The Role of Phytoplankton in the Assessment of the Ecological State of the Floodplain Lakes of the Irtysh River, Kazakhstan
Abstract
1. Introduction
2. Description of the Region
2.1. Site Description
2.2. Climate
2.3. Geomorphological Zoning and Geological Substrate
2.4. Description of the Surveyed Floodplain Lakes
3. Materials and Methods
3.1. Sampling
3.2. Sample Analysis
3.3. Bioindication
4. Results
4.1. Hydrophysical and Hydrochemical Characteristics
4.2. Phytoplankton
4.2.1. Species Composition
4.2.2. Quantitative Variables and Dominants
4.2.3. Structural Variables
4.3. Assessment of the Ecological State of Floodplain Lakes by Phytoplankton and Chemical Variables
4.3.1. Ratio of Indicator Species
4.3.2. Quantitative and Structural Variables
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JASP | Jeffreys’s Amazing Statistics Program |
| PI | Permanganate index |
| TDS | Total dissolved solids |
| RDA | Redundancy Discriminant Analysis |
Appendix A
| Species Name | Occurrence, % | ||||||
|---|---|---|---|---|---|---|---|
| Lakes * | |||||||
| BA | SH | KU | OR | SI | |||
| 2024 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | |
| Charophyta | |||||||
| Closterium acerosum Ehrenberg ex Ralfs, 1848 | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Closterium acutum Brébisson, 1848 | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Closterium parvulum Nägeli, 1849 | 67 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cosmarium punctulatum Brébisson, 1856 | 0 | 0 | 100 | 0 | 67 | 33 | 0 |
| Mougeotia sp. C. Agardh, 1824 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurastrum gracile Ralfs ex Ralfs, 1848 | 0 | 0 | 67 | 0 | 67 | 0 | 67 |
| Chlorophyta | |||||||
| Actinastrum aciculare Playfair, 1917 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Actinastrum hantzschii Lagerheim, 1882 | 0 | 100 | 33 | 33 | 0 | 0 | 67 |
| Ankistrodesmus fusiformis Corda, 1838 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus spiralis (W.B. Turner) Lemmermann, 1908 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Ankistrodesmus arcuatus Korshikov, 1953 | 0 | 100 | 33 | 0 | 67 | 100 | 100 |
| Ankyra ocellata (G. M. Smith) Fott, 1957 | 33 | 0 | 0 | 0 | 0 | 0 | 0 |
| Binuclearia lauterbornii (Schmidle) Proshkina-Lavrenko, 1966 | 0 | 100 | 0 | 0 | 0 | 0 | 100 |
