Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories
Abstract
1. Introduction
2. Materials and Methods
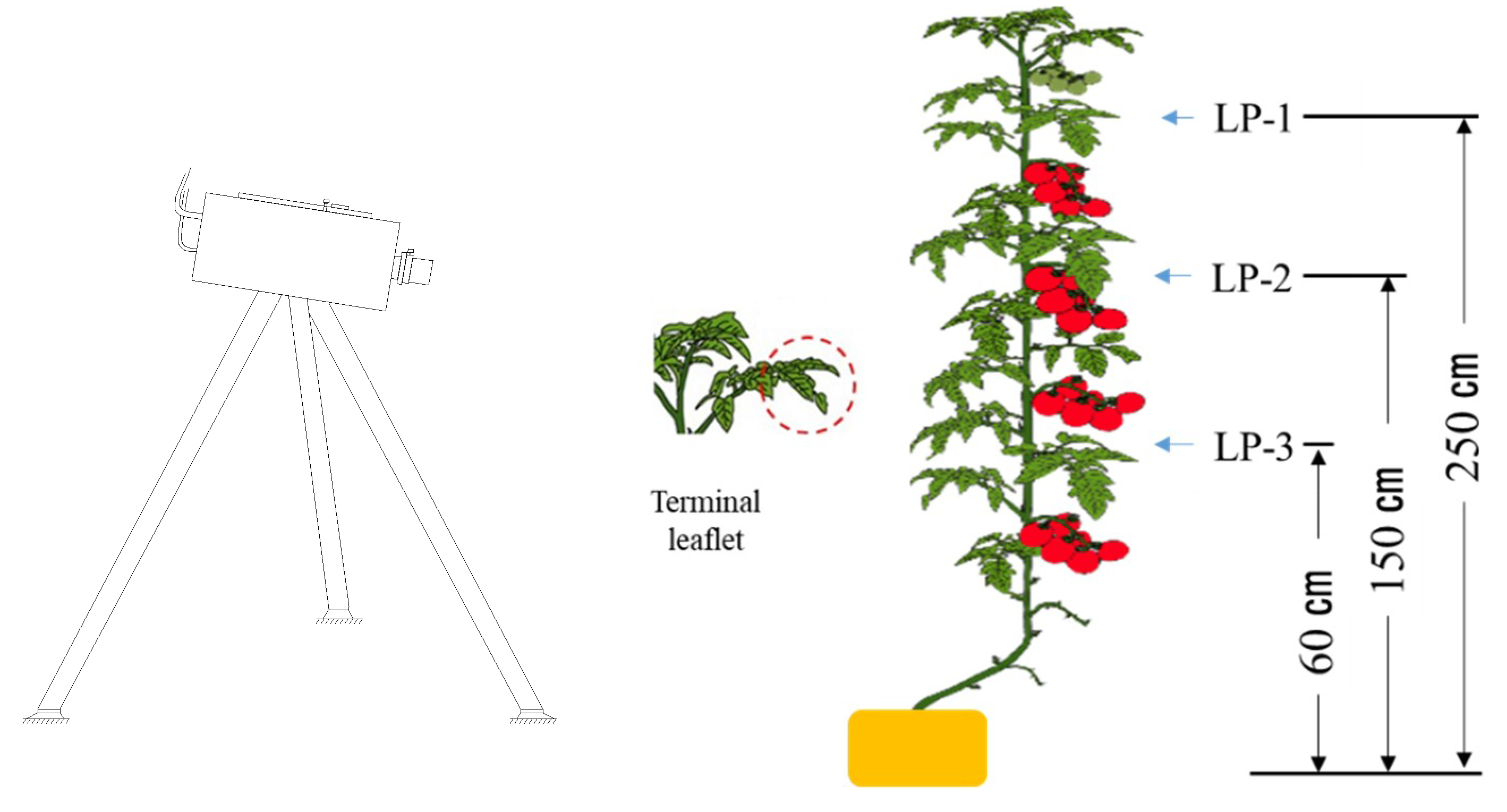
2.1. Research Site and Tomato Cultivation
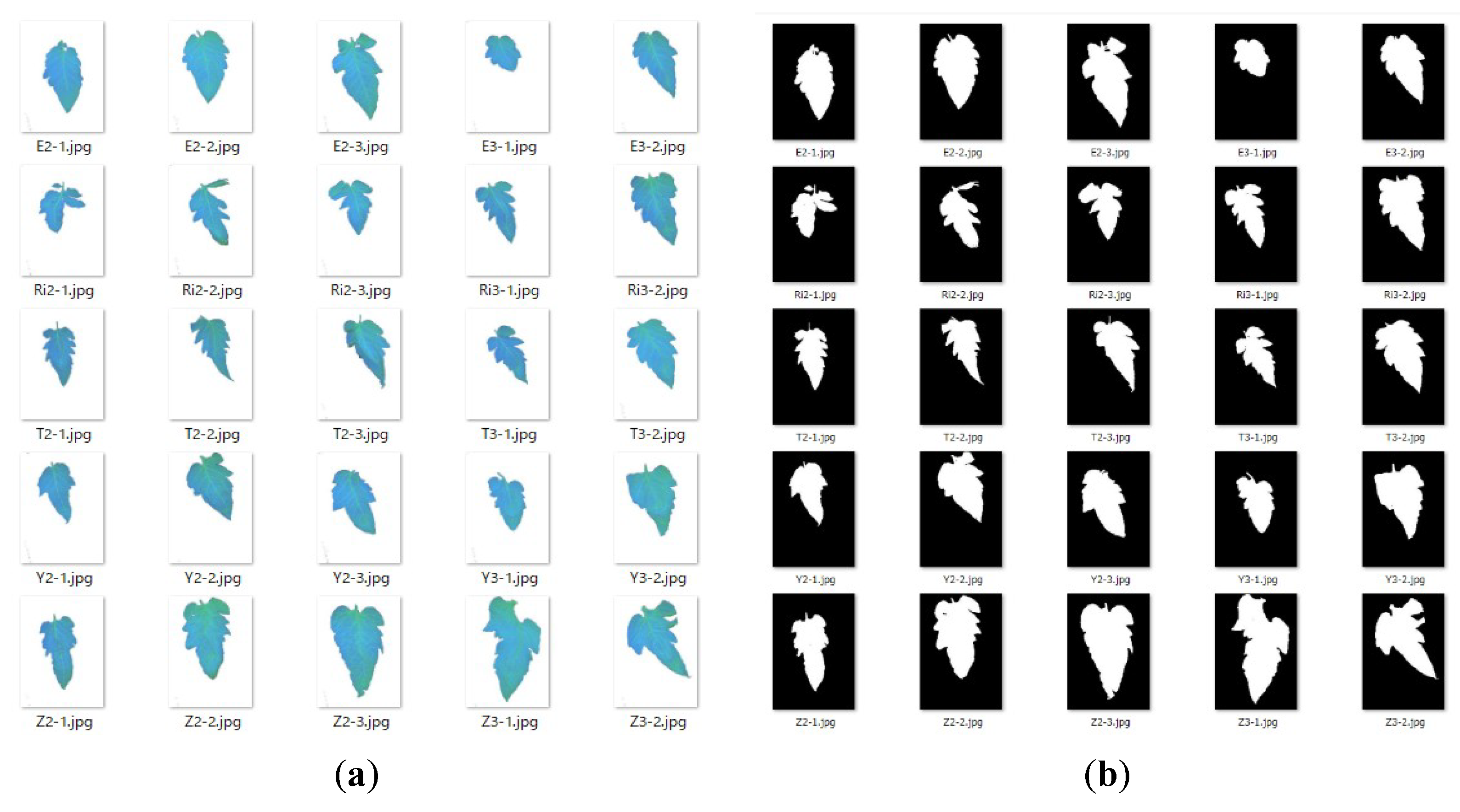
2.2. Tomato Leaf Sampling and Leaf Water Content Measurement
2.3. Hyperspectral Imaging and Data Analysis
2.3.1. Leaf Hyperspectral Measurement
