Microfluidically-Assisted Isolation and Characterization of Achromobacter spanius from Soils for Microbial Degradation of Synthetic Polymers and Organic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation
2.1.1. Soil Samples Description
2.1.2. Next Gen Sequencing of Soil Samples
2.2. Chemicals and Medium
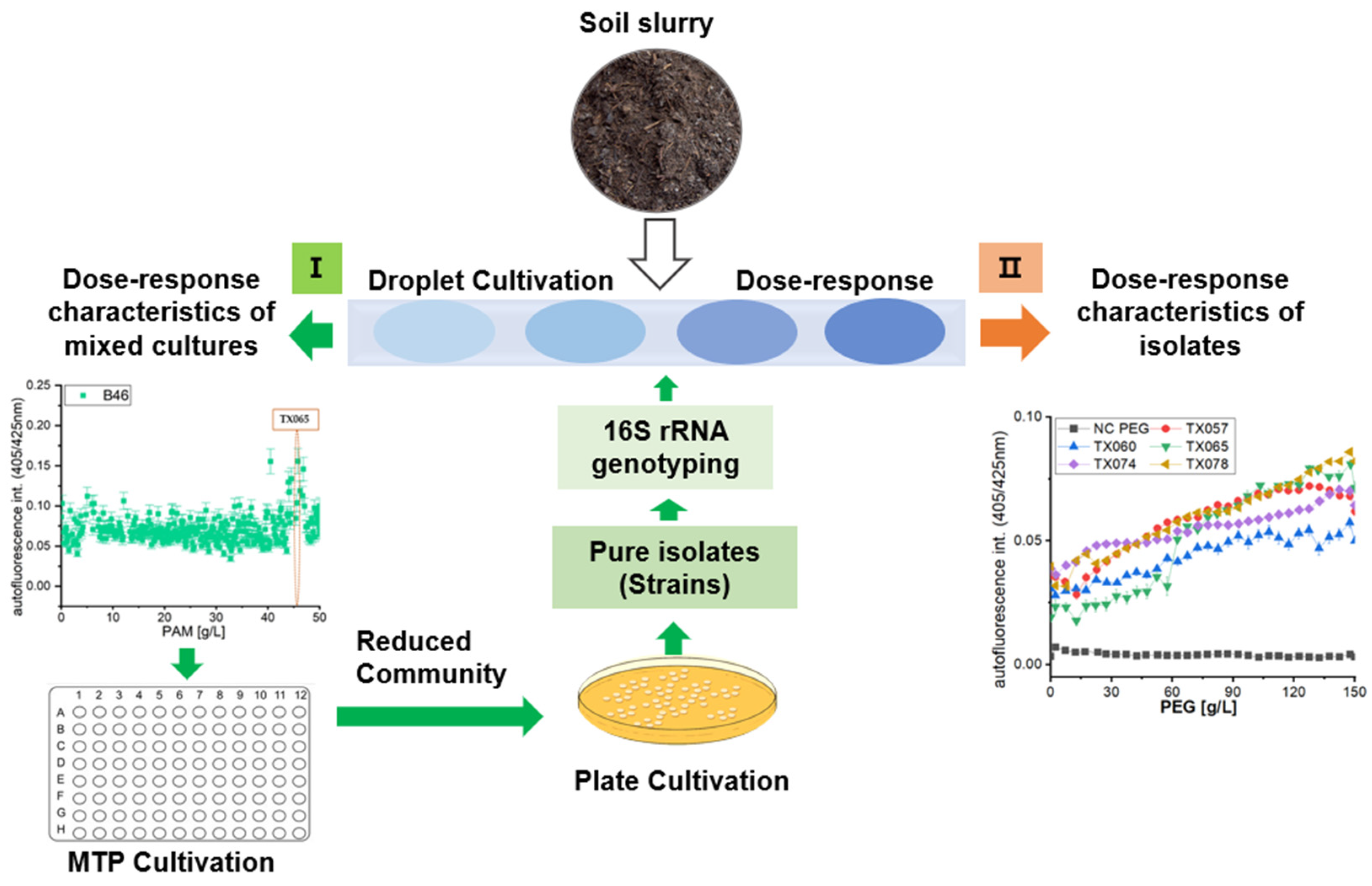
2.3. Microfluidic Cultivation Procedure
2.4. Single Strain Isolation and Identification
3. Results
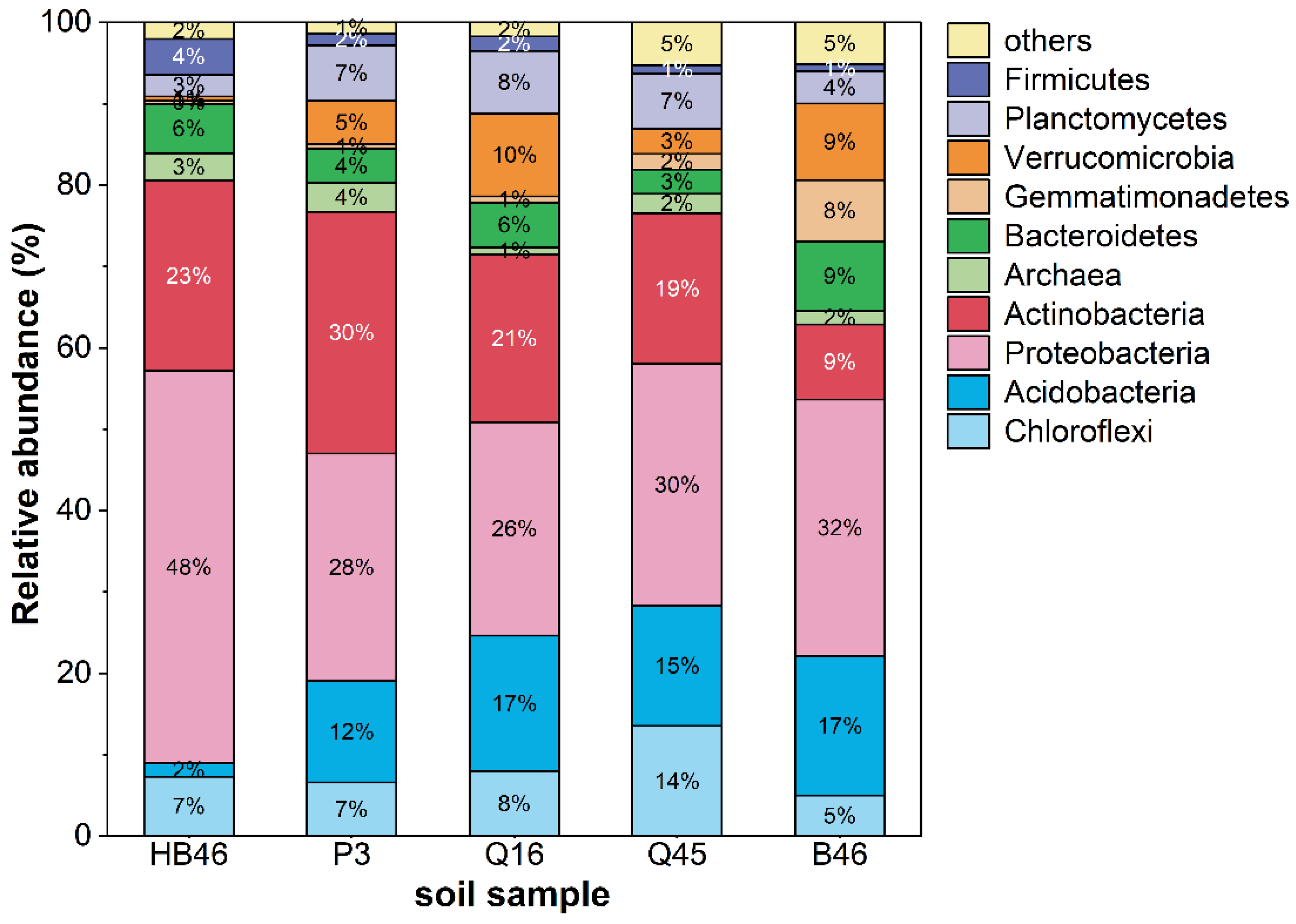
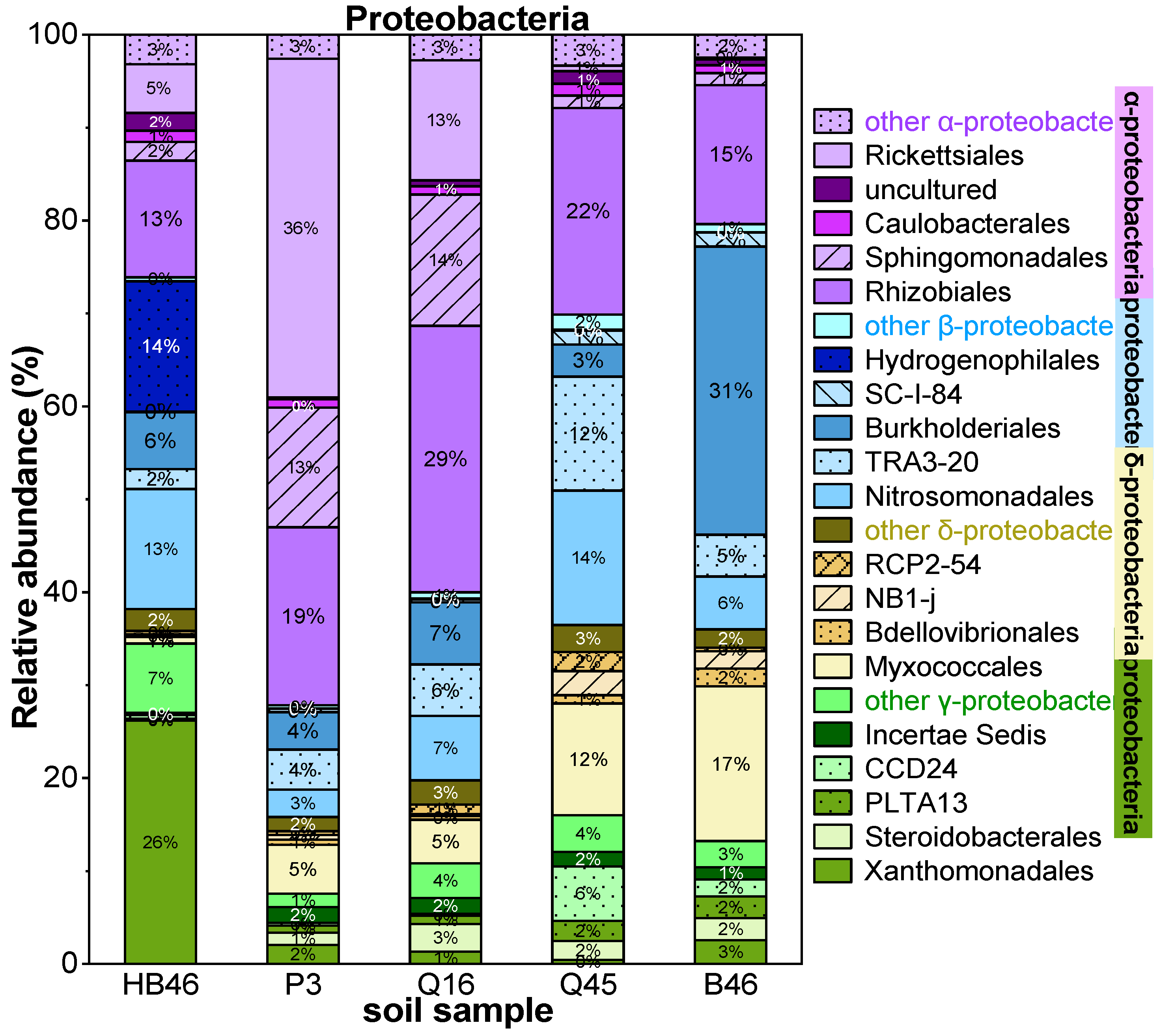
3.1. Characterization of Soil Samples
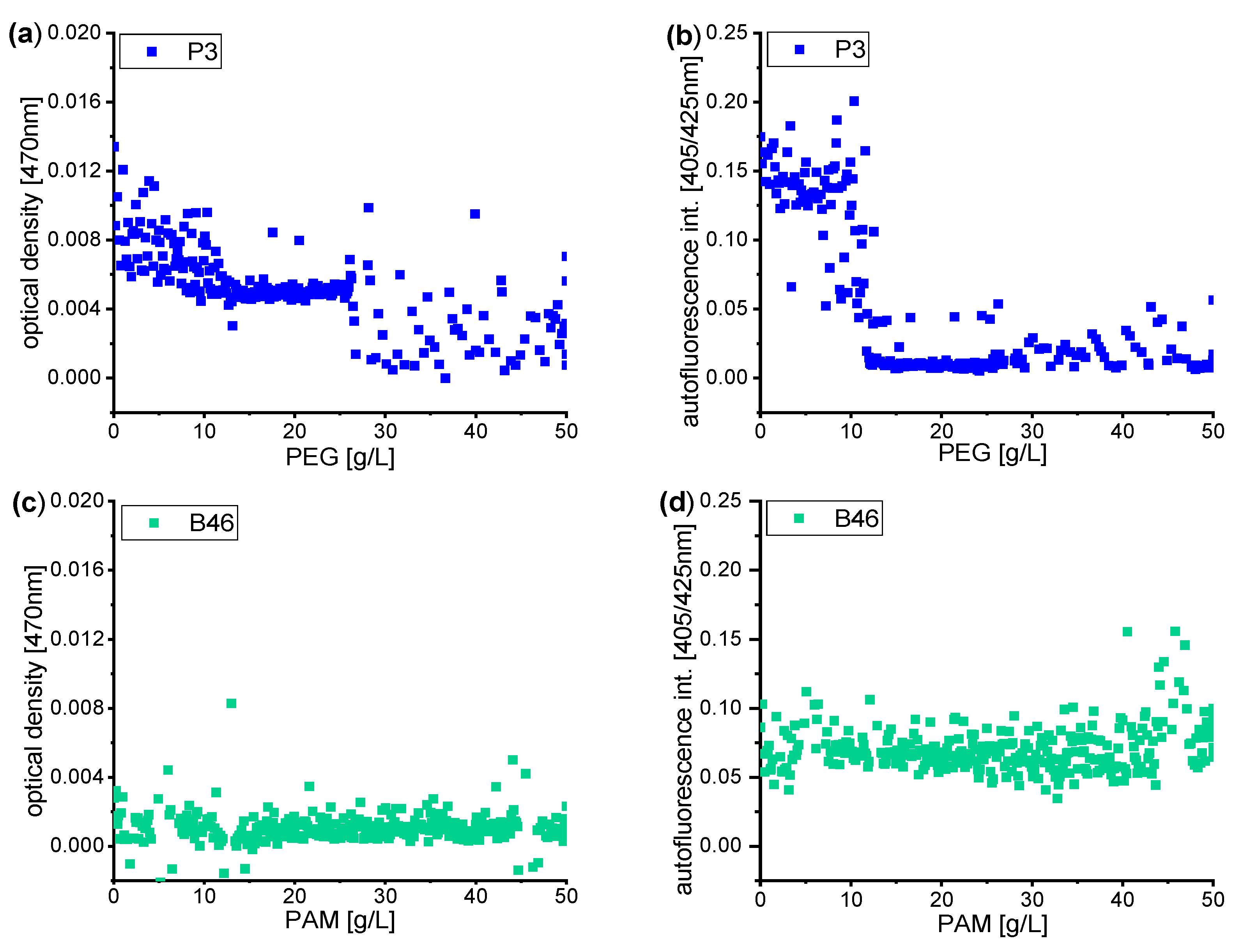
3.2. Dose-Response Functions of Stochastic Distributed Soil Microbial Community in Dependence of PEG and PAM
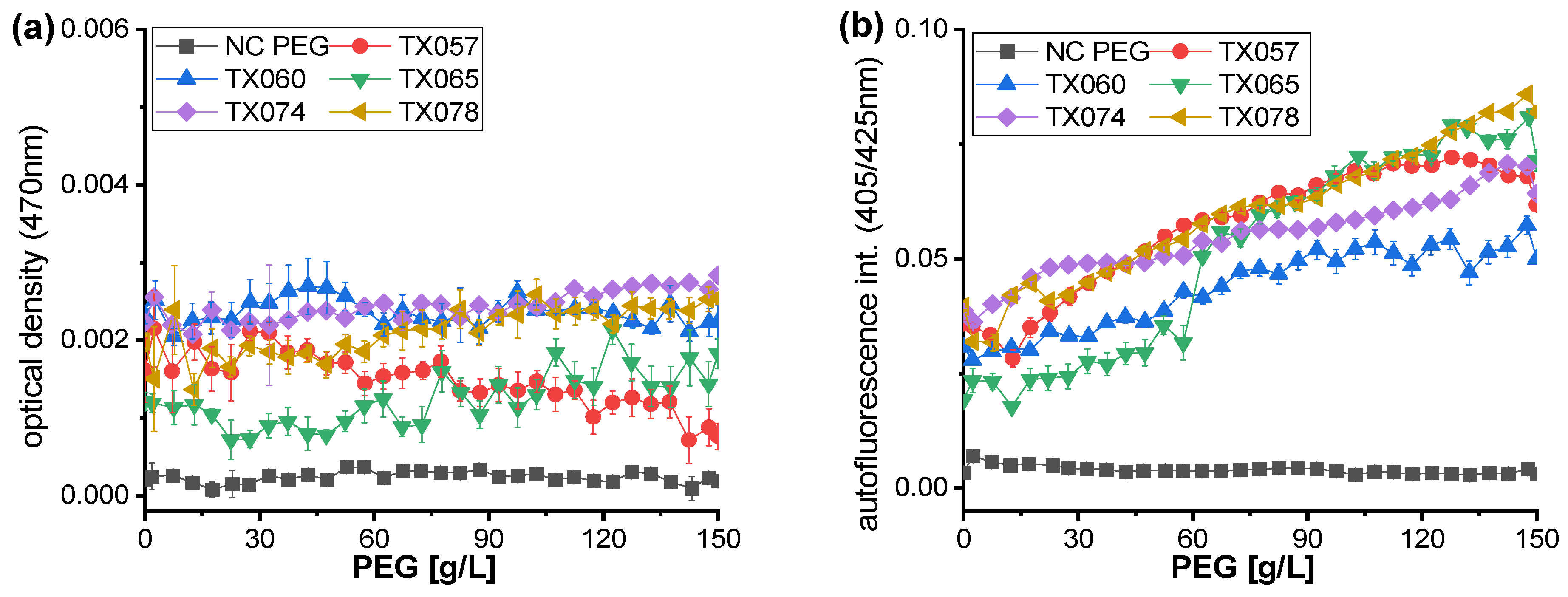
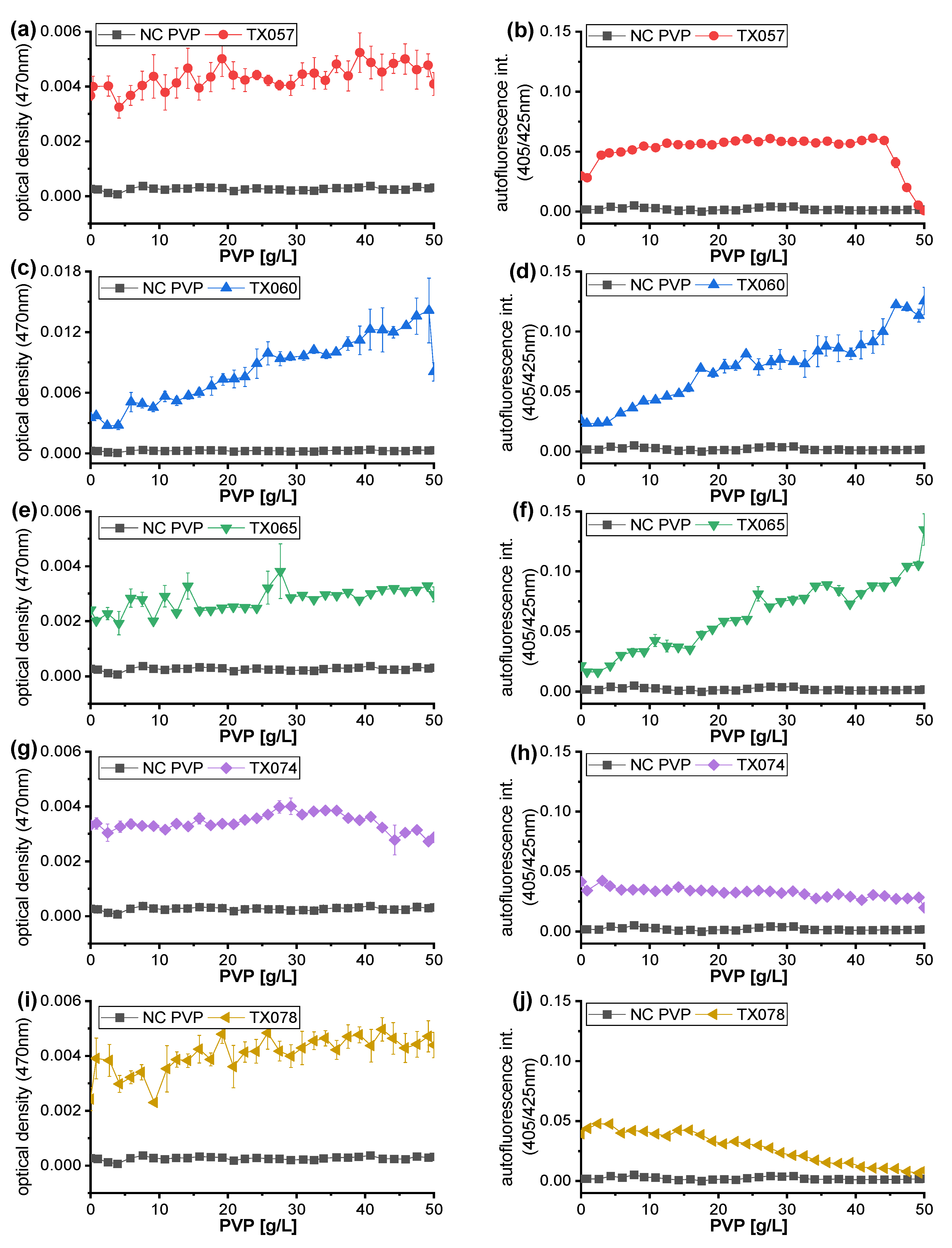
3.3. Highly-Resolved Microfluid Dose-Response Functions for Synthetic Polymer PEG, PVP and PAM
3.4. Highly Resolved Dose-Response Functions for Organic Solvents 1,4-Dioxane and Diglyme
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.C.; Karlsson, S. The influence of biotic and abiotic environments on the degradation of polyethylene. Prog. Polym. Sci. 1990, 15, 177–192. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Luqman, A.; Nugrahapraja, H.; Wahyuono, R.A.; Islami, I.; Haekal, M.H.; Fardiansyah, Y.; Putri, B.Q.; Amalludin, F.I.; Rofiqa, E.A.; Götz, F.; et al. Microplastic Contamination in Human Stools, Foods, and Drinking Water Associated with Indonesian Coastal Population. Environments 2021, 8, 138. [Google Scholar] [CrossRef]
