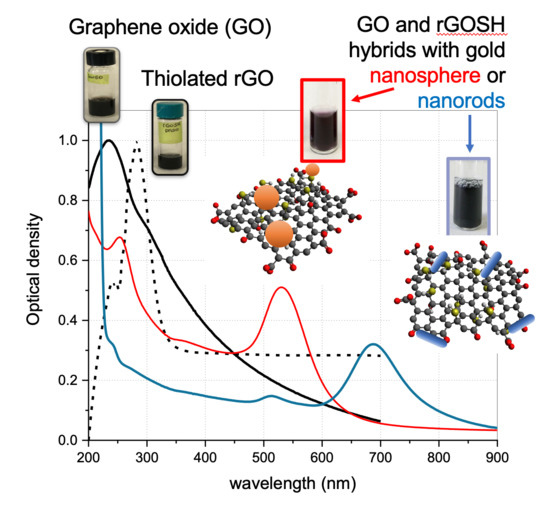
Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Base-Washed Graphene Oxide
2.3. Synthesis of Thiolated Reduced Graphene Oxide
2.4. Synthesis of Gold Nanospheres (AuNS)
2.5. Synthesis of Gold Nanorods (AuNR)
2.6. Synthesis of Hybrid GO@AuNS and GO@AuNR
2.7. Physicochemical Characterization
2.7.1. UV-Visible (UV-VIS) and Attenuated Total Reflectance FTIR (ATR-FTIR) Spectroscopies
2.7.2. Thermogravimetric Analysis (TGA)
2.7.3. Atomic Force Microscopy (AFM)
2.8. Computational Studies
2.9. Cell Cultures
3. Results and Discussions
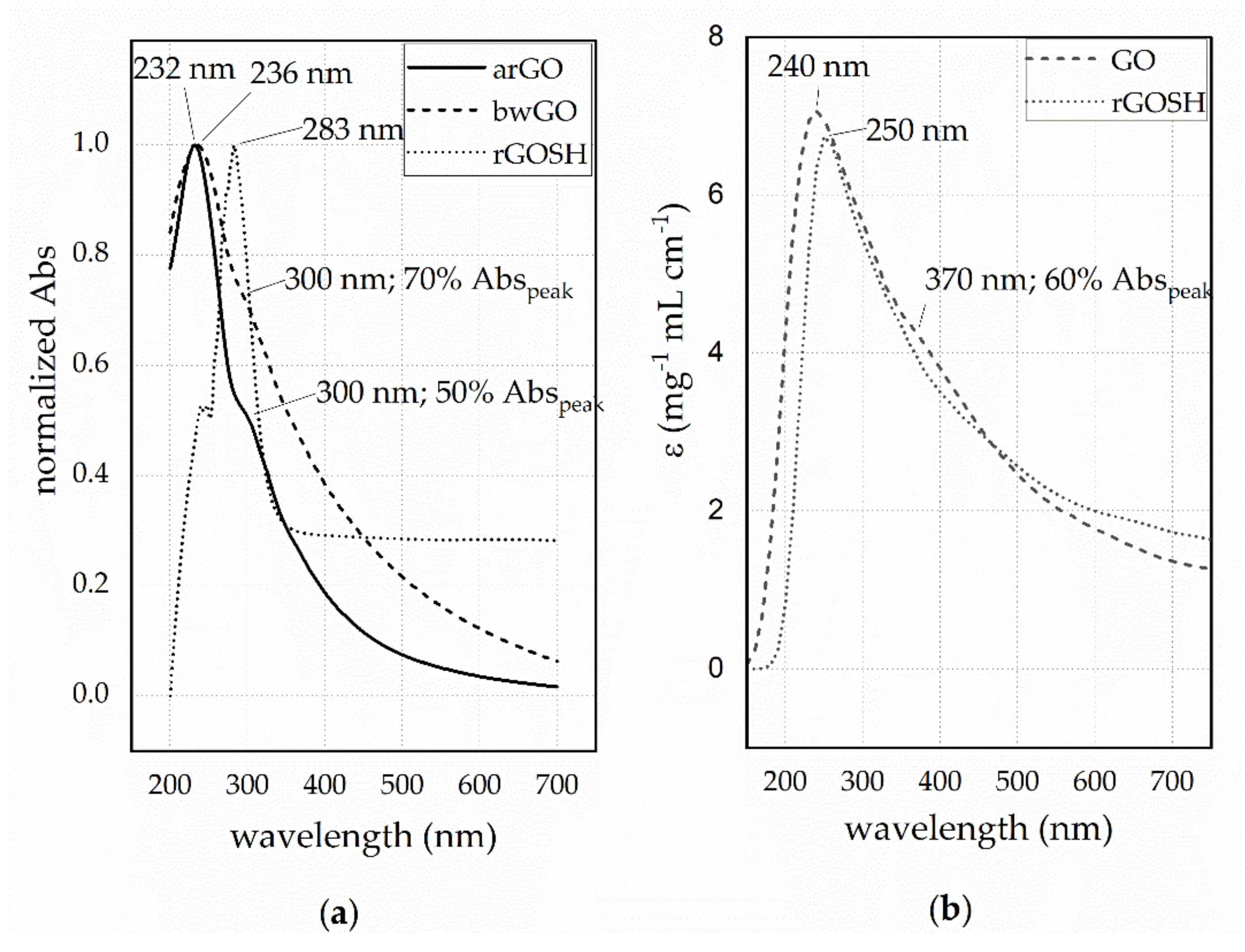
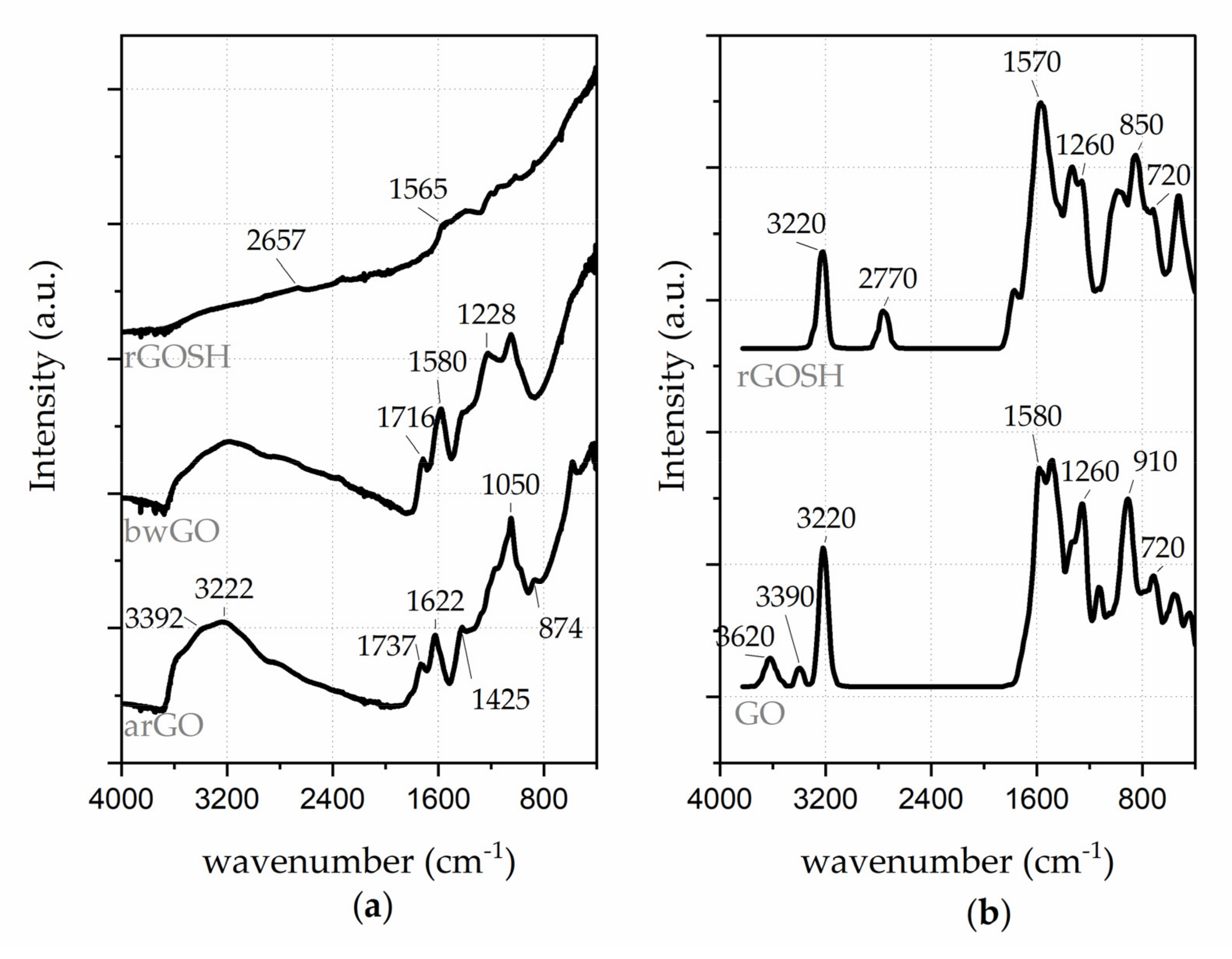
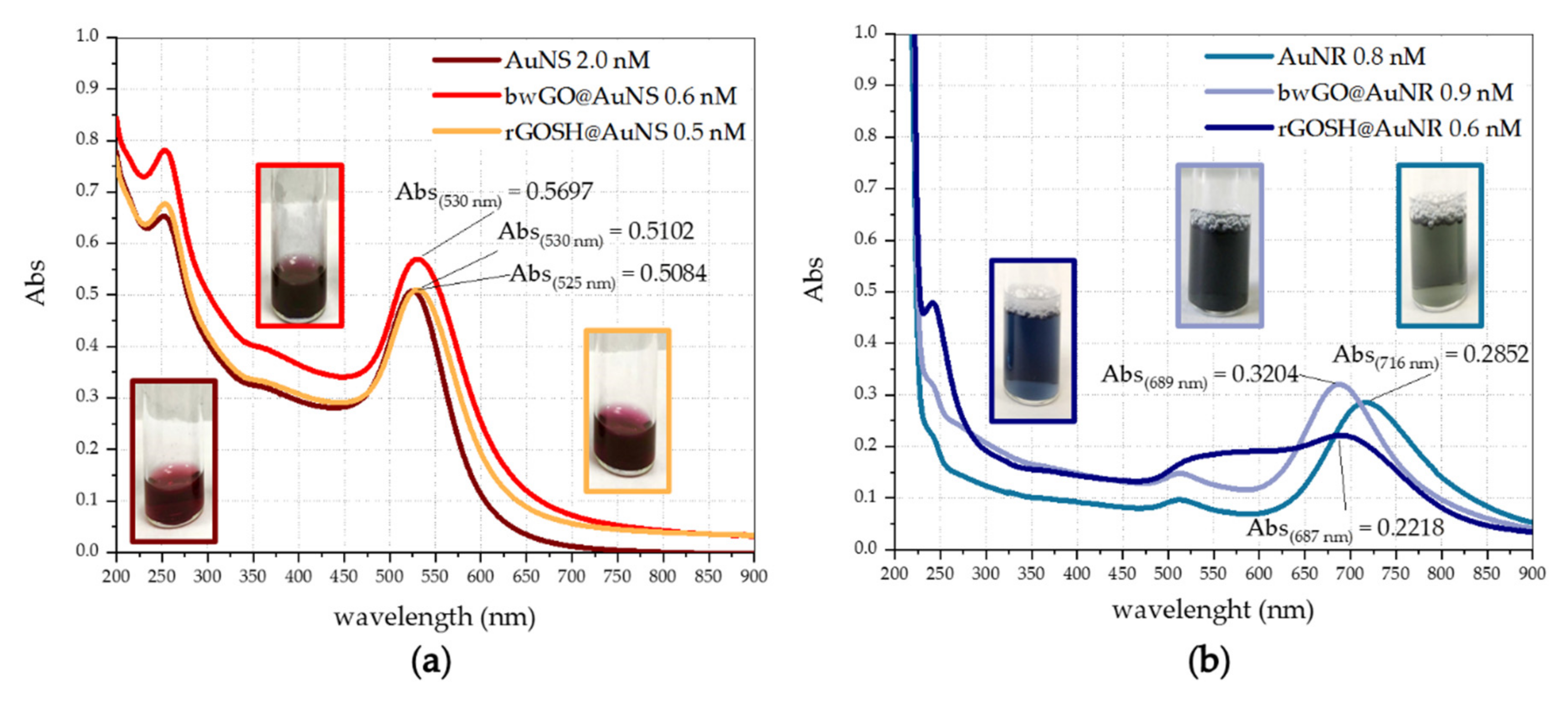
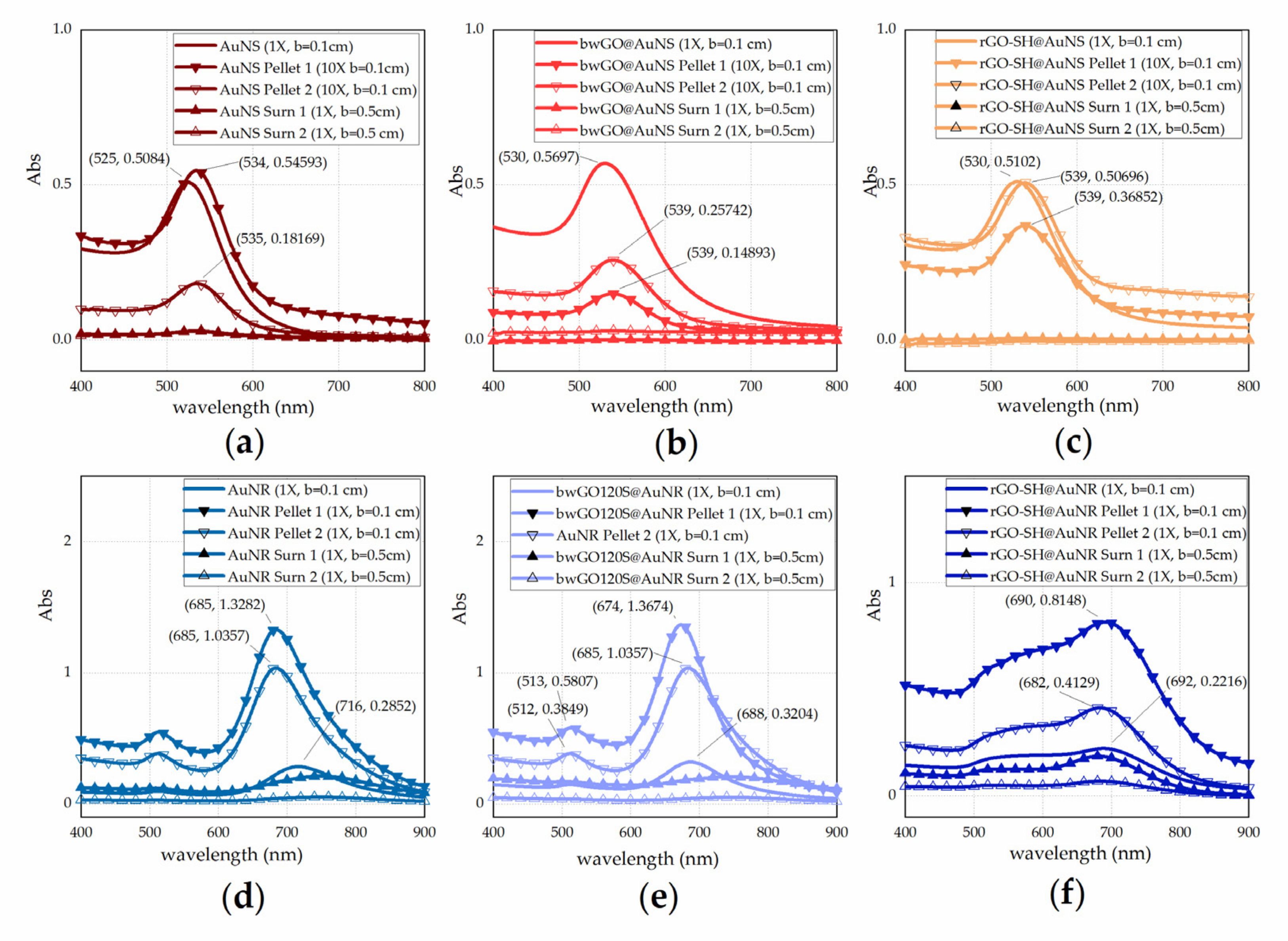
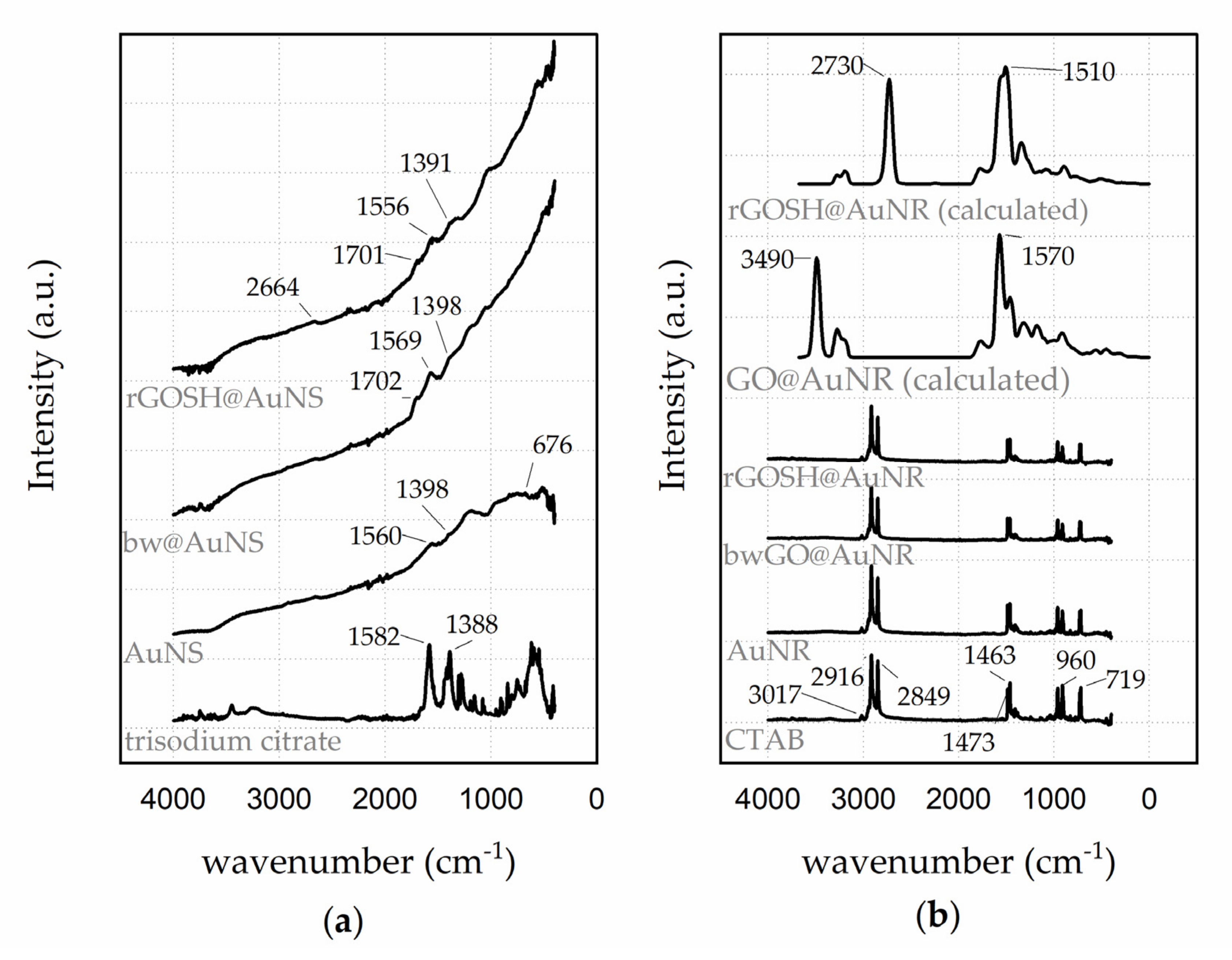
3.1. Physicochemical Characterisation UV-Visible Characterization of GO-Based Nanosheet Dispersions and Plasmonic Nanoplatforms
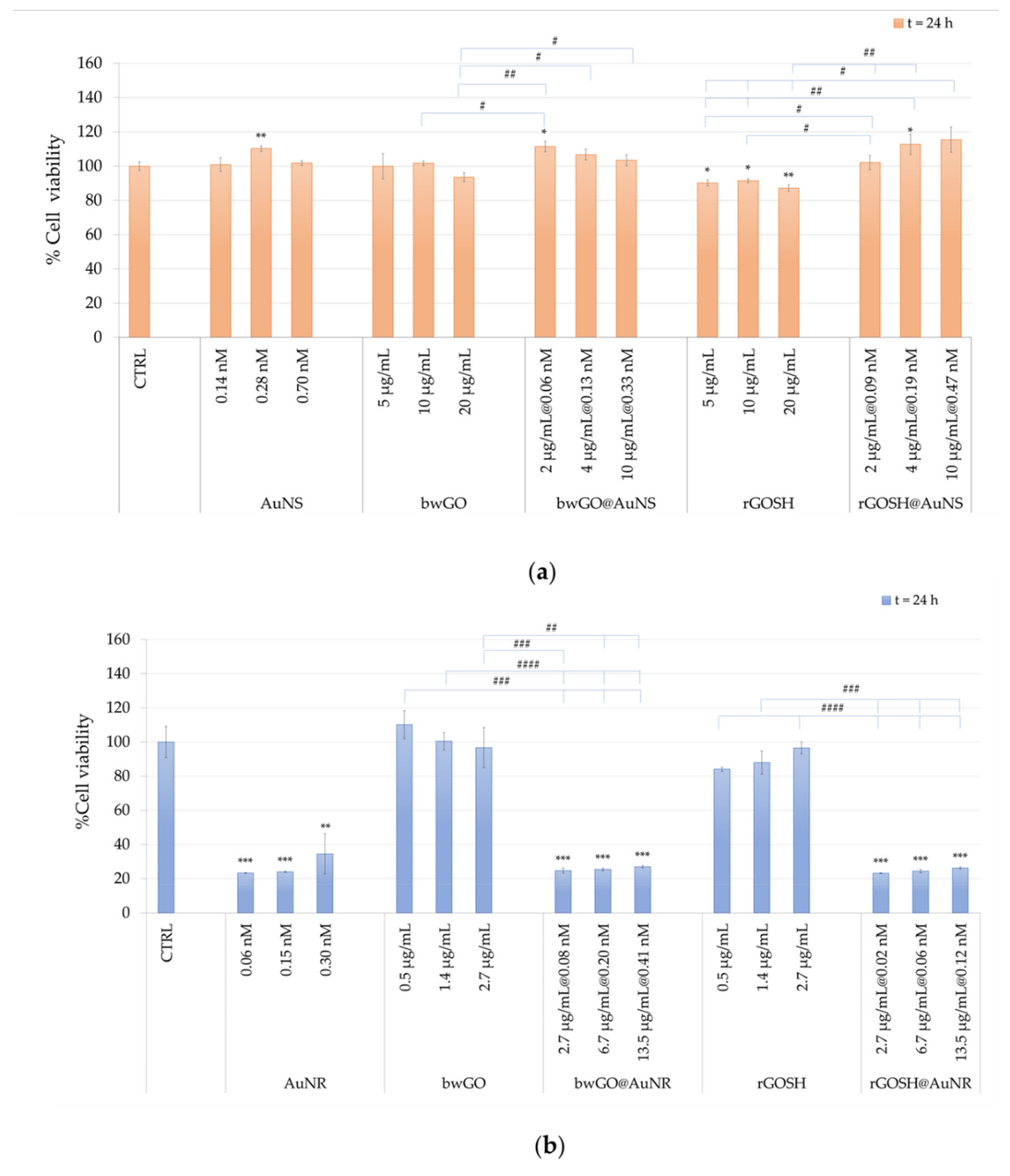
3.2. Biological Characterization of GO and GO@AuNP Nanocomposite Platforms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onaciu, A.; Jurj, A.; Moldovan, C.; Berindan-Neagoe, I. Theranostic Nanoparticles and Their Spectrum in Cancer. In Engineered Nanomaterials Safety; Intech. Open: London, UK, 2019. [Google Scholar]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Strano, G.; Zuccarello, L.; Satriano, C. Gold and Silver Nanoparticles for Applications in Theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Cheng, Z.; Jia, L.; Mo, S.; Liu, Z. Ultrahigh specific surface area of graphene for eliminating subcooling of water. Appl. Energy 2014, 130, 824–829. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Meric, I.; Han, M.Y.; Young, A.F.; Ozyilmaz, B.; Kim, P.; Shepard, K.L. Current saturation in zero-bandgap, top-gated graphene field-effect transistors. Nat. Nanotechnol. 2008, 3, 654–659. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Lee, G.H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor-Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A. Elastic properties of single-layered graphene sheet. Solid State Commun. 2009, 149, 91–95. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef]