| Chlorella vulgaris Beijerinck, 1890 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Chlorella vulgaris f. globosa V.M. Andreeva, 1975 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Chlorotetraedron incus (Teiling) Komárek & Kovácik, 1985 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Closteriopsis acicularis (Chodat) J.H.Belcher & Swale, 1962 | 0 | 67 | 0 | 0 | 67 | 0 | 0 |
| Closteriopsis longissima (Lemmermann) Lemmermann, 1899 | 0 | 0 | 0 | 0 | 33 | 100 | 0 |
| Coelastrum microporum Nägeli, 1855 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Coenochloris aquatica I. Kostikov, T. Darienko, A. Lukesová, & L. Hoffmann, 2002 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Coenochloris pyrenoidosa Korshikov, 1953 | 0 | 0 | 0 | 33 | 0 | 67 | 0 |
| Crucigenia fenestrata (Schmidle) Schmidle, 1900 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Crucigenia quadrata Morren, 1830 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| Desmodesmus armatus (Chodat) E.H. Hegewald, 2000 | 0 | 0 | 67 | 33 | 0 | 100 | 33 |
| Desmodesmus armatus var. bicaudatus (Guglielmetti) E.H.Hegewald, 2000 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| Desmodesmus communis (E.Hegewald) E.Hegewald, 2000 | 0 | 0 | 33 | 0 | 33 | 0 | 67 |
| Desmodesmus subspicatus (Chodat) E. Hegewald & A. W. F. Schmidt, 2000 | 0 | 33 | 33 | 0 | 0 | 100 | 33 |
| Eudorina elegans Ehrenberg, 1832 | 0 | 0 | 0 | 0 | 67 | 0 | 0 |
| Hyaloraphidium contortum Pascher & Korshikov, 1931 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| Kirchneriella lunaris (Kirchner) Möbius, 1894 | 0 | 0 | 0 | 0 | 67 | 0 | 0 |
| Lemmermannia tetrapedia (Kirchner) Lemmermann, 1904 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Monactinus simplex (Meyen) Corda, 1839 | 100 | 100 | 67 | 67 | 0 | 0 | 67 |
| Monoraphidium contortum (Thuret) Komárková-Legnerová, 1969 | 0 | 67 | 33 | 0 | 100 | 100 | 100 |
| Monoraphidium convolutum (Corda) Komárková-Legnerová, 1969 | 0 | 0 | 0 | 0 | 0 | 33 | 0 |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová, 1969 | 0 | 100 | 33 | 0 | 33 | 100 | 100 |
| Monoraphidium minutum (Nägeli) Komárková-Legnerová, 1969 | 0 | 0 | 0 | 0 | 33 | 0 | 0 |
| Monoraphidium pusillum (Printz) Komárková-Legnorová, 1969 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Mucidosphaerium pulchellum (H.C.Wood) C. Bock, Proschold & Krienitz, 2011 | 0 | 100 | 67 | 0 | 100 | 100 | 67 |
| Nephrochlamys subsolitaria (G.S.West) Korshikov, 1953 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Oocystis borgei J. W. Snow, 1903 | 0 | 100 | 0 | 33 | 0 | 33 | 0 |