- CUTRIGHT, T.J.; LEE, S. Remediation of PAH-Contaminated Soil Using Achromobacter sp. Energy Sources 1994, 16, 279–287. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Chyc, M.; Ryszka, P.; Latowski, D. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ. Sci. Pollut. Res. Int. 2016, 23, 11349–11356. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Q.; Zhang, L.; Wang, Y. Kinetics of the aerobic co-metabolism of 1,1-dichloroethylene by Achromobacter sp.: A novel benzene-grown culture. Biotechnol. Lett. 2014, 36, 1271–1278. [Google Scholar] [CrossRef]
- Das, G.; Bordoloi, N.K.; Rai, S.K.; Mukherjee, A.K.; Karak, N. Biodegradable and biocompatible epoxidized vegetable oil modified thermostable poly(vinyl chloride): Thermal and performance characteristics post biodegradation with Pseudomonas aeruginosa and Achromobacter sp. J. Hazard. Mater. 2012, 209, 434–442. [Google Scholar] [CrossRef]
- Ghaffarian, V.; Mousavi, S.M.; Bahreini, M.; Shoaei Parchin, N. Biodegradation of cellulose acetate/poly(butylene succinate) membrane. Int. J. Environ. Sci. Technol. 2017, 14, 1197–1208. [Google Scholar] [CrossRef]
- Haloi, S.; Medhi, T. Optimization and characterization of a glycolipid produced by Achromobacter sp. to use in petroleum industries. J. Basic Microbiol. 2019, 59, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chande, C.; Köhler, J.M. Microtoxicology by microfluidic instrumentation: A review. Lab Chip 2022, 22, 2600–2623. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xu, P.; Ma, L.; Chen, D.; Wang, J.; Dai, X.; Huang, L.; Du, W. One cell at a time: Droplet-based microbial cultivation, screening and sequencing. Mar. Life Sci. Technol. 2021, 3, 169–188. [Google Scholar] [CrossRef]
- Cao, J.; Kürsten, D.; Schneider, S.; Knauer, A.; Günther, P.M.; Köhler, J.M. Uncovering toxicological complexity by multi-dimensional screenings in microsegmented flow: Modulation of antibiotic interference by nanoparticles. Lab Chip 2012, 12, 474–484. [Google Scholar] [CrossRef]
