C.; Diedrich, D.; Coyle, E.F. Learning effects associated with maximal power testing: Implications for validity. Int. J. Sports Med. 2000, 21, 485–487. [Google Scholar] [CrossRef]
- Lee, N.H.; Jung, H.C.; Ok, G.; Lee, S. Acute effects of Kinesio taping on muscle function and self-perceived fatigue level in healthy adults. Eur. J. Sport Sci. 2017, 17, 757–764. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Caldwell, L.K.; DuPont, W.H.; Beeler, M.K.; Post, E.M.; Barnhart, E.C.; Hardesty, V.H.; Anders, J.P.; Borden, E.C.; Volek, J.S.; Kraemer, W.J. The effects of a Korean ginseng, GINST15, on perceptual effort, psychomotor performance, and physical performance in men and women. J. Sports Sci. Med. 2018, 17, 92. [Google Scholar]
- Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum. Psychopharmacol. 2010, 25, 462–471. [Google Scholar] [CrossRef]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J. Psychopharmacol. 2005, 19, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F.; Wesnes, K.A.; Scholey, A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: Comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav. 2004, 79, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physio. Behav. 2002, 75, 739–751. [Google Scholar] [CrossRef]
- Chen, X.; Lee, T.J.F. Ginsenosides-induced nitric oxide-mediated relaxation of the rabbit corpus cavernosum. Br. J. Pharmacol. 1995, 115, 15–18. [Google Scholar] [CrossRef]
- Vuksan, V.; Sievenpiper, J.L.; Koo, V.Y.; Francis, T.; Beljan-Zdravkovic, U.; Xu, Z.; Vidgen, E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch. Intern. Med. 2000, 160, 1009–1013. [Google Scholar] [CrossRef]
- Engels, H.J.; Kolokouri, I.; Cieslak, T.J., II; Wirth, J.C. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J. Strength Cond. Res. 2001, 15, 290–295. [Google Scholar]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef]
- Jung, H.L.; Kwak, H.E.; Kim, S.S.; Kim, Y.C.; Lee, C.D.; Byurn, H.K.; Kang, H.Y. Effects of Panax ginseng supplementation on muscle damage and inflammation after uphill treadmill running in humans. Am. J. Chin. Med. 2011, 39, 441–450. [Google Scholar] [CrossRef]
- Hou, C.W.; Lee, S.D.; Kao, C.L.; Cheng, I.S.; Lin, Y.N.; Chuang, S.J.; Chen, C.Y.; Ivy, J.L.; Haung, C.Y.; Kuo, C.H. Improved inflammatory balance of human skeletal muscle during exercise after supplementations of the ginseng-based steroid Rg1. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Flanagan, S.D.; DuPont, W.H.; Caldwell, L.K.; Hardesty, V.H.; Barnhart, E.C.; Beeler, M.K.; Post, E.M.; Vlek, J.S.; Kraemer, W.J. The effects of a Korean ginseng, GINST15, on hypo-pituitary-adrenal and oxidative activity induced by intense work stress. J. Med. Food 2018, 21, 104–112. [Google Scholar] [CrossRef]
- Lin, H.F.; Chou, C.C.; Chao, H.H.; Tanaka, H. Panax ginseng and Salvia miltiorrhiza supplementation during eccentric resistance training in middle-aged and older adults: A double-blind randomized control trial. Complement. Ther. Med. 2016, 29, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, D.A.; Petrides, J.S.; Tsigos, C.; Bina, S.; Kalogeras, K.T.; Wilder, R.; Gold, P.W.; Deuster, G.P.; Chrousos, G.P. Exercise stimulates interleukin-6 secretion: Inhibition by glucocorticoids and correlation with catecholamines. Am. J. Physiol. 1996, 271, E601–E605. [Google Scholar] [CrossRef] [PubMed]
- Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Nieman, D.C.; Henson, D.A.; Butterworth, D.E.; Schmitt, R.L.; Bailey, E.M.; Warren, B.J.; Utter, A.; Davis, J.M. Carbohydrate and the cytokine response to 2.5 h of running. J. Appl. Physiol. 1997, 82, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Youl, H.K.; Hwan, S.K.; Jun, W.L.; Byrne, H.K. Effects of ginseng ingestion on growth hormone, testosterone, cortisol, and insulin-like growth factor 1 responses to acute resistance exercise. J. Strength Cond. Res. 2002, 16, 179–183. [Google Scholar] [CrossRef]
| Variables | Base | Eccentric Exercise | 2 h | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|---|---|---|
| ✓ | Downhill Running and Drop Jump Exercise | ✓ | ✓ | ✓ |
| Variables | Mean (SD) |
|---|---|
| Age (year) | 27.1 (4.33) |
| Height (cm) | 174.8 (9.39) |
| Weight (kg) | 78.0 (13.70) |
| Resting Systolic Blood Pressure (mmHg) | 117.8 (17.84) |
| Resting Diastolic Blood Pressure (mmHg) | 73.4 (9.42) |
| Resting Heart Rate (beats/min) | 65.4 (7.96) |
| Body fat (%) | 12.7 (4.43) |
| VO2peak (ml/kg/min) | 44.9 (7.48) |
| Variables | Group | Base | 2 h | 48 h | 96 h | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| G | T | G × T | ||||||
| PVT Correctness (score) | Placebo | 36.7 | 37.7 | 37.7 | 37.3 | 0.568 | 0.498 | 0.969 |
| (SD) | −3.91 | −2.16 | −2.21 | −5.17 | ||||
| 95% CI | 33.9–39.5 | 36.2–39.2 | 36.1–39.3 | 33.6–41.0 | ||||
| WGE | 37.2 | 37.9 | 38.1 | 37.5 | ||||
| (SD) | −3.77 | −3.21 | −2.77 | −4.17 | ||||
| 95% CI | 34.5–39.9 | 35.6–40.2 | 36.1–40.1 | 34.5–40.5 | ||||
| PVT reaction time (msec) | Placebo | 335.3 | 338.8 | 340 | 346.1 | 0.073 | 0.355 | 0.626 |
| (SD) | −34.07 | −28.4 | −26.07 | −32 | ||||
| 95% CI | 311.0–359.7 | 318.5–359.1 | 321.4–358.7 | 323.2–369.0 | ||||
| WGE | 345.2 | 350.3 | 345.8 | 349.2 | ||||
| (SD) | −32.12 | −34.29 | −33.24 | −39.13 | ||||
| 95% CI | 322.3–368.2 | 325.7–374.8 | 322.0–369.6 | 321.2–377.2 | ||||
| DMS correctness (score) | Placebo | 26.5 | 25.2 * | 26.7 | 26.7 | 0.136 | 0.019 | 0.768 |
| (SD) | −2.68 | −3.68 | −3.68 | −3.56 | ||||
| 95% CI | 24.6–28.4 | 22.6–27.8 | 24.1–29.3 | 24.2–29.2 | ||||
| WGE | 27.3 | 26.0 * | 27.2 | 26.6 | ||||
| (SD) | −3.27 | −4 | −3.19 | −4.67 | ||||
| 95% CI | 25.0–29.6 | 23.1–28.9 | 24.9–29.5 | 23.3–29.9 | ||||
| DMS time (msec) | Placebo | 2942.1 | 2533.5 * | 2598.9 * | 2283.9 * | 0.272 | 0.025 | 0.416 |
| (SD) | −1948.13 | −1747.26 | −1495.75 | −1524.06 | ||||
| 95% CI | 1548.5–4335.7 | 1283.5–3783.4 | 1528.9–3668.9 | 1193.7–3374.2 | ||||
| WGE | 3335.8 | 2507.6 * | 2806.3 * | 2908.4 * | ||||
| (SD) | −1948.13 | −1390.8 | −1849.86 | −1535.01 | ||||
| 95% CI | 1846.8–4824.7 | 1512.7–3502.5 | 1483.0–4129.6 | 1810.3–4006.5 | ||||
| DMS reaction time (msec) | Placebo | 1784.9 | 1740.9 | 1711.7 | 1533.2 | 0.139 | 0.061 | 0.412 |
| (SD) | −715.58 | −790.94 | −516.6 | −518.58 | ||||
| 95% CI | 1273.0–2296.8 | 1175.1–2306.7 | 1342.1–2081.2 | 1162.3–1904.2 | ||||
| WGE | 1905.1 | 1693.3 | 1881.8 | 1823.8 | ||||
| (SD) | −351.92 | −366.75 | −759.4 | −629.23 | ||||
| 95% CI | 1653.3–2156.8 | 1431.6–1954.9 | 1338.5–2425.0 | 1373.7–2274.0 | ||||
| Variables | Group | Base | 2 h | 48 h | 96 h | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| G | T | G × T | ||||||
| Straight leg raise (°) | Placebo | 71.2 | 69.2 | 69.1 | 75.4 * | 0.29 | 0.03 | 0.412 |
| (SD) | −7.13 | −8.82 | −11.49 | −7.23 | ||||
| 95% CI | 65.5–77.1 | 61.7–75.9 | 59.2–77.7 | 69.6–81.3 | ||||
| WGE | 67.5 | 66.7 | 69.8 | 72.6 * | ||||