2.3.2. Image Processing for Hyperspectral Data
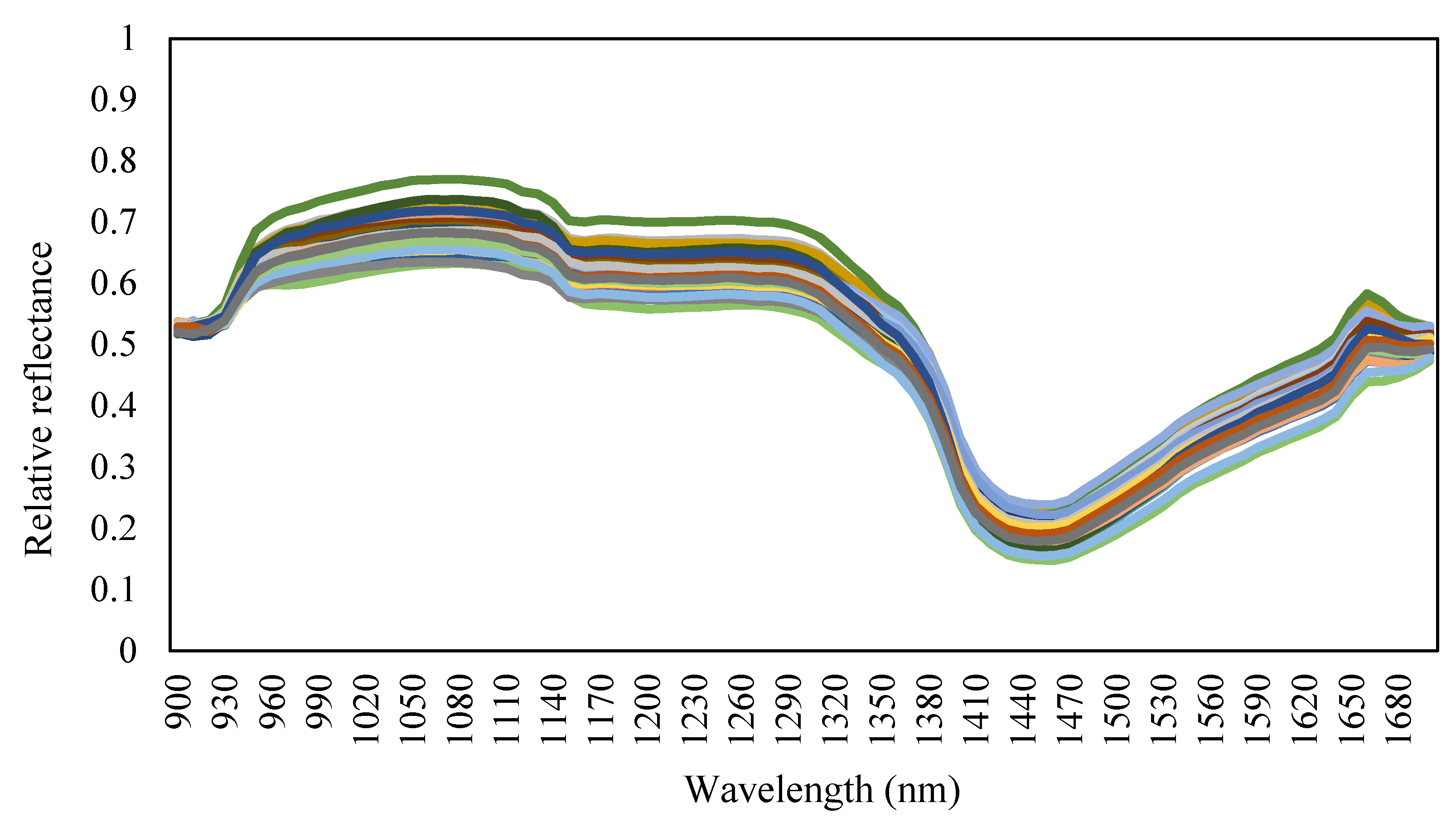
2.3.3. Hyperspectral Data Analysis and Waveband Selection
- : Spectrum of the tomato leaf sample, raw spectral data;
- : Spectrum of the standard reflector;
- Relative reflectance of the standard reflector;
- Standardized spectrum of the tomato leaf (i = 1–81 wavelength bands).