- Cao, J.; Kürsten, D.; Krause, K.; Kothe, E.; Martin, K.; Roth, M.; Köhler, J.M. Application of micro-segmented flow for two-dimensional characterization of the combinatorial effect of zinc and copper ions on metal-tolerant Streptomyces strains. Appl. Microbiol. Biotechnol. 2013, 97, 8923–8930. [Google Scholar] [CrossRef]
- Funfak, A.; Hartung, R.; Cao, J.; Martin, K.; Wiesmüller, K.-H.; Wolfbeis, O.S.; Köhler, J.M. Highly resolved dose–response functions for drug-modulated bacteria cultivation obtained by fluorometric and photometric flow-through sensing in microsegmented flow. Sens. Actuators B Chem. 2009, 142, 66–72. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Hsieh, K.; Wang, T.-H. Combinatorial nanodroplet platform for screening antibiotic combinations. Lab Chip 2022, 22, 621–631. [Google Scholar] [CrossRef]
- Cao, J.; Hafermann, L.; Köhler, J.M. Stochastically reduced communities-Microfluidic compartments as model and investigation tool for soil microorganism growth in structured spaces. Eng. Life Sci. 2017, 17, 792–800. [Google Scholar] [CrossRef]
- Cao, J.; Chande, C.; Kalensee, F.; Schüler, T.; Köhler, M. Microfluidically supported characterization of responses of Rhodococcus erythropolis strains isolated from different soils on Cu-, Ni-, and Co-stress. Braz. J. Microbiol. 2021, 52, 1405–1415. [Google Scholar] [CrossRef]
- Cao, J.; Kalensee, F.; Günther, P.M.; Köhler, J.M. Microsegmented flow-assisted miniaturized culturing for isolation and characterization of heavy metal-tolerant bacteria. Int. J. Environ. Sci. Technol. 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Schumann, P.; Kalensee, F.; Cao, J.; Criscuolo, A.; Clermont, D.; Köhler, J.M.; Meier-Kolthoff, J.P.; Neumann-Schaal, M.; Tindall, B.J.; Pukall, R. Reclassification of Haloactinobacterium glacieicola as Occultella glacieicola gen. nov., comb. nov., of Haloactinobacterium album as Ruania alba comb. nov, with