- Li, J.-L.; Tang, B.; Yuan, B.; Sun, L.; Wang, X.-G. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials 2013, 34, 9519–9534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, J.; Zhang, T.; Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 2018, 286, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Fu, Y.-Y.; Peng, Q.; Guo, S.-S.; Liu, G.; Li, J.; Yang, H.-H.; Chen, G.-N. Dye-enhanced graphene oxide for photothermal therapy and photoacoustic imaging. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Chua, C.K.; Pumera, M. Refinements to the structure of graphite oxide: Absolute quantification of functional groups via selective labelling. Nanoscale 2015, 7, 20256–20266. [Google Scholar] [CrossRef]
- Di Pietro, P.; Forte, G.; D’Urso, L.; Satriano, C. The hybrid nanobiointerface between nitrogen-doped graphene oxide and lipid membranes: A theoretical and experimental study. AIMS Mater. Sci. 2016, 4, 43–60. [Google Scholar] [CrossRef]
- Di Pietro, P.; Forte, G.; Snyders, R.; Satriano, C.; Bittencourt, C.; Thiry, D. Sulphur functionalizion of graphene oxide by radiofrequency plasma. Plasma Process. Polym. 2020. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Nguyen, T.D.; Tran, V.H.; Dang, T.T.H.; Tran, D.L. Functionalization of reduced graphene oxide by electroactive polymer for biosensing applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5. [Google Scholar] [CrossRef][Green Version]
- Jung, J.H.; Cheon, D.S.; Liu, F.; Lee, K.B.; Seo, T.S. A Graphene Oxide Based Immuno-biosensor for Pathogen Detection. Angew. Chemie Int. Ed. 2010, 49, 5708–5711. [Google Scholar] [CrossRef]
- Zeng, X.; Tu, W.; Li, J.; Bao, J.; Dai, Z. Photoelectrochemical Biosensor Using Enzyme-Catalyzed in Situ Propagation of CdS Quantum Dots on Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 16197–16203. [Google Scholar] [CrossRef]
- Wang, C.; Ravi, S.; Garapati, U.S.; Das, M.; Howell, M.; Mallela, J.; Alwarappan, S.; Mohapatra, S.S.; Mohapatra, S. Multifunctional chitosan magnetic-graphene (CMG) nanoparticles: A theranostic platform for tumor-targeted co-delivery of drugs, genes and MRI contrast agents. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Manna, A.K.; Pati, S.K.; Rao, C.N.R. A study of graphene decorated with metal nanoparticles. Chem. Phys. Lett. 2010, 497, 70–75. [Google Scholar] [CrossRef]
- Cucci, L.; Naletova, I.; Consiglio, G.; Satriano, C. A Hybrid Nanoplatform of Graphene Oxide/Nanogold for Plasmonic Sensing and Cellular Applications at the Nanobiointerface. Appl. Sci. 2019, 9, 676. [Google Scholar] [CrossRef]
- Gao, W. The Chemistry of Graphene Oxide. In Graphene Oxide; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Hu, Y.; Ye, M. Synthesis of hydrophilic and organophilic chemically modified graphene oxide sheets. J. Colloid Interface Sci. 2010, 352, 366–370. [Google Scholar] [CrossRef]