| Oocystis lacustris Chodat, 1897 | 0 | 100 | 0 | 100 | 0 | 33 | 0 |
| Oocystis submarina Lagerheim, 1886 | 0 | 0 | 100 | 100 | 67 | 33 | 33 |
| Pediastrum duplex Meyen, 1829 | 100 | 100 | 100 | 100 | 67 | 33 | 33 |
| Phacotus lenticularis (Ehrenberg) Diesing, 1866 | 0 | 0 | 33 | 33 | 33 | 100 | 0 |
| Pseudopediastrum boryanum (Turpin) E. Hegewald, 2005 | 0 | 0 | 67 | 0 | 0 | 0 | 0 |
| Raphidocelis sigmoidea Hindák, 1977 | 0 | 0 | 0 | 0 | 67 | 0 | 100 |
| Scenedesmus semipulcher Hortobágyi, 1960 | 0 | 67 | 67 | 33 | 67 | 100 | 67 |
| Scenedesmus ellipticus Corda, 1835 | 0 | 100 | 67 | 100 | 100 | 100 | 33 |
| Scenedesmus obtusus Meyen, 1829 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Scenedesmus quadricauda (Turpin) Brébisson, 1835 | 0 | 100 | 0 | 67 | 0 | 100 | 0 |
| Sphaerocystis planctonica (Korshikov) Bourrelly, 1974 | 0 | 0 | 33 | 33 | 0 | 33 | 67 |
| Stauridium tetras (Ehrenberg) E. Hegewald, 2005 | 0 | 0 | 100 | 100 | 0 | 0 | 0 |
| Tetradesmus lagerheimii M.J.Wynne & Guiry, 2016 | 0 | 100 | 0 | 100 | 0 | 67 | 0 |
| Tetradesmus incrassatulus (Bohlin) M. J. Wynne, 2016 | 0 | 33 | 33 | 0 | 33 | 0 | 0 |
| Tetradesmus obliquus (Turpin) M.J.Wynne, 2016 | 0 | 33 | 33 | 0 | 0 | 0 | 33 |
| Tetraedron caudatum (Corda) Hansgirg, 1888 | 0 | 67 | 0 | 0 | 0 | 0 | 67 |
| Tetraedron minimum (A. Braun) Hansgirg, 1889 | 0 | 33 | 0 | 0 | 0 | 0 | 33 |
| Tetraedron triangulare Korshikov, 1953 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Volvox globator Linnaeus, 1758 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyanobacteria | |||||||
| Anathece clathrata (West & G. S. West) Komárek, Kaštovský & Jezberová, 2011 | 0 | 100 | 100 | 33 | 0 | 0 | 100 |
| Aphanizomenon flos-aquae Ralfs ex Bornet & Flahault, 1886 | 100 | 100 | 33 | 0 | 33 | 100 | 100 |
| Aphanocapsa incerta (Lemmermann) G. Cronberg & Komárek, 1994 | 0 | 0 | 33 | 33 | 0 | 0 | 33 |
| Aphanocapsa planctonica Komárek & Anagnostidis, 1995 | 0 | 0 | 67 | 0 | 0 | 0 | 0 |
| Aphanocapsa delicatissima West & G. S. West, 1912 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Aphanothece elabens (Meneghini) Elenkin, 1936 | 0 | 100 | 100 | 0 | 67 | 0 | 100 |
| Chroococcus minimus (Keissler) Lemmermann, 1904 | 0 | 0 | 0 | 0 | 0 | 67 | 0 |
| Chroococcus minor (Kützing) Nägeli, 1849 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Chroococcus turgidus (Kützing) Nägeli, 1849 | 0 | 0 | 33 | 0 | 0 | 0 | 33 |