an emended description of the genus Ruania, recognition that the genus names Haloactinobacterium and Ruania are heterotypic synonyms and description of Occultella aeris sp. nov., a halotolerant isolate from surface soil sampled at an ancient copper smelter. Int. J. Syst. Evol. Microbiol. 2021, 71, 004769. [Google Scholar] [CrossRef]
- Cao, J.; Richter, F.; Kastl, M.; Erdmann, J.; Burgold, C.; Dittrich, D.; Schneider, S.; Köhler, J.M.; Groß, G.A. Droplet-Based Screening for the Investigation of Microbial Nonlinear Dose-Response Characteristics System, Background and Examples. Micromachines 2020, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Russo, D.A.; Xie, T.; Groß, G.A.; Zedler, J.A.Z. A droplet-based microfluidic platform enables high-throughput combinatorial optimization of cyanobacterial cultivation. Sci. Rep. 2022, 12, 15536. [Google Scholar] [CrossRef] [PubMed]
- Stucky, B.J. SeqTrace: A graphical tool for rapidly processing DNA sequencing chromatograms. J. Biomol. Tech. 2012, 23, 90–93. [Google Scholar] [CrossRef]
- Drancourt, M.; Berger, P.; Raoult, D. Systematic 16S rRNA gene sequencing of atypical clinical isolates identified 27 new bacterial species associated with humans. J. Clin. Microbiol. 2004, 42, 2197–2202. [Google Scholar] [CrossRef]
- Kawai, F. Biodegradation of Polyethers (Polyethylene Glycol, Polypropylene Glycol, Polytetramethylene glycol, and Others). In Biopolymers Online; Matsumura, S., Steinbüchel, A., Eds.; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-3-52-730290-1. [Google Scholar]
- Kawai, F.; Takeuchi, M. Taxonomical position of newly isolated polyethylene glycol-utilizing bacteria. J. Ferment. Bioeng. 1996, 82, 492–494. [Google Scholar] [CrossRef]
- Kohlweyer, U.; Thiemer, B.; Schräder, T.; Andreesen, J.R. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1. FEMS Microbiol. Lett. 2000, 186, 301–306. [Google Scholar] [CrossRef]