- Rajagopalan, B.; Chung, J.S. Reduced chemically modified graphene oxide for supercapacitor electrode. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y.-k. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Xu, L.Q.; Wang, L.; Zhang, B.; Lim, C.H.; Chen, Y.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Functionalization of reduced graphene oxide nanosheets via stacking interactions with the fluorescent and water-soluble perylene bisimide-containing polymers. Polymer 2011, 52, 2376–2383. [Google Scholar] [CrossRef]
- Pham, C.V.; Eck, M.; Krueger, M. Thiol functionalized reduced graphene oxide as a base material for novel graphene-nanoparticle hybrid composites. Chem. Eng. J. 2013, 231, 146–154. [Google Scholar] [CrossRef]
- Orth, E.S.; Fonsaca, J.E.S.; Almeida, T.G.; Domingues, S.H.; Ferreira, J.G.L.; Zarbin, A.J.G. Functionalized graphene oxide as a nanocatalyst in dephosphorylation reactions: Pursuing artificial enzymes. Chem. Commun. 2014, 50, 9891–9894. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Monothiolation and Reduction of Graphene OxideviaOne-Pot Synthesis: Hybrid Catalyst for Oxygen Reduction. ACS Nano 2015, 9, 4193–4199. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Sousa, I.; Buttenhauser, K.; Suchaoin, W.; Partenhauser, A.; Perrone, M.; Matuszczak, B.; Bernkop-Schnürch, A. Thiolated graphene oxide as promising mucoadhesive carrier for hydrophobic drugs. Int. J. Pharm. 2016, 509, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Highly sensitive electrochemical sensor based on environmentally friendly biomass-derived sulfur-doped graphene for cancer biomarker detection. Sens. Actuators B Chem. 2017, 241, 716–724. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Ryoo, S.-R.; Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Behaviors of NIH-3T3 Fibroblasts on Graphene/Carbon Nanotubes: Proliferation, Focal Adhesion, and Gene Transfection Studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef]
- Zhi, X.; Fang, H.; Bao, C.; Shen, G.; Zhang, J.; Wang, K.; Guo, S.; Wan, T.; Cui, D. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials 2013, 34, 5254–5261. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Chatterjee, N.; Eom, H.-J.; Choi, J. A systems toxicology approach to the surface functionality control of graphene–cell interactions. Biomaterials 2014, 35, 1109–1127. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Mittal, S.; Shukla, R.; Pandey, A.; Dhakate, S.; Pasricha, R.; Dhawan, A. Toxicity of Graphene in Normal Human Lung Cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Singh, M.K.; Kulkarni, P.P.; Sonkar, V.K.; Grácio, J.J.A.; Dash, D. Amine-Modified Graphene: Thrombo-Protective Safer Alternative to Graphene Oxide for Biomedical Applications. ACS Nano 2012, 6, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Wang, A.; Pu, K.; Dong, B.; Liu, Y.; Zhang, L.; Zhang, Z.; Duan, W.; Zhu, Y. Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells. J. Appl. Toxicol. 2013, 33, 1156–1164. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated Functional Group Density on Graphene Oxide: Its Effect on Cell Toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2007, 26, 83–90. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold Nanocages: Bioconjugation and Their Potential Use as Optical Imaging Contrast Agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.; Zagho, M.; Nasrallah, G.; Elzatahry, A. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomed. 2018, 13, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Verde, V.; Longo, A.; Cucci, L.M.; Sanfilippo, V.; Magrì, A.; Satriano, C.; Anfuso, C.D.; Lupo, G.; La Mendola, D. Anti-Angiogenic and Anti-Proliferative Graphene Oxide Nanosheets for Tumor Cell Therapy. Int. J. Mol. Sci. 2020, 21, 5571. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; Caporarello, N.; Anfuso, C.D.; Lupo, G.; Magrì, A.; La Mendola, D.; Satriano, C. Immobilization of Neurotrophin Peptides on Gold Nanoparticles by Direct and Lipid-Mediated Interaction: A New Multipotential Therapeutic Nanoplatform for CNS Disorders. ACS Omega 2017, 2, 4071–4079. [Google Scholar] [CrossRef]
- Cucci, L.M.; Munzone, A.; Naletova, I.; Magrì, A.; La Mendola, D.; Satriano, C. Gold nanoparticles functionalized with angiogenin-mimicking peptides modulate cell membrane interactions. Biointerphases 2018, 13. [Google Scholar] [CrossRef]
- Naletova, I.; Cucci, L.M.; D’Angeli, F.; Anfuso, C.D.; Magrì, A.; La Mendola, D.; Lupo, G.; Satriano, C. A Tunable Nanoplatform of Nanogold Functionalised with Angiogenin Peptides for Anti-Angiogenic Therapy of Brain Tumours. Cancers 2019, 11, 1322. [Google Scholar] [CrossRef]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Trachuk, L.A.; Mel’nikov, A.G. The effect of the size, shape, and structure of metal nanoparticles on the dependence of their optical properties on the refractive index of a disperse medium. Opt. Spectrosc. 2005, 98, 77–83. [Google Scholar] [CrossRef]
