Optical Birefringence Growth Driven by Magnetic Field in Liquids: The Case of Dibutyl Phosphate/Propylamine System
Abstract
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Method
2.2.1. Sample Preparation
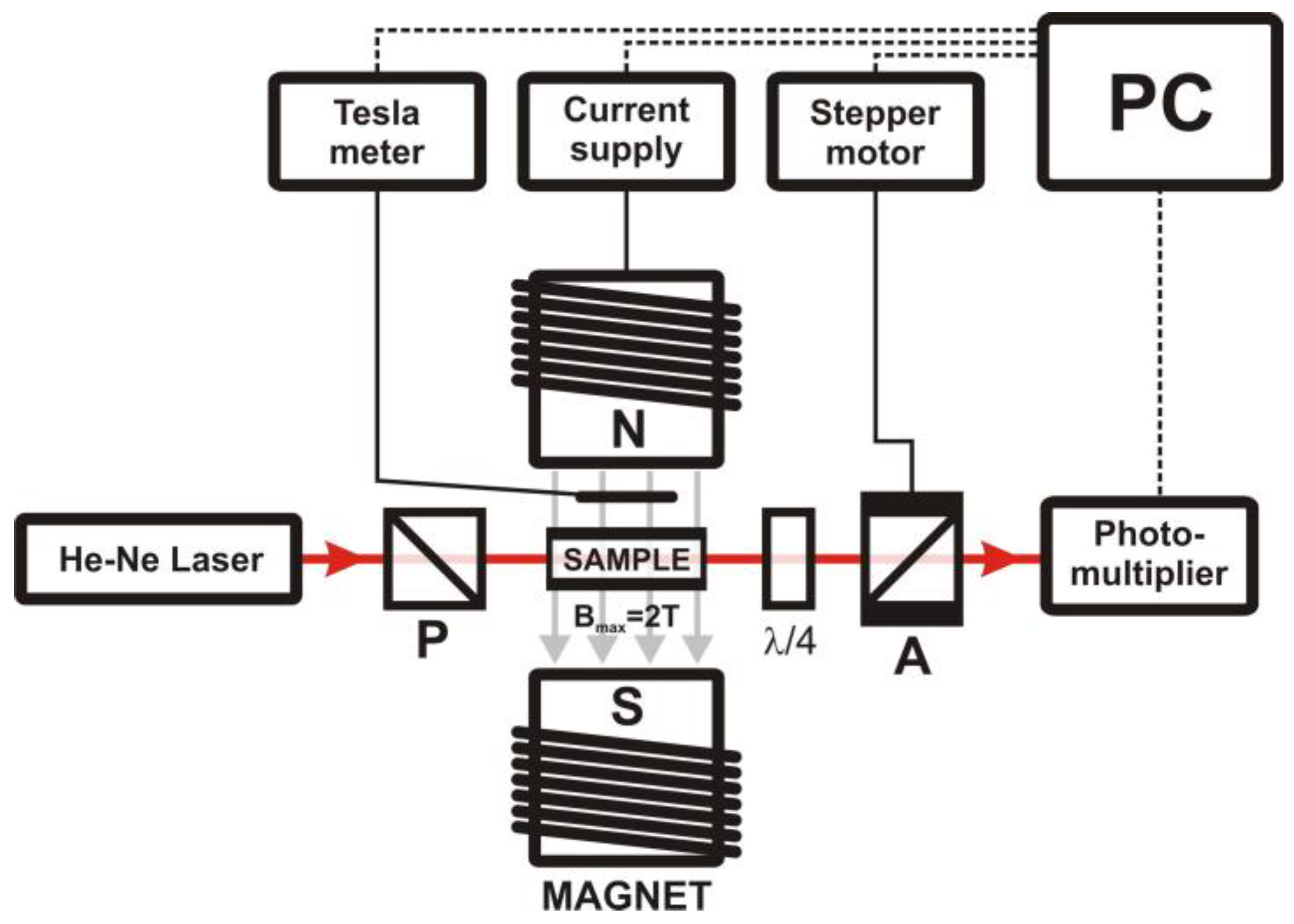
2.2.2. Measurement of Magnetically-Induced Optical Birefringence
3. Theoretical Background
3.1. Magnetically Induced Birefringence in Liquids
3.2. Magnetically Induced Birefringence in Absence of Permanent Magnetic Dipoles
3.3. Sign of Induced Birefringence
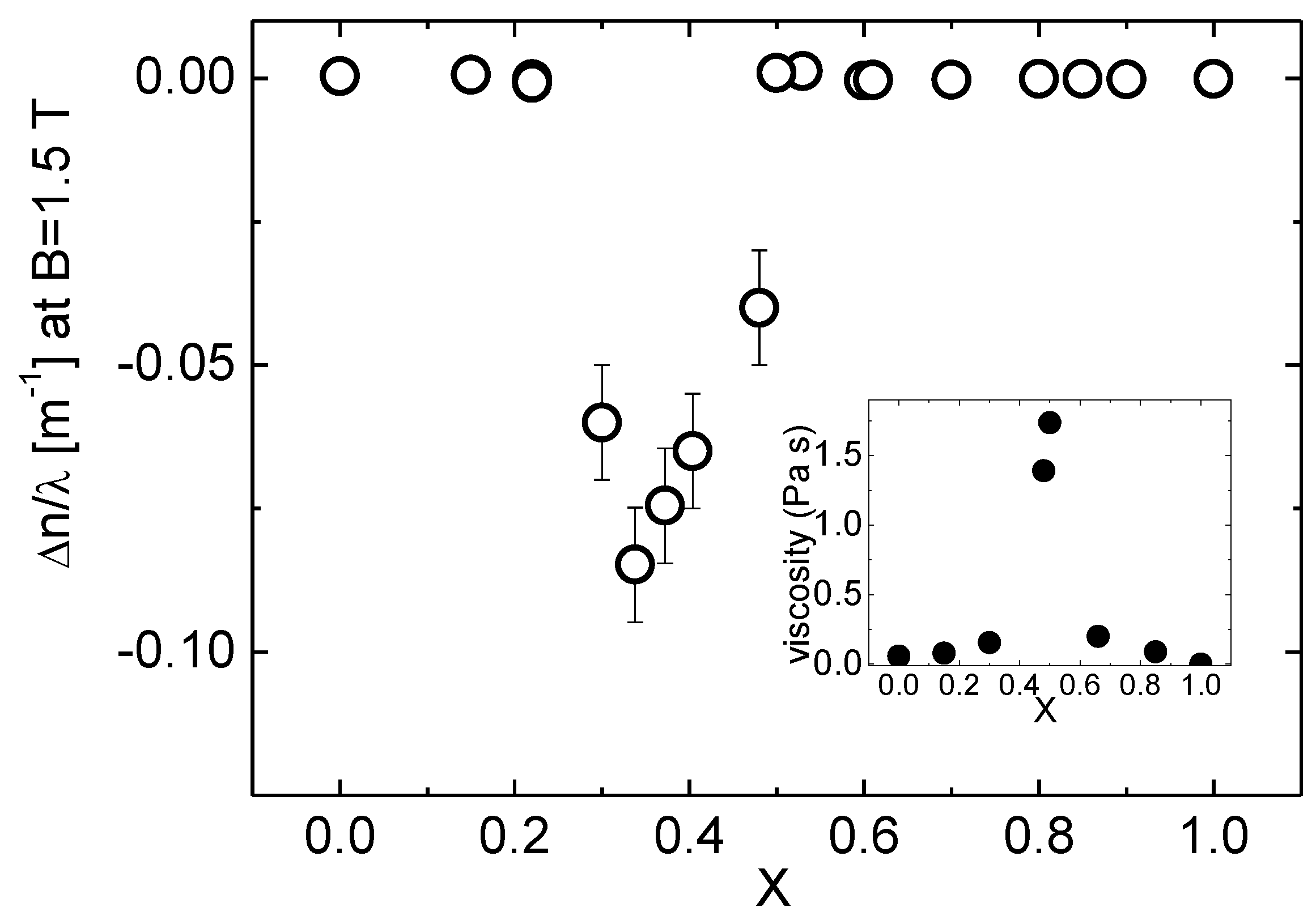
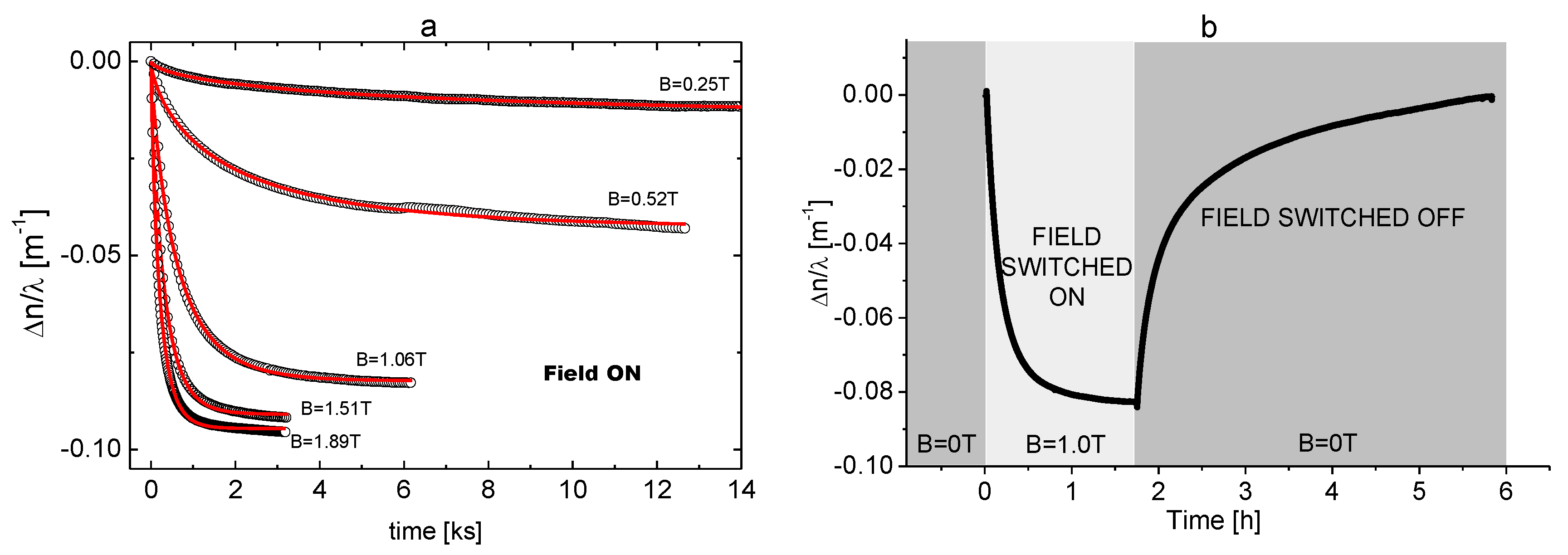
4. Results
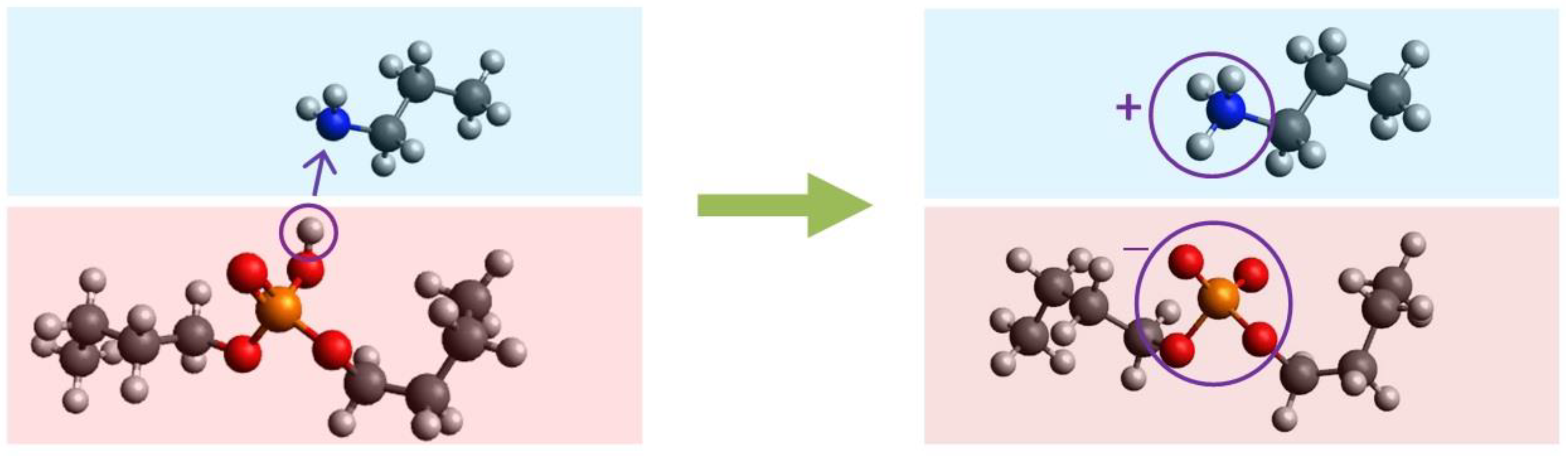
5. Discussion
5.1. Data
5.2. Modeling
6. Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calandra, P.; Caschera, D.; Turco Liveri, V.; Lombardo, D. How self-assembly of amphiphilic molecules can generate complexity in the nanoscale. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 164–183. [Google Scholar] [CrossRef]
- Calandra, P.; Mandanici, A.; Turco Liveri, V. Self-assembly in surfactant-based mixtures driven by acid–base reactions: Bis(2-ethylhexyl) phosphoric acid–noctylamine systems. RSC Adv. 2013, 3, 5148–5155. [Google Scholar] [CrossRef]
- Yamada, M.; Honma, I.J. Anhydrous Protonic Conductivity of a Self-Assembled Acid−Base Composite Material. Phys. Chem. B 2004, 108, 5522–5526. [Google Scholar] [CrossRef]
- Calandra, P.; Turco Liveri, V.; Riello, P.; Freris, I.; Mandanici, A. Self-assembly in surfactant-based liquid mixtures: Octanoic acid/Bis(2-ethylhexyl)amine systems. J. Colloid Interf. Sci. 2012, 367, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Honma, I. Anhydrous solid state proton conductor based on enzimidazole/monododecyl phosphate molecular hybrids. Solid State Ion. 2005, 176, 979–984. [Google Scholar] [CrossRef]