3. Results and Discussion
3.1. Tomato Leaf Water Status
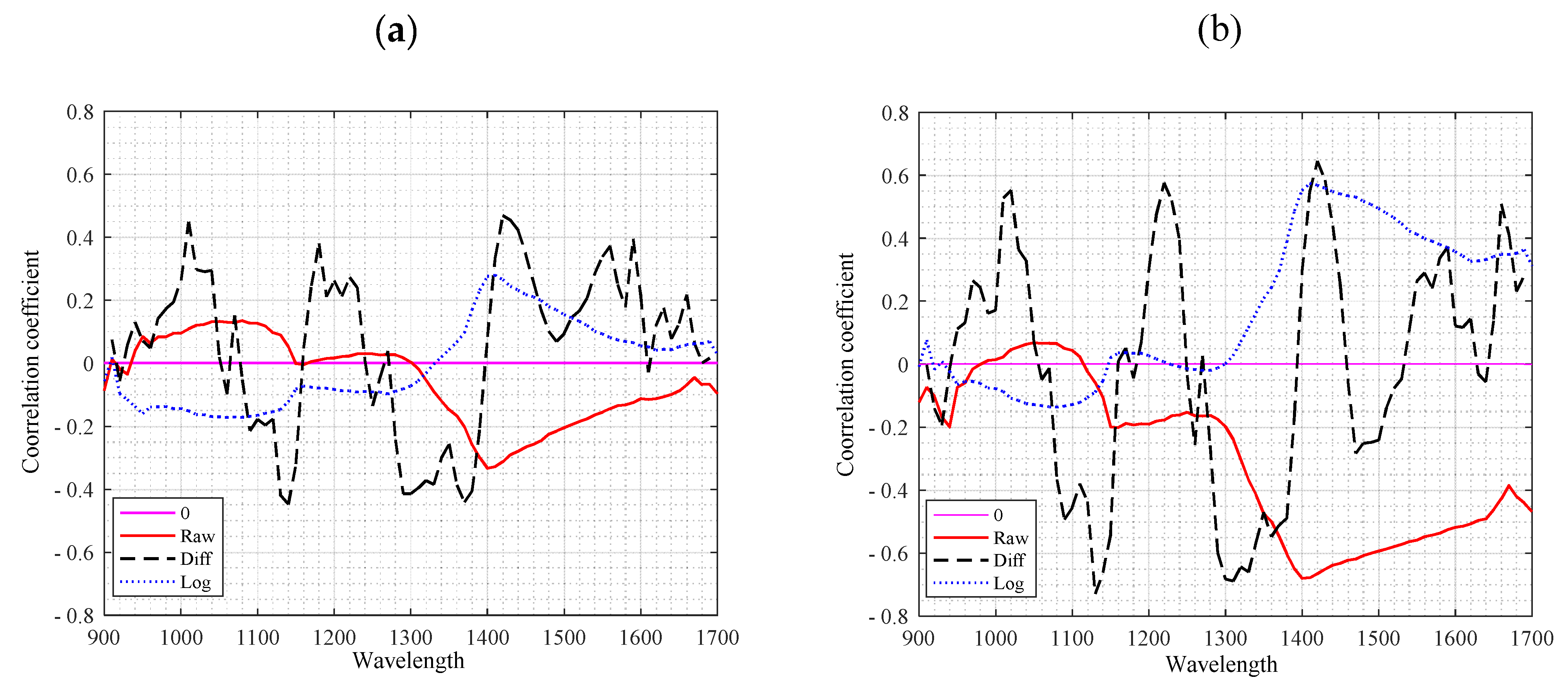
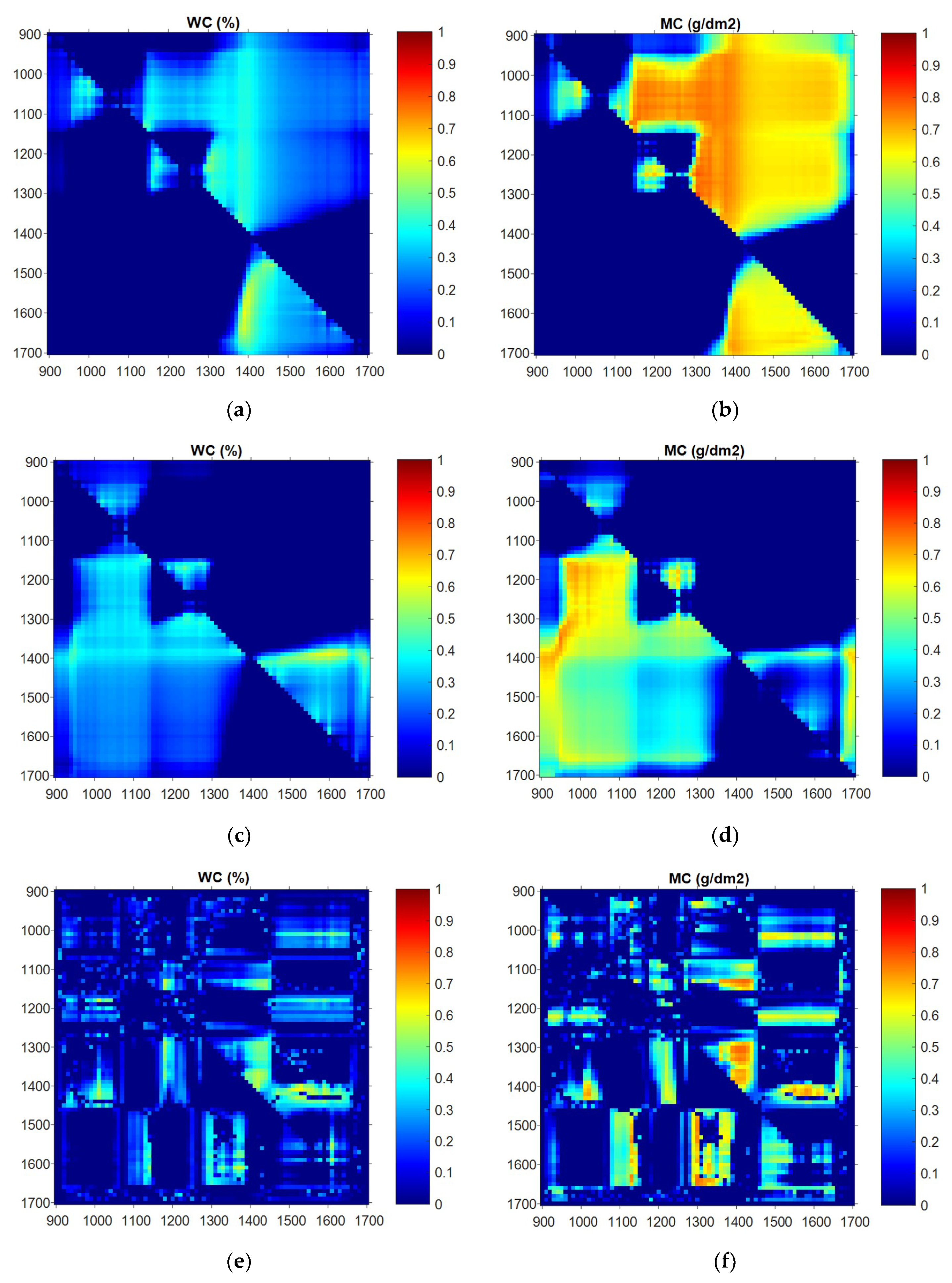
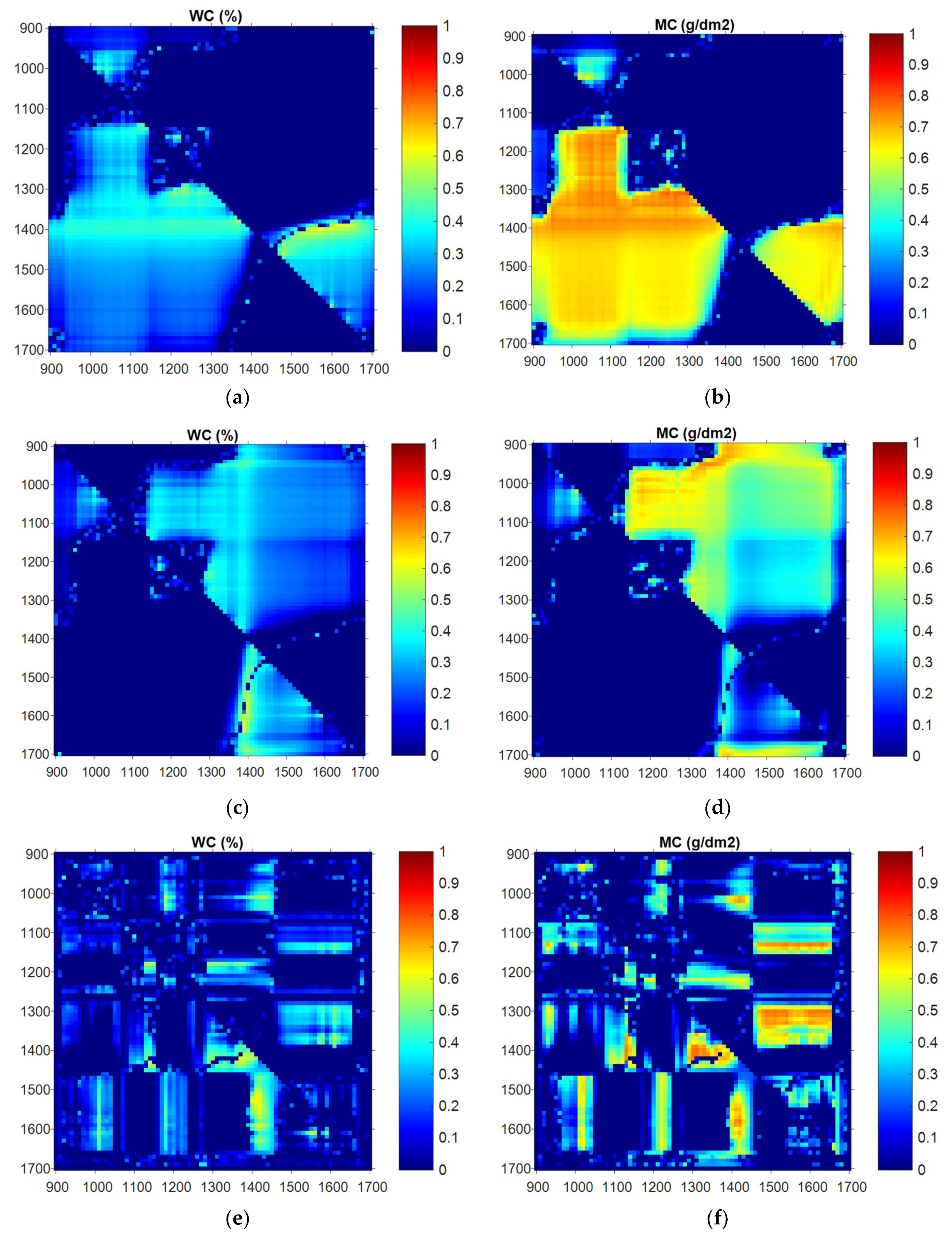
3.2. Relationship between Individual Wavelength and Water Status
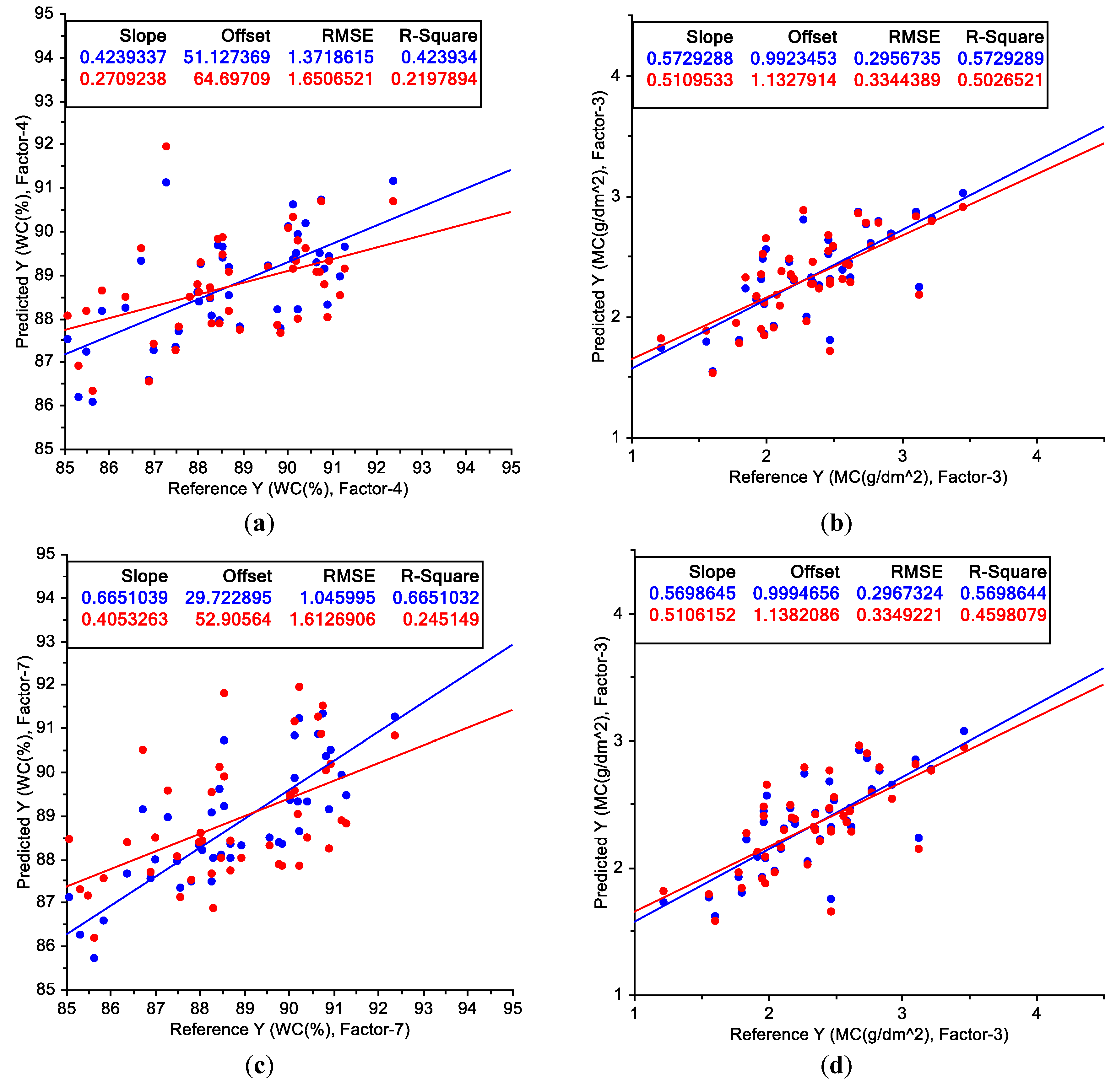
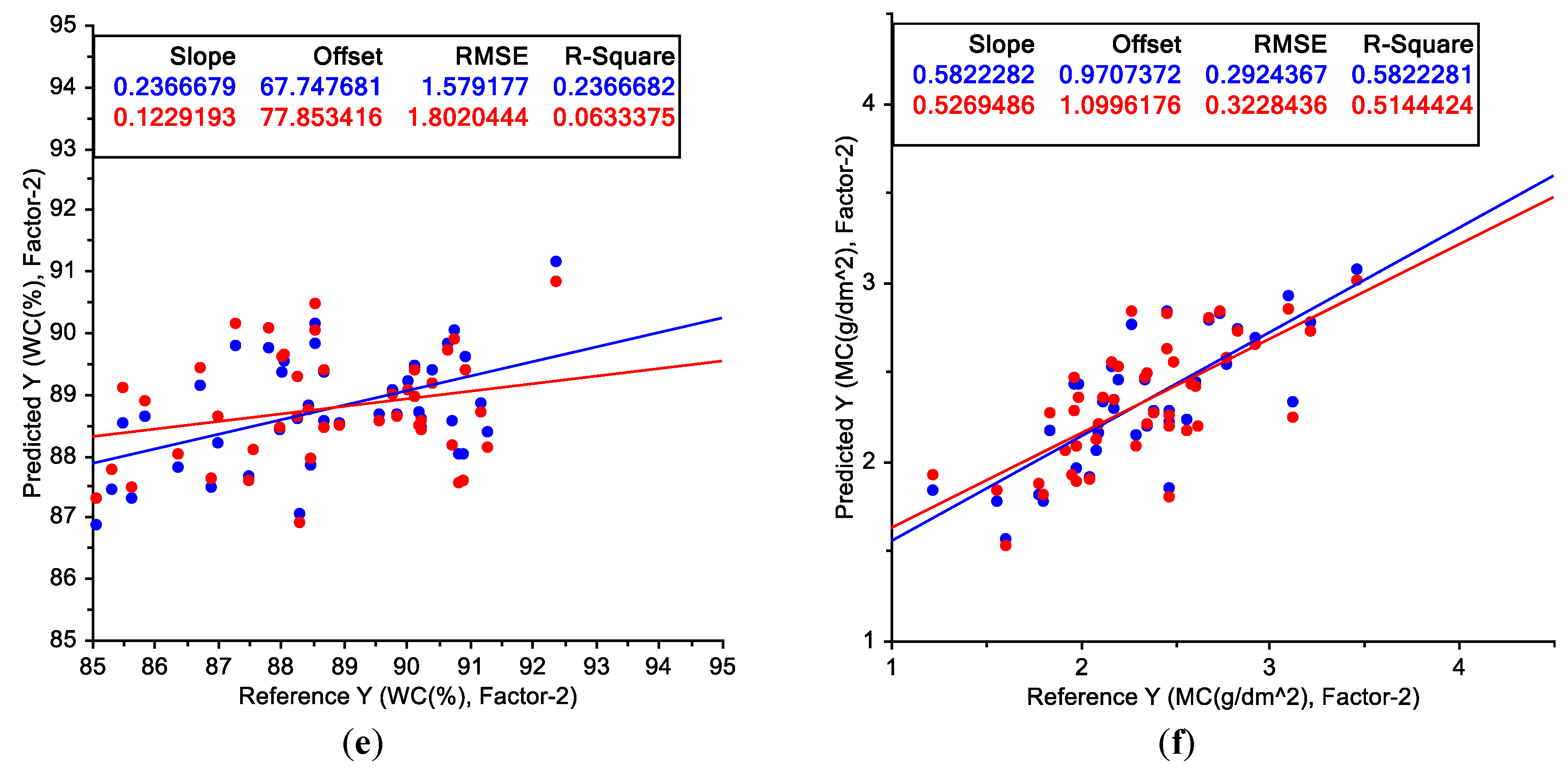
3.3. Partial Least-Squares Regression Applied to Wavelength and Water Status
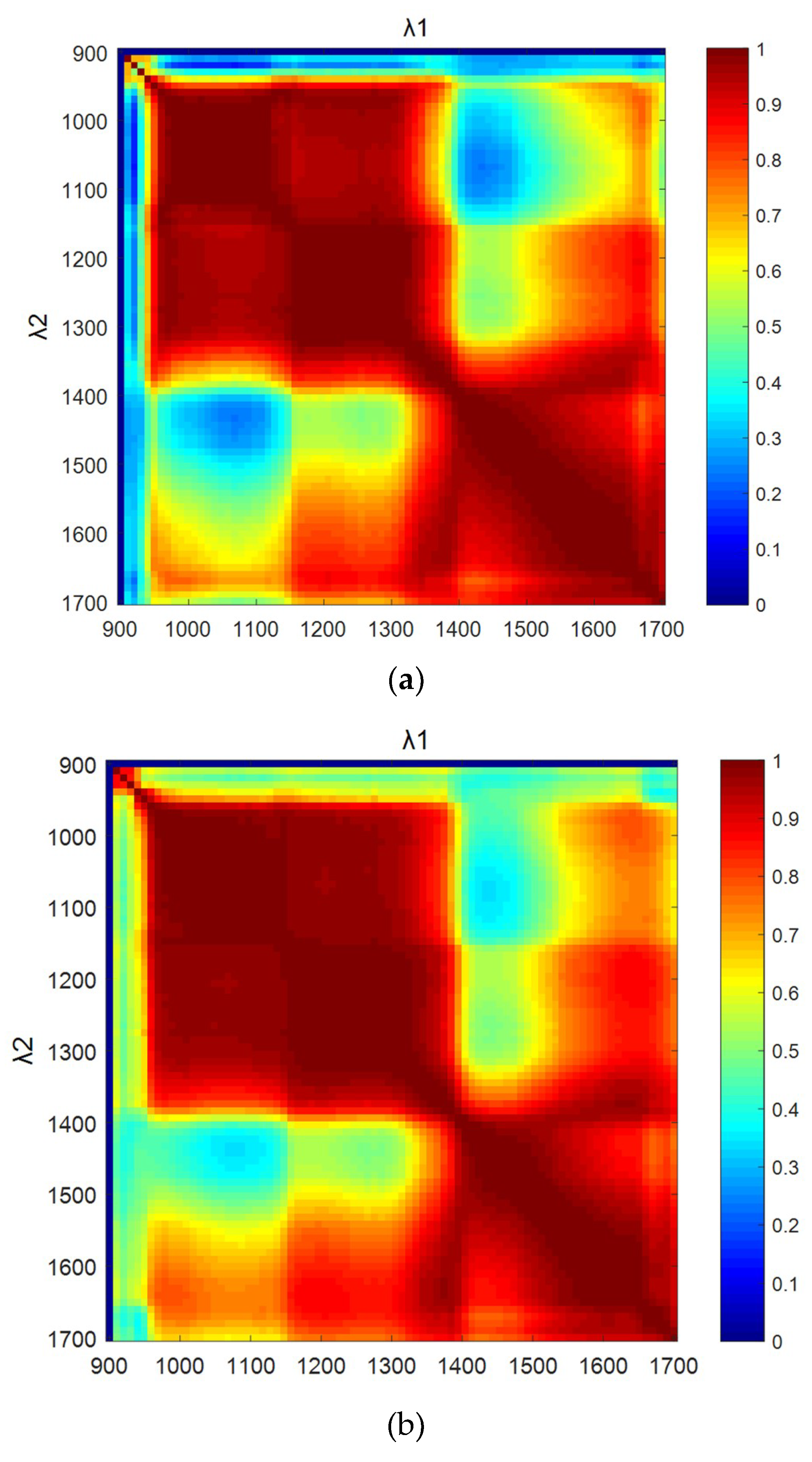
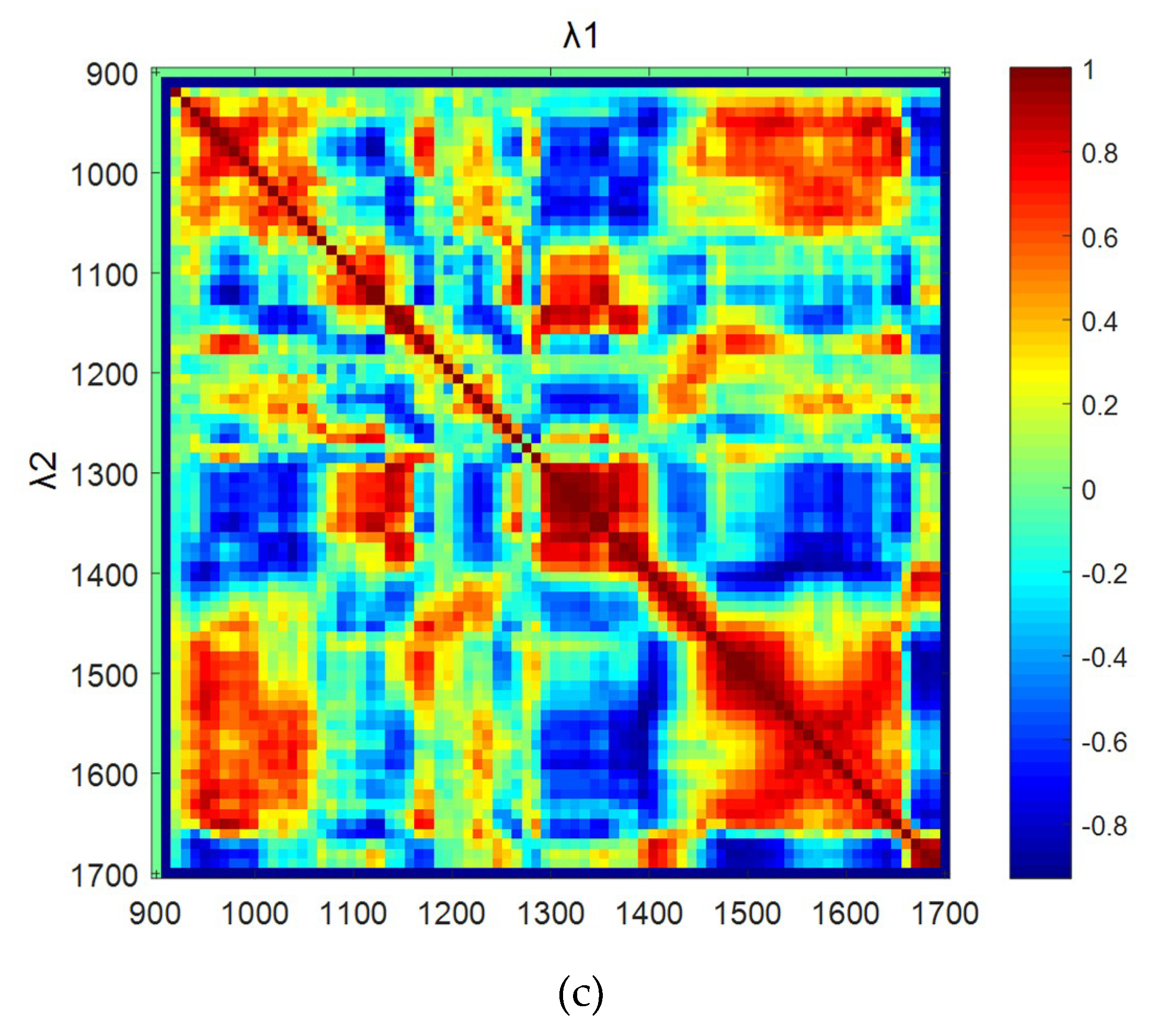
3.4. Autocorrelation Coefficient of Wavelength Bands
3.5. Application of NDVI and TBI to Wavelength and Water Status
3.5.1. NDVI
3.5.2. TBI
3.5.3. Characteristic Wavelength Bands
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amigo, J. Data Handling in Science and Technology. Hyperspectral Imaging; Elsevier: Amsterdam, The Netherlands, 2019; Volume 32, ISBN 978-0444639776. [Google Scholar]
- Pu, R. Hyperspectral Remote Sensing: Fundamentals and Practices; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Zhao, T.; Nakano, A. Agricultural Product Authenticity and Geographical Origin Traceability. Jpn. Agric. Res. Q. JARQ 2018, 52, 115–122. [Google Scholar] [CrossRef]
- Heuvelink, E.; Kierkels, T. Plant Physiology in Greenhouses; Horti-Text: Woerden, The Netherlands, 2015; p. 127. [Google Scholar]
- Zhang, J.; Xu, Y.; Yao, F.; Wang, P.; Guo, W.; Li, L.; Yang, L. Advances in estimation methods of vegetation water content based on optical remote sensing techniques. Sci. China Technol. Sci. 2010, 53, 1159–1167. [Google Scholar] [CrossRef]