- Murphy, C.J.; Jana, N.R. Controlling the aspect ratio of inorganic nanorods and nanowires. Adv. Mater. 2002, 14, 80–82. [Google Scholar] [CrossRef]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhu, J. Graphene−Metal Particle Nanocomposites. J. Phys. Chem. C 2008, 112, 19841–19845. [Google Scholar] [CrossRef]
- Pan, H.; Low, S.; Weerasuriya, N.; Wang, B.; Shon, Y.-S. Morphological transformation of gold nanoparticles on graphene oxide: Effects of capping ligands and surface interactions. Nano Converg. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, N.K.; Mandal, B.K.; Ranjan, S.; Dasgupta, N. Bioinspired gold nanoparticles decorated reduced graphene oxide nanocomposite using Syzygium cumini seed extract: Evaluation of its biological applications. Mater. Sci. Eng. C 2018, 93, 191–205. [Google Scholar] [CrossRef]
- Sun, B.; Wu, J.; Cui, S.; Zhu, H.; An, W.; Fu, Q.; Shao, C.; Yao, A.; Chen, B.; Shi, D. In situ synthesis of graphene oxide/gold nanorods theranostic hybrids for efficient tumor computed tomography imaging and photothermal therapy. Nano Res. 2016, 10, 37–48. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Colusso, A.; Litti, L.; Ostric, A.; Meneghetti, M.; Da Ros, T. Thiolated Graphene Oxide Nanoribbons as Templates for Anchoring Gold Nanoparticles: Two-Dimensional Nanostructures for SERS. ChemPlusChem 2019, 84, 862–871. [Google Scholar] [CrossRef]
- Thomas, H.R.; Day, S.P.; Woodruff, W.E.; Vallés, C.; Young, R.J.; Kinloch, I.A.; Morley, G.W.; Hanna, J.V.; Wilson, N.R.; Rourke, J.P. Deoxygenation of Graphene Oxide: Reduction or Cleaning? Chem. Mater. 2013, 25, 3580–3588. [Google Scholar] [CrossRef]
- Consiglio, G.; Di Pietro, P.; D’Urso, L.; Forte, G.; Grasso, G.; Sgarlata, C.; Cossement, D.; Snyders, R.; Satriano, C. Surface tailoring of polyacrylate-grafted graphene oxide for controlled interactions at the biointerface. J. Colloid Interface Sci. 2017, 506, 532–542. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11. [Google Scholar] [CrossRef]
- He, Y.Q.; Liu, S.P.; Kong, L.; Liu, Z.F. A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2861–2866. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Werts, M.H.V. Resonant light scattering spectroscopy of gold, silver and gold–silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 2013, 138, 583–592. [Google Scholar] [CrossRef]
- Zhang, H.; Hussain, I.; Brust, M.; Cooper, A.I. Emulsion-Templated Gold Beads Using Gold Nanoparticles as Building Blocks. Adv. Mater. 2004, 16, 27–30. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Murphy, C.J. Quantitation of Metal Content in the Silver-Assisted Growth of Gold Nanorods. J. Phys. Chem. B 2006, 110, 3990–3994. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Dependence of the Enhanced Optical Scattering Efficiency Relative to That of Absorption for Gold Metal Nanorods on Aspect Ratio, Size, End-Cap Shape, and Medium Refractive Index. J. Phys. Chem. B 2005, 109, 20331–20338. [Google Scholar] [CrossRef]