- Calandra, P.; Nicotera, I.; Oliviero Rossi, C.; Turco Liveri, V. Dynamical properties of self-assembled surfactant-based mixtures: Triggering of 1D anomalous diffusion in bis(2-ethylhexyl) phosphoric acid/n-octylamine systems. Langmuir 2013, 29, 14848–14854. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; de Caro, T.; Caschera, D.; Lombardo, D.; Todaro, L.; Turco Liveri, V. Spectroscopic and structural characterization of pure and FeCl3-containing tri-n-butyl phosphate. Colloid Polym. Sci. 2015, 293, 597–603. [Google Scholar] [CrossRef]
- Nicotera, I.; Oliviero Rossi, C.; Turco Liveri, V.; Calandra, P. Decoupling of dynamic processes in surfactant-based liquid mixtures: The case of lithium-containing bis(2-ethylhexyl)phosphoric acid/bis(2-ethylhexyl)amine systems. Langmuir 2014, 30, 8336–8341. [Google Scholar] [CrossRef]
- Nicotera, I.; Oliviero Rossi, C.; Simari, C.; Turco Liveri, V.; Calandra, P. Bis(2-etylhexyl) phosphoric acid/bis(2-etylhexyl)amine mixtures as solvent media for lithium ions: A dynamical study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 447–453. [Google Scholar] [CrossRef]
- Corici, L.; Caschera, D.; Cseh, L.; De Luca, G.; Szerb, E.I.; Calandra, P. Amphiphiles as novel solvents for photochromics: Stability and photophysical properties. Mol. Cryst. Liq. Cryst. 2019, 684, 24–36. [Google Scholar] [CrossRef]
- Calandra, P.; Turco Liveri, V.; Ruggirello, A.M.; Licciardi, M.; Lombardo, D.; Mandanici, A. Anti-Arrhenian behaviour of conductivity in octanoic acid–bis(2-ethylhexyl)amine systems: A physico-chemical study. J. Mater. Chem. C 2015, 3, 3198–3210. [Google Scholar] [CrossRef]
- Turco Liveri, V.; Lombardo, D.; Pochylski, M.; Calandra, P. Molecular association of small amphiphiles: Origin of ionic liquid properties in dibutyl phosphate/propylamine binary mixtures. J. Mol. Liq. 2018, 263, 274–281. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef]
- Maiuolo, L.; Russo, B.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Tallarida, M.A.; De Nino, A. Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition: Role of Er(OTf)3, ionic liquid and water. Tetrahedron Lett. 2019, 60, 672–674. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-Functionalized 1,2,3-Triazoles Using Ionic Liquid/Iron(III) Chloride as an Efficient and Reusable Homogeneous Catalyst. Catalysts 2018, 8, 364. [Google Scholar]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-Lopez, D.; Russo, B.; Algieri, V. Efficient Organocatalyst Supported on a Simple Ionic Liquid as a Recoverable System for the Asymmetric Diels–Alder Reaction in the Presence of Water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Greavesa, T.L.; Drummond, C.J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 2008, 37, 1709–1726. [Google Scholar] [CrossRef]
- Pochylski, M.; Oliviero Rossi, C.; Nicotera, I.; Turco Liveri, V.; Calandra, P. Nano-demixing as a novel strategy for magnetic field responsive systems: The case of dibutylphosphate/bis(2-ethylhexyl)amine systems. RSC Adv. 2016, 6, 26696–26708. [Google Scholar] [CrossRef]
- Pochylski, M.; Turco Liveri, V.; Calandra, P. Dibutyl phosphate/propylamine mixtures show supra-molecular slow building up under magnetic field. Atti Accademia Peloritana Pericolanti 2019, 97, A21. [Google Scholar] [CrossRef]
- Pochylski, M.; Iwaszkiewicz-Kostka, I.; Kaczmarek, M.S.; Woźniak, Z.; Drozdowski, H. Molecular orientation in binary liquid mixtures from excess Cotton-Mouton constant. J. Mol. Liq. 2016, 224, 146–150. [Google Scholar] [CrossRef]
- Koralewski, M.; Pochylski, M.; Mitróová, Z.; Timko, M.; Kopčanský, P.; Melníková, L. Magnetic birefringence of natural and synthetic ferritin. J. Magn. Magn. Mater. 2011, 323, 2413–2417. [Google Scholar] [CrossRef]
- Koralewski, M.; Pochylski, M.; Gierszewski, J. Magnetic birefringence of iron oxyhydroxide nanoparticles stabilised by sucrose. J. Magn. Magn. Mater. 2011, 323, 1140–1144. [Google Scholar] [CrossRef]
- Levy, O. Dielectric response and electro-optical effects in suspensions of anisotropic particles. Phys. Rev. E 2002, 66, 011404. [Google Scholar] [CrossRef] [PubMed]
- Pochylski, M.; Calandra, P.; Aliotta, F.; Ponterio, R.C. Electrically induced birefringence in nanoparticle dispersions for electro-rheological applications. J. Phys. D Appl. Phys. 2014, 47, 465301. [Google Scholar] [CrossRef]