- Yi, Q.; Bao, A.; Qi, W.; Zhao, J. Estimation of leaf water content in cotton by means of hyperspectral indices. Comput. Electron. Agric. 2013, 90, 144–151. [Google Scholar] [CrossRef]
- Danson, F.M.; Bowyer, P. Estimating live fuel moisture content from remotely sensed reflectance. Remote Sens. Environ. 2004, 92, 309–321. [Google Scholar] [CrossRef]
- Jabbari, E.; Kim, D.H.; Lee, L.P. Handbook of Biomimetics and Bioinspiration: Biologically Driven Engineering of Materials, Processes, Devices, and Systems; World Scientific Publishing Company: Singapore, 2014; p. 1462. [Google Scholar]
- Tong, Q.; Zhang, B.; Zheng, L. Hyperspectral Remote Sensing; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Zhao, T.; Komatsuzaki, M.; Okamoto, H.; Sakai, K. Cover Crop Nutrient and Biomass Assessment System Using Portable Hyperspectral Camera and Laser Distance Sensor. Eng. Agric. Environ. Food 2010, 3, 105–112. [Google Scholar] [CrossRef]
- Ting, H.; Jing, W.; Zongjian, L.; Ye, C. Spectral features of soil moisture. Acta Pedol. Sin. 2006, 43, 1027–1032, (In Chinese with English abstract). [Google Scholar]
- Zheng, Y.; Zhang, T.; Zhang, J.; Chen, X.; Shen, X. Influence of Smooth, 1st Derivative and Baseline Correction on the Near-Infrared Spectrum Analysis with PLS. Spectrosc. Spectr. Anal. 2004, 1546–1548. [Google Scholar]