- He, H.; Klinowski, J.; Forster, M.; Lerf, A. A new structural model for graphite oxide. Chem. Phys. Lett. 1998, 287, 53–56. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Naletova, I.; Satriano, C.; Curci, A.; Margiotta, N.; Natile, G.; Arena, G.; La Mendola, D.; Nicoletti, V.G.; Rizzarelli, E. Cytotoxic phenanthroline derivatives alter metallostasis and redox homeostasis in neuroblastoma cells. Oncotarget 2018, 9, 36289–36316. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.-J.; Feng, J.; Ren, Y.-m.; Song, L.-P.; Wei, F. Facile Synthesis of Graphene Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano 2010, 5, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, S.; Luo, X.; Zou, M.; Huang, S. Ultraviolet-visible spectroscopy of graphene oxides. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile Synthesis of Soluble Graphene via a Green Reduction of Graphene Oxide in Tea Solution and Its Biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Renewal of sp2 bonds in graphene oxides via dehydrobromination. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Wang, G.; Niu, Z.; Yang, J.; Wu, W. Effect of oxidation debris on spectroscopic and macroscopic properties of graphene oxide. Carbon 2014, 76, 203–211. [Google Scholar] [CrossRef]
- Thomas, H.R.; Marsden, A.J.; Walker, M.; Wilson, N.R.; Rourke, J.P. Sulfur-Functionalized Graphene Oxide by Epoxide Ring-Opening. Angew. Chem. Int. Ed. 2014, 53, 7613–7618. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Frogley, M.D.; Wang, C.; Cinque, G.; Barber, A.H. Polarised infrared microspectroscopy of edge-oriented graphene oxide papers. Vib. Spectrosc. 2014, 75, 178–183. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Venkataraghavan, R.; Kasturi, T.R. Contribution to the Infrared Spectra of Organosulphur Compounds. Can. J. Chem. 1964, 42, 36–42. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M.; Stener, M.; Guo, Y.; Chen, S.; Crespo, P.; García, M.A.; Hernando, A.; Pengo, P.; Pasquato, L. Physico-Chemical Characteristics of Gold Nanoparticles. In Comprehensive Analytical Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 66, pp. 81–152. [Google Scholar]