- Lopes, C.M.; Felisberti, M.I. Mechanical behaviour and biocompatibility of poly(1-vinyl-2-pyrrolidinone)–gelatin IPN hydrogels. Biomaterials 2003, 24, 1279–1284. [Google Scholar] [CrossRef]
- Mark, H.F.; Bikales, N.M.; Overberger, C.G.; Menges, G. Encyclopedia of Polymer Science and Engineering; Wiley-Interscience: New York, NY, USA, 1986; Volume 4, ISBN 978-0-47-188099-8. [Google Scholar]
- Julinová, M.; Kupec, J.; Houser, J.; Slavík, R.; Marusincová, H.; Cervenáková, L.; Klívar, S. Removal of polyvinylpyrrolidone from wastewater using different methods. Water Environ. Res. 2012, 84, 2123–2132. [Google Scholar] [CrossRef]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some aspects of the properties and degradation of polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef] [PubMed]
- Kay-Shoemake, J.L.; Watwood, M.E.; Sojka, R.E.; Lentz, R.D. Polyacrylamide as a substrate for microbial amidase in culture and soil. Soil Biol. Biochem. 1998, 30, 1647–1654. [Google Scholar] [CrossRef]
- Yu, F.; Fu, R.; Xie, Y.; Chen, W. Isolation and characterization of polyacrylamide-degrading bacteria from dewatered sludge. Int. J. Environ. Res. Public Health 2015, 12, 4214–4230. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, A.; Shiraishi, H.; Nishikawa, M.; Yamamoto, T.; Nakasugi, O.; Okumura, T.; Kenmotsu, K.; Fukui, H.; Nagase, M.; Kawagoshi, Y. Organic components in leachates from hazardous waste disposal sites. Waste Manag. Res. 1999, 17, 186–197. [Google Scholar] [CrossRef]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments. Microbes Environ. 2018, 33, 332–335. [Google Scholar] [CrossRef]