- Cloutis, E.A. Hyperspectral geological remote sensing: Evaluation of analytical techniques. Int. J. Remote Sens. 1996, 17, 2215–2242. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Rueda, C.A.; Ustin, S.L. Water content estimation in vegetation with MODIS reflectance data and model inversion methods. Remote Sens. Environ. 2003, 85, 109–124. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B.N. Detection of changes in leaf water content using near and middle-infrared reflectance. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Deering, D.W. Rangeland Reflectance Characteristics Measured by Aircraft and Spacecraft Sensors. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1978. [Google Scholar]
- Hardinsky, M.A.; Lemas, V.; Smart, R.M. The influence of soil salinity, growth form, and leaf moisture on the spectral reflectance of Spartina alternifolia canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.J. NMDI: A normalized multi-band drought index for monitoring soil and vegetation moisture with satellite remote sensing. Geophys. Res. Lett. 2007, 34, L20405. [Google Scholar] [CrossRef]
- Fensholt, R.; Sandholt, I. Derivation of a shortwave infrared water stress index from MODIS nearand shortwave infrared data in a semiarid environment. Remote Sens. Environ. 2003, 87, 111–121. [Google Scholar] [CrossRef]
- Higashide, T.; Oshio, T.; Nukaya, T.; Yasuba, K.; Nakano, A.; Suzuki, K.; Ohmori, H.; Kaneko, S. Light Transmission of a Greenhouse (NARO Tsukuba Factory Farm) Built to Meet Building and Fire Standards. Bull. Natl. Inst. Veg. & Tea Sci. 2013, 13, 27–33. [Google Scholar]
- Pu, R.L.; Gong, P. Hyperspectral Remote Sensing and Its Applications; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef]