- Hu, C.; Rong, J.; Cui, J.; Yang, Y.; Yang, L.; Wang, Y.; Liu, Y. Fabrication of a graphene oxide–gold nanorod hybrid material by electrostatic self-assembly for surface-enhanced Raman scattering. Carbon 2013, 51, 255–264. [Google Scholar] [CrossRef]
- Wulandari, P.; Nagahiro, T.; Fukada, N.; Kimura, Y.; Niwano, M.; Tamada, K. Characterization of citrates on gold and silver nanoparticles. J. Colloid Interface Sci. 2015, 438, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, T.J.; Myneni, S.C.B. Speciation of aqueous Ni(II)-carboxylate and Ni(II)-fulvic acid solutions: Combined ATR-FTIR and XAFS analysis. Geochim. Cosmochim. Acta 2004, 68, 3441–3458. [Google Scholar] [CrossRef]
- Tyagi, H.; Kushwaha, A.; Kumar, A.; Aslam, M. A Facile pH Controlled Citrate-Based Reduction Method for Gold Nanoparticle Synthesis at Room Temperature. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, B.; Shadjou, N.; Afsharan, H.; Eskandani, M.; Nozad Charoudeh, H.; Rashidi, M.-R. Reduced graphene oxide decorated with gold nanoparticle as signal amplification element on ultra-sensitive electrochemiluminescence determination of caspase-3 activity and apoptosis using peptide based biosensor. Bioimpacts 2016, 6, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Chen, X.; Wang, L.; Chai, Y.; Yang, C.; Zhao, J. An Improved Approach for Synthesis of Positively Charged Silver Nanoparticles. Chem. Lett. 2005, 34, 100–101. [Google Scholar] [CrossRef]
- Viana, R.B.; da Silva, A.B.F.; Pimentel, A.S. Infrared Spectroscopy of Anionic, Cationic, and Zwitterionic Surfactants. Adv. Phys. Chem. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.-J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Liang, L.; Wei, M.; Hu, W.; Li, X.; Huang, Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Seeded High Yield Synthesis of Short Au Nanorods in Aqueous Solution. Langmuir 2004, 20, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Evidence for Bilayer Assembly of Cationic Surfactants on the Surface of Gold Nanorods. Langmuir 2001, 17, 6368–6374. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.; Orendorff, C.J. Surfactant-Directed Synthesis and Optical Properties of One-Dimensional Plasmonic Metallic Nanostructures. MRS Bull. 2011, 30, 349–355. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface chemistry of gold nanorods: Origin of cell membrane damage and cytotoxicity. Nanoscale 2013, 5. [Google Scholar] [CrossRef]
- Ito, E.; Yip, K.W.; Katz, D.; Fonseca, S.B.; Hedley, D.W.; Chow, S.; Xu, G.W.; Wood, T.E.; Bastianutto, C.; Schimmer, A.D.; et al. Potential Use of Cetrimonium Bromide as an Apoptosis-Promoting Anticancer Agent for Head and Neck Cancer. Mol. Pharmacol. 2009, 76, 969–983. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]