| (SD) | 7.32 | −5.44 | −7.13 | −4.5 | ||||
| 95% CI | 62.3–72.7 | 62.8–70.6 | 64.7–74.9 | 69.4–75.8 | ||||
| Vertical Jump (cm) | Placebo | 53.5 | 50.4 | 51.5 | 51.1 | 0.205 | 0.324 | 0.307 |
| (SD) | −6.93 | −7.92 | −9.12 | −6.62 | ||||
| 95% CI | 47.4–57.9 | 43.5–56.1 | 43.5–57.7 | 45.4–56.0 | ||||
| WGE | 50.6 | 50.1 | 49.8 | 51.2 | ||||
| (SD) | −8.62 | −7.06 | −6.76 | −7.79 | ||||
| 95% CI | 44.4–56.7 | 45.1–55.2 | 44.9–54.6 | 45.6–56.8 | ||||
| Isometric Leg extension (kg) | Placebo | 44.1 | 40.9 | 43.2 | 43.3 | 0.818 | 0.259 | 0.832 |
| (SD) | −9.3 | −9.49 | −11.1 | −11.9 | ||||
| 95% CI | 37.5–50.8 | 34.1–47.7 | 35.2–51.1 | 34.8–51.8 | ||||
| WGE | 44.9 | 42.1 | 42.2 | 43.3 | ||||
| (SD) | −11.04 | −13.04 | −9.02 | −9.75 | ||||
| 95% CI | 37.0–52.8 | 32.8–51.4 | 35.7–48.7 | 36.3–50.2 | ||||
| Isometric Leg Flexion (kg) | Placebo | 35.5 | 34.8 | 36.8 | 36.2 | 0.671 | 0.173 | 0.911 |
| (SD) | −8.77 | −7.54 | −7.48 | −6.99 | ||||
| 95% CI | 29.2–41.8 | 29.4–40.2 | 31.4–42.1 | 31.2–41.2 | ||||
| WGE | 35.5 | 33.5 | 36.5 | 35.5 | ||||
| (SD) | −8.21 | −6.81 | −8.9 | −6.39 | ||||
| 95% CI | 29.6–41.4 | 28.6–38.4 | 30.2–42.9 | 30.9–40.0 | ||||
| Mean Power (Watts/kg) | Placebo | 13.9 | 13.9 | 13.6 | 13.9 | 0.45 | 0.447 | 0.389 |
| (SD) | −1.11 | −1.71 | −1.58 | −1.36 | ||||
| 95% CI | 13.1–14.7 | 12.7–15.1 | 12.4–14.7 | 12.9–14.9 | ||||
| WGE | 12.7 | 13.8 | 13.8 | 14.2 | ||||
| (SD) | −4.33 | −1.56 | −1.34 | −1.36 | ||||
| 95% CI | 9.6–15.8 | 12.7–14.9 | 12.9–14.8 | 13.2–15.1 | ||||
| Peak Power (Watts/kg) | Placebo | 14.1 | 14.1 | 13.7 | 14 | 0.583 | 0.452 | 0.786 |
| (SD) | −1.15 | −1.73 | −1.62 | −1.33 | ||||
| 95% CI | 13.3–14.9 | 12.9–15.3 | 12.5–14.9 | 13.1–15.0 | ||||
| WGE | 14.1 | 14 | 14 | 14.3 | ||||
| (SD) | −1.63 | −1.56 | −1.31 | −1.35 | ||||
| 95% CI | 13.0–15.3 | 12.9–15.1 | 13.0–14.9 | 13.3–15.3 | ||||
| Variables | Group | Base | 2 h | 48 h | 96 h | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| G | T | G × T | ||||||
| Perceived Muscle Soreness | Placebo | 0.6 | 3.0 * | 2.3 | 0.8 | 0.922 | 0.001 | 0.462 |
| (SD) | (0.70) | (2.16) | (1.89) | (1.03) | ||||
| 95% CI | 0.1–1.1 | 1.5–4.5 | 0.9–3.7 | 0.1–1.5 | ||||
| WGE | 0.4 | 3.5 * | 1.8 | 1.2 | ||||
| (SD) | (0.47) | (2.34) | (1.75) | (1.69) | ||||
| 95% CI | 0.0–0.7 | 1.8–5.2 | 0.5–3.0 | 0.0–2.4 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.C.; Lee, N.H.; Kim, Y.C.; Lee, S. The Effects of Wild Ginseng Extract on Psychomotor and Neuromuscular Performance Recovery Following Acute Eccentric Exercise: A Preliminary Study. Appl. Sci. 2020, 10, 5839. https://doi.org/10.3390/app10175839
Jung HC, Lee NH, Kim YC, Lee S. The Effects of Wild Ginseng Extract on Psychomotor and Neuromuscular Performance Recovery Following Acute Eccentric Exercise: A Preliminary Study. Applied Sciences. 2020; 10(17):5839. https://doi.org/10.3390/app10175839
Chicago/Turabian StyleJung, Hyun Chul, Nan Hee Lee, Young Chan Kim, and Sukho Lee. 2020. "The Effects of Wild Ginseng Extract on Psychomotor and Neuromuscular Performance Recovery Following Acute Eccentric Exercise: A Preliminary Study" Applied Sciences 10, no. 17: 5839. https://doi.org/10.3390/app10175839
APA StyleJung, H. C., Lee, N. H., Kim, Y. C., & Lee, S. (2020). The Effects of Wild Ginseng Extract on Psychomotor and Neuromuscular Performance Recovery Following Acute Eccentric Exercise: A Preliminary Study. Applied Sciences, 10(17), 5839. https://doi.org/10.3390/app10175839