- Sun, D.-W. Hyperspectral Imaging for Food Quality Analysis and Control; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Ceccato, P.; Flasse, S.; Tarantola, S.; Jacquemoud, S.; Gregoire, J.M. Detecting vegetation leaf water content using reflectance in the optical domain. Remote Sens. Environ. 2001, 77, 22–33. [Google Scholar] [CrossRef]
- Basantia, N.C.; Kamruzzaman, M.; Nollet, L.M.; Leo, M.L. Hyperspectral Imaging Analysis and Applications for Food Quality; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–112. [Google Scholar]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral absorption features as indicators of water status in Quercus Agrifolia leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Zhang, J.H.; Guo, W.J. Studying on spectral characteristics of winter wheat with different soil moisture condition. In Proceedings of the 2nd International Symposium on Recent Advances in Quantitative Remote Sensing: RAQRS’II, Valencia, Spain, 25–29 September2006. [Google Scholar]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Schlerf, M.; Atzberger, C.; Hill, J. Remote sensing of forest biophysical variables using HyMap imaging spectrometer data. Remote Sens. Environ. 2005, 95, 177–194. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.F.; Jackson, T.J. Vegetation water content estimation for corn and soybeans using spectral indices derived from MODIS near- and short-wave infrared bands. Remote Sens. Environ. 2005, 98, 222–236. [Google Scholar] [CrossRef]