- Gielen, J.C.; Shklyarevskiy, I.O.; Schenning, A.P.H.J.; Christianen, P.C.M.; Maan, J.C. Using magnetic birefringence to determine the molecular arrangement of supramolecular nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, B.J.; Lim, H.-K.; Kang, K.; Yoo, S.H.; Gong, J.; Yoon, E.; Lee, J.; Choi, I.S.; Kim, H.; et al. Magnetotactic molecular architectures from self-assembly of β-peptide foldamers. Nat. Commun. 2015, 6, 8747. [Google Scholar] [CrossRef]
- Rikken, R.S.M.; Kerkenaar, H.H.M.; Nolte, R.J.M.; Maan, J.C.; van Hest, J.C.M.; Christianen, P.C.M.; Wilson, D.A. Probing morphological changes in polymersomes with magnetic birefringence. Chem. Commun. 2014, 50, 5394–5396. [Google Scholar] [CrossRef]
- Levy, O. Electro-optical properties of suspensions of anisotropic particles. Phys. B Condens. Matter 2003, 338, 44–47. [Google Scholar] [CrossRef]
- Socoliuc, V.; Raşa, M.; Sofonea, V.; Bica, D.; Osvath, L.; Luca, D. Agglomerate formation in moderately concentrated ferrofluids from static magneto-optical measurements. J. Magn. Magn. Mater. 1999, 191, 241–248. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gazeau, F.; Roger, J.; Pons, J.N.; Salis, M.F.; Perzynski, R.; Bacri, J.C. Binding of biological effectors on magnetic nanoparticles measured by a magnetically induced transient birefringence experiment. Phys. Rev. E 2002, 65, 031404. [Google Scholar] [CrossRef]
- Ku, B.Y.; Chan, M.L.; Ma, Z.; Horsley, D.A. Frequency-domain birefringence measurement of biological binding to magnetic nanoparticles. J. Magn. Magn. Mater. 2008, 320, 2279–2283. [Google Scholar] [CrossRef] [PubMed]
- Köber, M.; Moros, M.; Grazú, V.; De La Fuente, J.M.; Luna, M.; Briones, F. Transient magnetic birefringence for determining magnetic nanoparticle diameters in dense, highly light scattering media. Nanotechnology 2012, 23, 155501. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Durall, B.; Frankowiak, G. Magnetic birefringence in a liquid crystal: An experiment for the advanced undergraduate laboratory. Am. J. Phys. 2000, 63, 248. [Google Scholar] [CrossRef]