| Chrysosporum bergii (Ostenfeld) E. Zapomelová, O. Skácelová, P. Pumann, R. Kopp & E. Janecek, 2012 | 100 | 0 | 0 | 0 | 100 | 100 | 33 |
| Coelomoron pusillum (Van Goor) Komárek, 1988 | 0 | 0 | 33 | 33 | 0 | 0 | 0 |
| Coelosphaerium kuetzingianum Nägeli, 1849 | 0 | 0 | 100 | 67 | 0 | 100 | 67 |
| Dolichospermum sigmoideum (Nygaard) Wacklin, L. Hoffmann & Komárek, 2009 | 0 | 0 | 0 | 0 | 67 | 0 | 0 |
| Dolichospermum spiroides (Klebahn) Wacklin, L. Hoffmann & Komárek, 2009 | 100 | 33 | 33 | 0 | 0 | 67 | 100 |
| Glaucospira laxissima (G. S. West) Simic, Komárek & Dordevic, 2014 | 0 | 0 | 0 | 0 | 0 | 33 | 0 |
| Gomphosphaeria aponina Kützing, 1836 | 0 | 100 | 67 | 0 | 0 | 67 | 0 |
| Leptolyngbya angustissima (West & G. S. West) Anagnostidis & Komárek, 1988 | 0 | 100 | 0 | 0 | 0 | 67 | 33 |
| Leptolyngbya tenuis (Gomont) Anagnostidis & Komárek, 1988 | 0 | 100 | 33 | 0 | 0 | 67 | 33 |
| Limnococcus limneticus (Lemmermann) Komárková, Jezberová, O. Komárek & Zapomelová, 2010 | 0 | 0 | 33 | 67 | 0 | 0 | 33 |
| Merismopedia elegans A. Braun ex Kützing, 1849 | 0 | 0 | 33 | 0 | 33 | 0 | 0 |
| Merismopedia glauca (Ehrenberg) Kützing, 1845 | 0 | 33 | 67 | 67 | 33 | 67 | 0 |
| Merismopedia minima G. Beck, 1897 | 0 | 67 | 100 | 33 | 33 | 33 | 33 |
| Merismopedia tenuissima Lemmermann, 1898 | 0 | 0 | 33 | 0 | 67 | 0 | 0 |
| Merismopedia tranquilla (Ehrenberg) Trevisan, 1845 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Microcystis aeruginosa (Kützing) Kützing, 1846 | 0 | 100 | 100 | 0 | 67 | 33 | 100 |
| Microcystis pulverea (H. C. Wood) Forti, 1907 | 0 | 100 | 100 | 0 | 0 | 100 | 100 |
| Microcystis wesenbergii (Komárek) Komárek ex Komárek, 2009 | 0 | 0 | 67 | 0 | 0 | 0 | 67 |
| Microcystis ichthyoblabe (G. Kunze) Kützing, 1843 | 0 | 33 | 0 | 100 | 0 | 0 | 0 |
| Planktolyngbya contorta (Lemmermann) Anagnostidis & Komárek, 1988 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| Pseudanabaena limnetica (Lemmermann) Komárek, 1974 | 0 | 0 | 33 | 0 | 33 | 0 | 0 |
| Pseudanabaena mucicola (Naumann & Huber-Pestalozzi) Schwabe, 1964 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Rhabdoderma lineare Schmidle & Lauterborn, 1900 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Romeria leopoliensis (Raciborski) Koczwara, 1932 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Snowella atomus Komárek & Hindák, 1988 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Spirulina laxa G.M.Smith, 1916 | 0 | 0 | 0 | 0 | 0 | 33 | 0 |