- Galvão, L.S.; Formaggio, A.R.; Tisot, D.A. Discrimination of sugarcane varieties in Southeastern Brazil with EO-1 Hyperion data. Remote Sens. Environ. 2005, 94, 523–534. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Q.; Zheng, C. Best hyperspectral indices for tracing leaf water status as determined from leaf dehydration experiments. Ecol. Indic. 2015, 54, 96–107. [Google Scholar] [CrossRef]
- José, R.; Riaño, D.; Carlisle, E.; Ustin, S.; Smart, D. Evaluation of Hyperspectral Reflectance Indexes to Detect Grapevine Water Status in Vineyards. Am. J. Enol. Viticult. 2007, 58, 302–317. [Google Scholar]
- Yamamoto, H.; Suzuki, Y.; Kojima, T.; Hayakawa, S.; Inoue, Y.; Tanaka, M. Estimation of Leaf Water Content of Plants by Spectral Reflectance of Near Infrared Range. J. Remote Sens. Soc. Jpn. 1994, 14, 293–301. [Google Scholar]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. Plant water parameters and the remote sensing R 1300/R 1450 leaf water index: Controlled condition dynamics during the development of water deficit stress. Irrig. Sci. 2009, 27, 357–365. [Google Scholar] [CrossRef]
- Margaret, K. Hyperspectral Remote Sensing of Tropical and Sub-Tropical Forests; CRC Press_Taylor & Francis Ltd.: Boca Raton, FL, USA, 2008; p. 350. [Google Scholar]

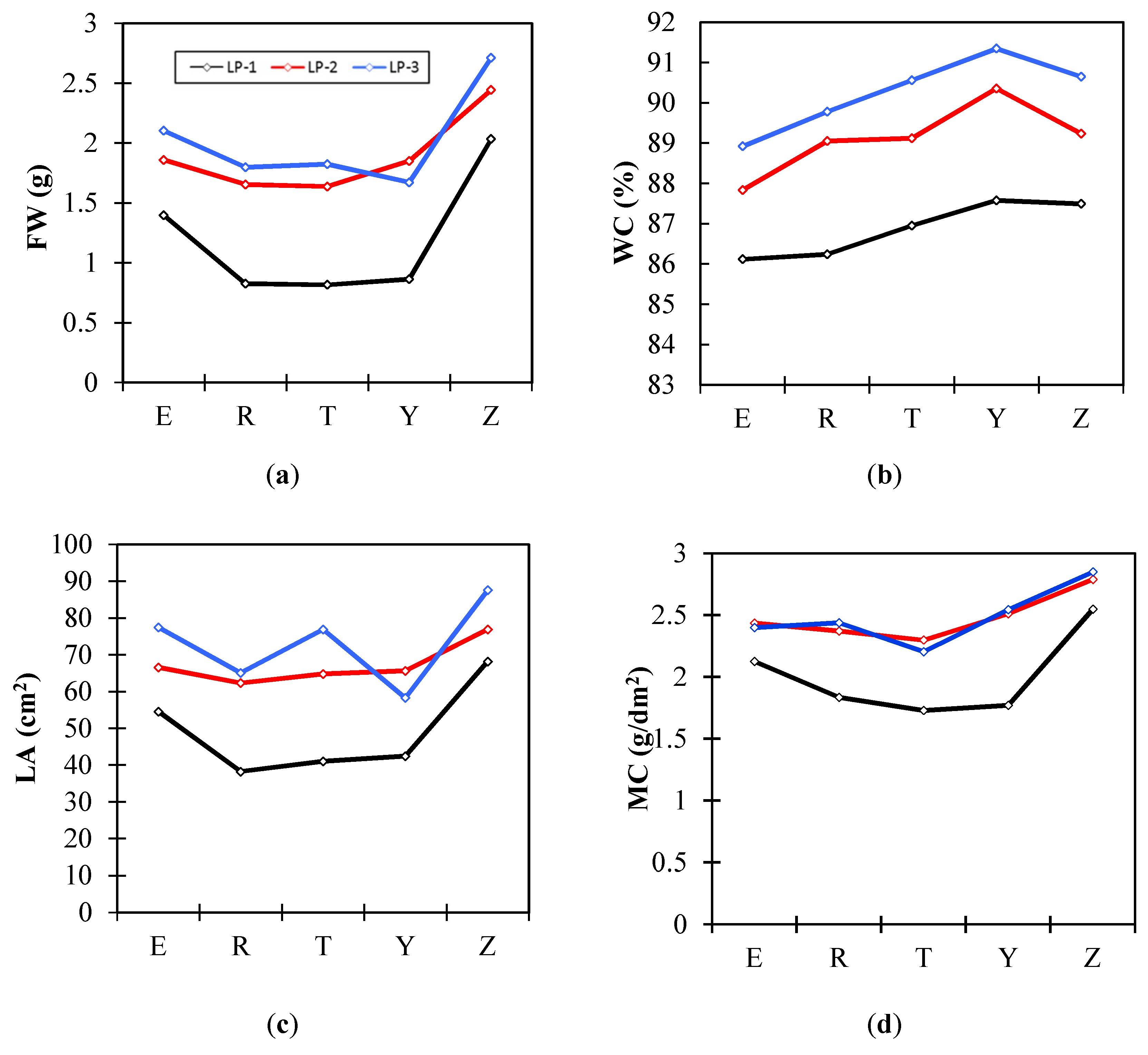
| FW (g) | WC (%) | LA (cm2) | FW/LA (g/dm2) | MC (g/dm2) | |
|---|---|---|---|---|---|
| LP-1 | 1.19 b | 86.88 c | 48.96 b | 2.30 b | 2.00 b |
| LP-2 | 1.89 a | 89.12 b | 67.31 a | 2.75 a | 2.48 a |
| LP-3 | 2.02 a | 90.25 a | 73.11 a | 2.79 a | 2.49 a |