- Sagong, H.-Y.; Kim, S.; Lee, D.; Hong, H.; Lee, S.H.; Seo, H.; Kim, K.-J. Structural and functional characterization of an auxiliary domain-containing PET hydrolase from Burkholderiales bacterium. J. Hazard. Mater. 2022, 429, 128267. [Google Scholar] [CrossRef]
- Dey, A.S.; Bose, H.; Mohapatra, B.; Sar, P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., Isolated From Waste Dumpsite and Drilling Fluid. Front. Microbiol. 2020, 11, 603210. [Google Scholar] [CrossRef]
- Adelowo, O.O.; Alagbe, S.O.; Ayandele, A.A. Time-Dependent Stability of Used Engine Oil Degradation by Cultures of Pseudomonas fragi and Achromobacter aerogens. Afr. J. Biotechnol. 2006, 5, 2476–2479. [Google Scholar]
- Sałek, K.; Zgoła-Grześkowiak, A.; Kaczorek, E. Modification of surface and enzymatic properties of Achromobacter denitrificans and Stenotrophomonas maltophilia in association with diesel oil biodegradation enhanced with alkyl polyglucosides. Colloids Surf. B Biointerfaces 2013, 111, 36–42. [Google Scholar] [CrossRef]
- Gumuscu, B.; Tekinay, T. Effective biodegradation of 2,4,6-trinitrotoluene using a novel bacterial strain isolated from TNT-contaminated soil. Int. Biodeterior. Biodegrad. 2013, 85, 35–41. [Google Scholar] [CrossRef]
- Bordoloi, N.K.; Rai, S.K.; Chaudhuri, M.K.; Mukherjee, A.K. Deep-desulfurization of dibenzothiophene and its derivatives present in diesel oil by a newly isolated bacterium Achromobacter sp. to reduce the environmental pollution from fossil fuel combustion. Fuel Process. Technol. 2014, 119, 236–244. [Google Scholar] [CrossRef]
- Pawelczyk, S.; Abraham, W.-R.; Harms, H.; Müller, S. Community-based degradation of 4-chorosalicylate tracked on the single cell level. J. Microbiol. Methods 2008, 75, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, O.N.; Brown, L.M.; Radwan, O.; Bowen, L.L.; Gunasekera, T.S.; Mueller, S.S.; West, Z.J.; Striebich, R.C. Metagenomic characterization reveals complex association of soil hydrocarbon-degrading bacteria. Int. Biodeterior. Biodegrad. 