| Synechocystis aquatilis Sauvageau, 1892 | 0 | 0 | 0 | 67 | 0 | 33 | 33 |
| Woronichinia naegeliana (Unger) Elenkin, 1933 | 0 | 0 | 33 | 100 | 33 | 0 | 67 |
| Dinoflagellata | |||||||
| Ceratium hirundinella (O. F. Müller) Dujardin, 1841 | 0 | 0 | 0 | 67 | 100 | 100 | 100 |
| Peridiniopsis quadridens (F. Stein) Bourrelly, 1968 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Peridinium cinctum (O. F. Müller) Ehrenberg, 1832 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Euglenophyta | |||||||
| Euglena viridis (O. F. Müller) Ehrenberg, 1830 | 0 | 33 | 0 | 0 | 67 | 0 | 100 |
| Lepocinclis acus (O. F. Müller) B. Marin & Melkonian, 2003 | 0 | 100 | 0 | 0 | 0 | 0 | 33 |
| Lepocinclis oxyuris (Schmarda) B. Marin & Melkonian, 2003 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky, 1877 | 0 | 33 | 33 | 0 | 0 | 0 | 0 |
| Phacus longicauda (Ehrenberg) Dujardin, 1841 | 0 | 100 | 67 | 0 | 0 | 67 | 67 |
| Trachelomonas hispida (Perty) F. Stein, 1878 | 0 | 100 | 67 | 100 | 33 | 33 | 67 |
| Trachelomonas oblonga Lemmermann, 1899 | 0 | 67 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas oblonga var. attenuata Playfair, 1915 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Trachelomonas volvocina (Ehrenberg) Ehrenberg, 1834 | 0 | 0 | 33 | 100 | 0 | 0 | 0 |
| Heterokontophyta | |||||||
| Amphora ovalis (Kützing) Kützing, 1844 | 0 | 0 | 67 | 0 | 0 | 0 | 33 |
| Asterionella formosa Hassall, 1850 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Aulacoseira granulata (Ehrenberg) Simonsen, 1979 | 0 | 0 | 0 | 0 | 100 | 0 | 33 |
| Aulacoseira italica (Ehrenberg) Simonsen, 1979 | 33 | 0 | 0 | 0 | 67 | 0 | 0 |
| Cocconeis placentula Ehrenberg, 1838 | 33 | 0 | 33 | 0 | 67 | 0 | 33 |
| Cymbella cistula (Ehrenberg) O. Kirchner, 1878 | 0 | 33 | 0 | 0 | 0 | 0 | 0 |
| Cymbella tumida (Brébisson) Van Heurck, 1880 | 0 | 0 | 0 | 0 | 33 | 0 | 0 |
| Cymbopleura lata (Grunow ex Cleve) Krammer, 2003 | 0 | 0 | 0 | 0 | 33 | 0 | 0 |
| Diatoma vulgaris Bory, 1824 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Dinobryon divergens O.E. Imhof, 1887 | 0 | 0 | 0 | 0 | 0 | 0 | 33 |
| Dinobryon sertularia Ehrenberg, 1834 | 0 | 0 | 0 | 100 | 0 | 0 | 67 |
| Epithemia turgida (Ehrenberg) Kützing, 1844 | 0 | 0 | 33 | 0 | 0 | 67 | 0 |
| Epithemia adnata (Kützing) Brébisson, 1838 | 0 | 0 | 0 | 0 | 67 | 0 | 0 |
| Fragilaria capucina Desmazières, 1830 | 0 | 0 | 0 | 67 | 0 | 67 | 0 |
| Gyrosigma acuminatum (Kützing) Rabenhorst, 1853 | 0 | 33 | 33 | 0 | 0 | 0 | 0 |