- Lombardo, D.; Munaò, G.; Calandra, P.; Pasqua, L.; Caccamo, M.T. Evidence of pre-micellar aggregates in aqueous solution of amphiphilic PDMS–PEO block copolymer. Phys. Chem. Chem. Phys. 2019, 22, 11983–11991. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, M.A.; Lombardo, D. Structural characterization in mixed lipid membrane systems by neutron and X-ray scattering. Biochim. Biophys. Acta BBA Gen. Subj. 2017, 1861, 3700–3717. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, M.A.; Janich, M.; Hildebrand, A.; Strunz, P.; Neubert, R.H.H.; Lombardo, D. Structural transition in aqueous lipid/bile salt [DPPC/NaDC] supramolecular aggregates: SANS and DLS study. Chem. Phys. 2013, 424, 93–99. [Google Scholar] [CrossRef]
- Sackmann, E. Physical basis of self-organization and function of membranes: Physics of vesicles. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 1, pp. 213–303. [Google Scholar]
- Katsaras, J.; Gutberlet, T. Lipid Bilayers: Structure and Interactions; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Lombardo, D.; Calandra, P.; Bellocco, E.; Lagana, G.; Barreca, D.; Magazù, S.; Wanderlingh, U.; Kiselev, M.A. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2769–2777. [Google Scholar] [CrossRef]
- Muthu, M.S.; Feng, S.S. Theranostic liposomes for cancer diagnosis and treatment: Current development and pre-clinical success. Expert Opin. Drug Deliv. 2013, 10, 151–155. [Google Scholar] [CrossRef]
- Yavlovich, A.; Smith, B.; Gupta, K.; Blumenthal, R.; Puri, A. Light-sensitive lipid-based nanoparticles for drug delivery: Design principles and future considerations for biological applications. Mol. Membr. Biol. 2010, 27, 364–381. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Magazù, S.; Wanderlingh, U.; Barreca, D.; Pasqua, L.; Kiselev, M.A. Soft nanoparticles charge expression within lipid membranes: The case of amino terminated dendrimers in bilayers vesicles. Colloids Surf. B Biointerfaces 2018, 170, 609–616. [Google Scholar] [CrossRef]
- Calandra, P. Managing Complexity in Material Science: The Role of Imagination. In New Trends in Physics Education Research; Nova Science Publishers: New York, NY, USA, 2018; Chapter 1; ISBN 978-1-53613-893-1. [Google Scholar]