| Experimental | Theoretical | |||
|---|---|---|---|---|
| Sample | εpeak (π→π*) (mg−1 mL cm−1) | εshoulder (n→π*) (mg−1 mL cm−1) | εpeak (π→π*) (mg−1 mL cm−1) | εshoulder (n→π*) (mg−1 mL cm−1) |
| arGO | 71.7 | 37.0 | - | - |
| bwGO | 24.5 | 18.2 | 7.1 | 5.1 |
| rGO-SH | 47.0 | - | 6.7 | - |
| Step 1 | Step 2 | Step 3 | |||||
|---|---|---|---|---|---|---|---|
| Sample | (Δ °C) | Weight Loss (%) | (Δ °C) | Weight Loss (%) | (Δ °C) | Weight Loss (%) | Residue at 800 °C (%) |
| arGO | 50–110 | 4.2 | 111–300 | 44.5 | 360–450 | 10.7 | 34.0 |
| bwGO | 50–110 | 7.6 | 120–300 | 22.8 | 460–610 | 11.6 | 40.6 |
| rGO-SH | 50–110 | 2.9 | 150–340 | 7.6 | 430–800 | 51.0 | 34.5 |
| Sample | λmax (nm) | FWHM (nm) | D (nm)/ | R |
|---|---|---|---|---|
| AuNS | 525 | 53 | 27 | |
| bwGO@AuNS | 530 | 63 | 41 | |
| rGOSH@AuNS | 530 | 62 | 41 | |
| AuNR | 716 | 111 | 3.12 | |
| bwGO@AuNR | 689 | 86 | 2.83 | |
| rGOSH@AuNR | 687 | - | 2.81 |
| Sample | z (nm) | r |
|---|---|---|
| AuNS | 14 ± 2 | 0.75 ± 0.02 |
| bwGO@AuNS | 12 ± 2 | 0.98 ± 0.02 |
| rGOSH@AuNS | 15 ± 2 | 0.88 ± 0.02 |
| AuNR | 6 ± 2 6 ± 2 | 0.60 ± 0.02 0.88 ± 0.02 |
| bwGO@AuNR | 8 ± 2 7 ± 2 | 0.60 ± 0.02 0.84 ± 0.02 |
| rGOSH@AuNR | 7 ± 2 | 0.98 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasella, P.; Sanfilippo, V.; Bonaccorso, C.; Cucci, L.M.; Consiglio, G.; Nicosia, A.; Mineo, P.G.; Forte, G.; Satriano, C. Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Appl. Sci. 2020, 10, 5529. https://doi.org/10.3390/app10165529
Tomasella P, Sanfilippo V, Bonaccorso C, Cucci LM, Consiglio G, Nicosia A, Mineo PG, Forte G, Satriano C. Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Applied Sciences. 2020; 10(16):5529. https://doi.org/10.3390/app10165529
Chicago/Turabian StyleTomasella, Pascal, Vanessa Sanfilippo, Carmela Bonaccorso, Lorena Maria Cucci, Giuseppe Consiglio, Angelo Nicosia, Placido Giuseppe Mineo, Giuseppe Forte, and Cristina Satriano. 2020. "Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles" Applied Sciences 10, no. 16: 5529. https://doi.org/10.3390/app10165529
APA StyleTomasella, P., Sanfilippo, V., Bonaccorso, C., Cucci, L. M., Consiglio, G., Nicosia, A., Mineo, P. G., Forte, G., & Satriano, C. (2020). Theranostic Nanoplatforms of Thiolated Reduced Graphene Oxide Nanosheets and Gold Nanoparticles. Applied Sciences, 10(16), 5529. https://doi.org/10.3390/app10165529