| Melosira inflexa (Roth) Guiry, 2019 | 67 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula cincta (Ehrenberg) Ralfs, 1861 | 67 | 0 | 0 | 33 | 0 | 0 | 0 |
| Navicula cryptocephala Kützing, 1844 | 33 | 33 | 0 | 0 | 0 | 0 | 0 |
| Navicula lanceolata Ehrenberg, 1838 | 67 | 0 | 33 | 0 | 33 | 0 | 0 |
| Navicula minima Grunow, 1880 | 0 | 0 | 0 | 0 | 33 | 0 | 0 |
| Navicula radiosa Kützing, 1844 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Navicula rhynchocephala Kützing, 1844 | 0 | 0 | 67 | 33 | 0 | 33 | 0 |
| Navicula tripunctata (O. F. Müller) Bory, 1822 | 0 | 33 | 33 | 0 | 0 | 0 | 0 |
| Nitzschia acicularis (Kützing) W. Smith, 1853 | 0 | 33 | 33 | 100 | 100 | 100 | 0 |
| Nitzschia linearis W. Smith, 1853 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Nitzschia palea (Kützing) W.Smith, 1856 | 0 | 0 | 33 | 0 | 0 | 0 | 0 |
| Nitzschia longissima (Brébisson ex Kützing) Grunow, 1862 | 0 | 0 | 0 | 0 | 0 | 67 | 0 |
| Pseudokephyrion entzii W. Conrad, 1939 | 0 | 0 | 33 | 0 | 0 | 0 | 67 |
| Sellaphora pupula (Kützing) Mereschkovsky, 1902 | 67 | 0 | 0 | 0 | 0 | 0 | 0 |
| Staurosira construens Ehrenberg, 1843 | 0 | 0 | 0 | 0 | 33 | 67 | 0 |
| Staurosira subsalina (Hustedt) Lange-Bertalot, 2004 | 0 | 0 | 0 | 33 | 0 | 0 | 0 |
| Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal & Kociolek, 2022 | 0 | 0 | 100 | 0 | 67 | 67 | 100 |
| Stephanodiscus hantzschii Grunow, 1880 | 0 | 0 | 67 | 0 | 67 | 0 | 33 |
| Tryblionella hantzschiana Grunow, 1862 | 0 | 0 | 67 | 0 | 33 | 0 | 33 |
| Ulnaria ulna (Nitzsch) Compère, 2001 | 0 | 0 | 0 | 67 | 0 | 100 | 0 |
| Ulnaria ulna var. spathulifera (Grunow) Aboal, 2003 | 0 | 0 | 0 | 0 | 0 | 33 | 0 |
| Total: | 16 | 60 | 61 | 40 | 47 | 49 | 60 |
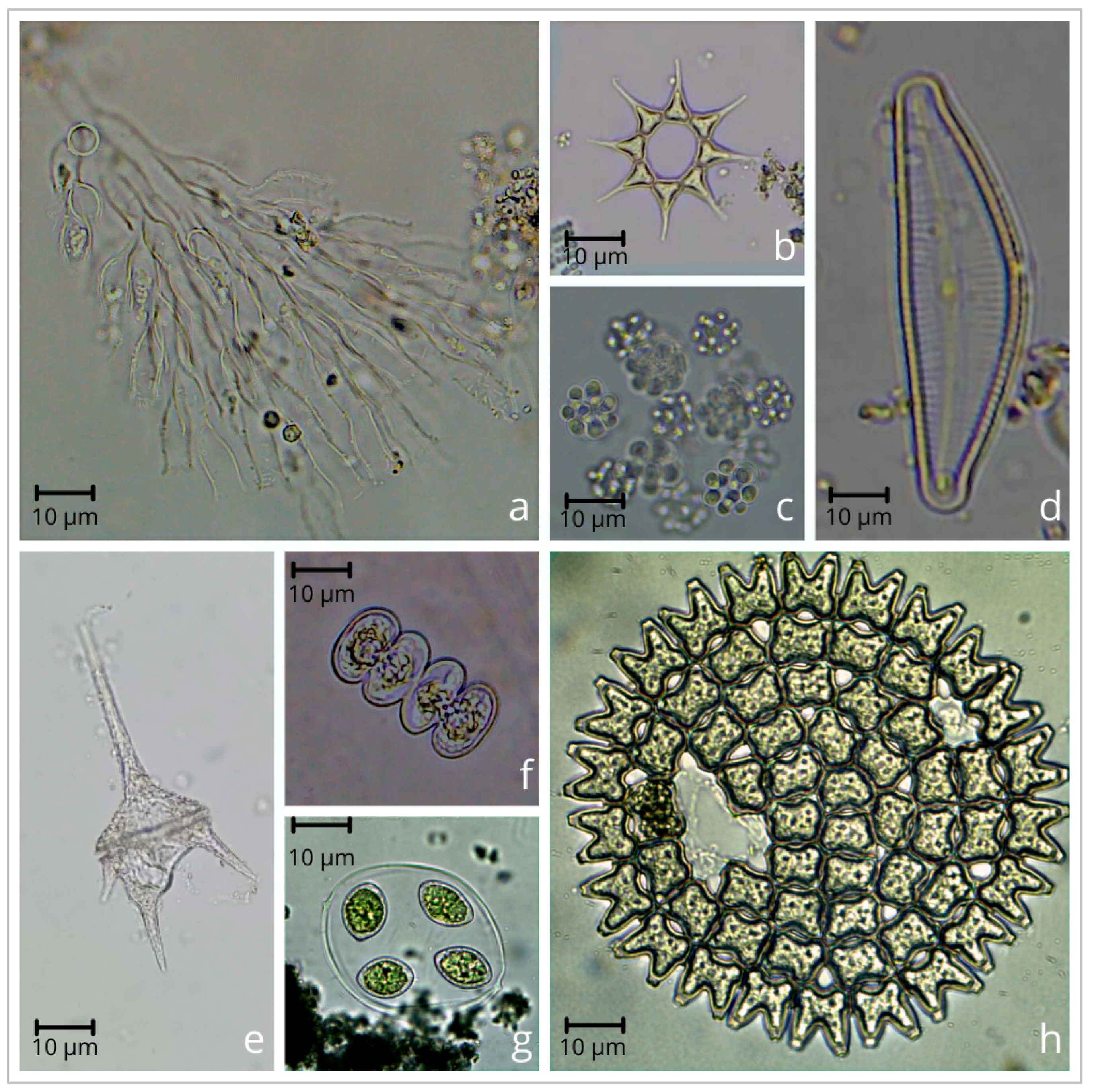
| Abbreviation, Station Number and Sampling Year * | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phyla name | BA- 1-24 | BA- 2-24 | BA- 3-24 | SH- 1-24 | SH- 2-24 | SH- 3-24 | KU- 1-23 | KU- 2-23 | KU- 3-23 | KU- 1-24 | KU- 2-24 | KU- 3-24 | OR- 1-23 | OR- 2-23 | OR- 3-23 | OR- 1-24 | OR- 2-24 | OR- 3-24 | SI- 1-23 | SI- 2-23 | SI- 3-23 |
| Charophyta | 2 | 2 | 1 | 0 | 0 | 0 | 3 | 2 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Chlorophyta | 3 | 3 | 4 | 25 | 24 | 21 | 11 | 14 | 6 | 14 | 9 | 9 | 9 | 11 | 11 | 14 | 14 | 15 | 12 | 14 | 14 |
| Cyanobacteria | 3 | 3 | 3 | 11 | 10 | 13 | 12 | 11 | 14 | 6 | 7 | 6 | 4 | 9 | 7 | 10 | 10 | 12 | 12 | 13 | 14 |