2021, 157, 105161. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and Use of pH and Electrical Conductivity for Soil Quality Analysis. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1997; pp. 169–185. ISBN 978-0-89-118944-2. [Google Scholar]
- Kaczorek, E.; Sałek, K.; Guzik, U.; Dudzińska-Bajorek, B.; Olszanowski, A. The impact of long-term contact of Achromobacter sp. 4(2010) with diesel oil–Changes in biodegradation, surface properties and hexadecane monooxygenase activity. Int. Biodeterior. Biodegrad. 2013, 78, 7–16. [Google Scholar] [CrossRef]


| Soil Sample | Location | GPS Coordinates (Gauss-Krueger) | Description | Collection Date |
|---|---|---|---|---|
| P3 | Pößneck | 4472.292/5616.998 | prehistorical mining site | 4 November 2013 |
| Q16 | Morungen | 4446.336/5708.593 | pre-industrial mining site | 9 November 2013 |
| B46 | Sondershausen, Ole Burg | 4418.862/5690.465 | prehistorical rampart | 20 May 2017 |
| HB46 | Jena | 4471.400/5643.9 | Archaeological excavation historical tannery area | 16 July 2019 |
| Q45 | Morungen | 4446.306/5708.705 | pre-industrial mining site | 30 December 2015 |
| Effector Group | Name | Concentration Range | Medium |
|---|---|---|---|
| Synthetic polymer | PEG600 | 0–50 g/L | AM without glucose |
| PVP | 0–50 g/L | AM without glucose | |
| PAM | 0–50 g/L | AM without glucose | |
| Organic solvent | 1,4-dioxiane | 0–25% | AM without glucose |
| Diglyme | 0–25% | AM without glucose |
| Soil Sample | pH-Value | Conductivity [µS/cm] | Number of Reads | Number of Reads with the Type of the Genus |
|---|---|---|---|---|
| Q45 | 7.43 | 818.3 | 177,340 | 81,701 |
| Q16 | 7.49 | 219.0 | 144,409 | 85,235 |
| B46 | 7.7 | 491.3 | 132,607 | 69,322 |
| P3 | 7.82 | 245.7 | 133,725 | 67,819 |