- Yadav, P.S.; Gulec, S.; Jena, A.; Tang, S.; Yadav, S.; Katoshevski, D.; Tadmor, R. Interfacial modulus and surfactant coated surfaces. Surf. Topogr. Metrol. Prop. 2018, 6, 045007. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, X. Tuning the amphiphilicity of building blocks: Controlled self-assembly and disassembly for functional supramolecular materials. Adv. Mater. 2009, 21, 2849–2864. [Google Scholar] [CrossRef]
- Lombardo, D.; Micali, N.; Villari, V.; Kiselev, M.A. Large structures in diblock copolymer micellar solution. Phys. Rev. E 2004, 70, 021402. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J. Intermolecular and Surface Forces, 2nd ed.; Elsevier Science: Burlington, MA, USA, 2011. [Google Scholar]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers: Self-Assembly and Applications (Studies in Surface Science and Catalysis); Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; ISBN 0-444-82441-3. [Google Scholar]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pochylski, M.; Lombardo, D.; Calandra, P. Optical Birefringence Growth Driven by Magnetic Field in Liquids: The Case of Dibutyl Phosphate/Propylamine System. Appl. Sci. 2020, 10, 164. https://doi.org/10.3390/app10010164
Pochylski M, Lombardo D, Calandra P. Optical Birefringence Growth Driven by Magnetic Field in Liquids: The Case of Dibutyl Phosphate/Propylamine System. Applied Sciences. 2020; 10(1):164. https://doi.org/10.3390/app10010164
Chicago/Turabian StylePochylski, Mikolaj, Domenico Lombardo, and Pietro Calandra. 2020. "Optical Birefringence Growth Driven by Magnetic Field in Liquids: The Case of Dibutyl Phosphate/Propylamine System" Applied Sciences 10, no. 1: 164. https://doi.org/10.3390/app10010164
APA StylePochylski, M., Lombardo, D., & Calandra, P. (2020). Optical Birefringence Growth Driven by Magnetic Field in Liquids: The Case of Dibutyl Phosphate/Propylamine System. Applied Sciences, 10(1), 164. https://doi.org/10.3390/app10010164