| Regression Models | Data Sets | WC (%) | MC (g/dm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Wavelength Bands (nm) | R2 | r2 | RMSE | Wavelength Bands (nm) | R2 | r2 | RMSE | ||
| NDVI | RAW | 1390, 1600 | 0.6060 | 0.3672 | 1.44 | 1300, 1310 | 0.8042 | 0.6467 | 0.2689 |
| LOG | 1390, 1600 | 0.6080 | 0.3696 | 1.44 | 950, 1350 | 0.7550 | 0.5699 | 0.2967 | |
| DIFF | 1370, 1610 | 0.5643 | 0.3185 | 1.49 | 1310, 1410 | 0.7733 | 0.5980 | 0.2869 | |
| TBI | RAW | 1400, 1570 | 0.5950 | 0.3540 | 1.45 | 1300, 1310 | 0.7886 | 0.6389 | 0.2720 |
| LOG | 1390, 1620 | 0.5851 | 0.3430 | 1.47 | 950, 1350 | 0.7500 | 0.5707 | 0.2971 | |
| DIFF | 1410, 1520 | 0.6369 | 0.4057 | 1.39 | 1310, 1400 | 0.7716 | 0.5972 | 0.2878 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Nakano, A.; Iwaski, Y.; Umeda, H. Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories. Appl. Sci. 2020, 10, 4665. https://doi.org/10.3390/app10134665
Zhao T, Nakano A, Iwaski Y, Umeda H. Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories. Applied Sciences. 2020; 10(13):4665. https://doi.org/10.3390/app10134665
Chicago/Turabian StyleZhao, Tiejun, Akimasa Nakano, Yasunaga Iwaski, and Hiroki Umeda. 2020. "Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories" Applied Sciences 10, no. 13: 4665. https://doi.org/10.3390/app10134665
APA StyleZhao, T., Nakano, A., Iwaski, Y., & Umeda, H. (2020). Application of Hyperspectral Imaging for Assessment of Tomato Leaf Water Status in Plant Factories. Applied Sciences, 10(13), 4665. https://doi.org/10.3390/app10134665