| HB46 | no data | no data | 245,077 | 201,476 |
| Strain Code | TX057 | TX060 | TX065 | TX074 | TX078 |
|---|---|---|---|---|---|
| Soil sample | P3 | Q16 | B46 | HB46 | Q45 |
| Effector | PEG600 | PEG600 | PAM | PAM | PAM |
| Effector conc. | 41 g/L | 46 g/L | 46 g/L | 32 g/L | 28 g/L |
| Sequencing result | Achromobacter spanius | Achromobacter spanius | Achromobacter spanius | Achromobacter spanius | Achromobacter spanius |
| Query cover | 100% | 100% | 100% | 99% | 100% |
| Percent identity | 98.71% | 99.28% | 97.99% | 97.56% | 99.35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Köhler, J.M.; Heyder, S.; Günther, P.M.; Cao, J. Microfluidically-Assisted Isolation and Characterization of Achromobacter spanius from Soils for Microbial Degradation of Synthetic Polymers and Organic Solvents. Environments 2022, 9, 147. https://doi.org/10.3390/environments9120147
Xie T, Köhler JM, Heyder S, Günther PM, Cao J. Microfluidically-Assisted Isolation and Characterization of Achromobacter spanius from Soils for Microbial Degradation of Synthetic Polymers and Organic Solvents. Environments. 2022; 9(12):147. https://doi.org/10.3390/environments9120147
Chicago/Turabian StyleXie, Ting, J. Michael Köhler, Stefan Heyder, P. Mike Günther, and Jialan Cao. 2022. "Microfluidically-Assisted Isolation and Characterization of Achromobacter spanius from Soils for Microbial Degradation of Synthetic Polymers and Organic Solvents" Environments 9, no. 12: 147. https://doi.org/10.3390/environments9120147
APA StyleXie, T., Köhler, J. M., Heyder, S., Günther, P. M., & Cao, J. (2022). Microfluidically-Assisted Isolation and Characterization of Achromobacter spanius from Soils for Microbial Degradation of Synthetic Polymers and Organic Solvents. Environments, 9(12), 147. https://doi.org/10.3390/environments9120147