| Dinoflagellata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 3 |
| Euglenophyta | 0 | 0 | 0 | 6 | 4 | 5 | 0 | 2 | 4 | 2 | 2 | 2 | 0 | 2 | 1 | 1 | 1 | 1 | 4 | 2 | 2 |
| Heterokontophyta | 3 | 4 | 4 | 2 | 0 | 3 | 9 | 2 | 8 | 5 | 7 | 6 | 2 | 9 | 11 | 6 | 7 | 8 | 3 | 5 | 5 |
| Total | 11 | 12 | 12 | 44 | 38 | 42 | 35 | 31 | 34 | 28 | 26 | 23 | 17 | 34 | 32 | 32 | 33 | 38 | 34 | 37 | 38 |
| № | Abbreviation | Charophyta | Chlorophyta | Cyanobacteria | Dinoflagellata | Euglenophyta | Heterokontophyta | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | BA-1-24 | 3.3 | 6101.7 | 2040.0 | 0.0 | 0.0 | 145.0 | 8290.0 |
| 2 | BA-2-24 | 1.7 | 50,233.3 | 1303.3 | 0.0 | 0.0 | 100.0 | 51,638.3 |
| 3 | BA-3-24 | 0.0 | 13,055.0 | 845.0 | 0.0 | 0.0 | 6.7 | 14,450.0 |
| 4 | SH-1-24 | 0.0 | 6505.0 | 30,180.0 | 0.0 | 250.0 | 40.0 | 36,975.0 |
| 5 | SH-2-24 | 0.0 | 6563.3 | 19,875.0 | 0.0 | 145.0 | 0.0 | 26,583.3 |
| 6 | SH-3-24 | 0.0 | 3441.7 | 10,970.0 | 0.0 | 18.3 | 20.0 | 14,450.0 |
| 7 | KU-1-23 | 10.0 | 7793.3 | 237,886.7 | 0.0 | 0.0 | 328.3 | 246,018.3 |
| 8 | KU-2-23 | 3.3 | 6653.3 | 173,416.7 | 0.0 | 23.3 | 143.3 | 180,240.0 |
| 9 | KU-3-23 | 10.0 | 3616.7 | 76,816.7 | 0.0 | 11.7 | 170.0 | 80,625.0 |
| 10 | KU-1-24 | 0.0 | 436.7 | 495.0 | 3.3 | 6.7 | 1200.0 | 2141.7 |
| 11 | KU-2-24 | 0.0 | 448.3 | 1505.0 | 10.0 | 6.7 | 2023.3 | 3993.3 |
| 12 | KU-3-24 | 0.0 | 543.3 | 1525.0 | 0.0 | 10.0 | 1691.7 | 3770.0 |
| 13 | OR-1-23 | 0.0 | 163.3 | 473.3 | 11.7 | 0.0 | 15.0 | 663.3 |
| 14 | OR-2-23 | 10.0 | 2008.3 | 5000.0 | 60.0 | 13.3 | 230.0 | 7321.7 |
| 15 | OR-3-23 | 20.0 | 1281.7 | 2280.0 | 50.0 | 10.0 | 326.7 | 3968.3 |
| 16 | OR-1-24 | 0.0 | 490.0 | 3405.0 | 1.7 | 50.0 | 358.3 | 4305.0 |
| 17 | OR-2-24 | 0.0 | 1018.3 | 3926.7 | 1.7 | 10.0 | 226.7 | 5183.3 |
| 18 | OR-3-24 | 0.0 | 1313.3 | 4853.3 | 15.0 | 15.0 | 250.0 | 6446.7 |
| 19 | SI-1-23 | 40.0 | 1703.3 | 15,938.3 | 80.0 | 41.7 | 146.7 | 17,950.0 |
| 20 | SI-2-23 | 10.0 | 2483.3 | 15,996.7 | 220.0 | 18.3 | 240.0 | 18,968.3 |
| 21 | SI-3-23 | 0.0 | 2048.3 | 16,176.7 | 220.0 | 120.0 | 455.0 | 19,020.0 |
| № | Abbreviation | Charophyta | Chlorophyta | Cyanobacteria | Dinoflagellata | Euglenophyta | Heterokontophyta | Total |
|---|---|---|---|---|---|---|---|---|
| 1 | BA-1-24 | 71.8 | 13,078.5 | 2365.4 | 0.0 | 0.0 | 316.0 | 15,831.6 |
| 2 | BA-2-24 | 35.9 | 109,693.3 | 1178.4 | 0.0 | 0.0 | 1020.0 | 111,927.6 |
| 3 | BA-3-24 | 0.0 | 27,938.7 | 944.9 | 0.0 | 0.0 | 26.2 | 28,909.8 |
| 4 | SH-1-24 | 0.0 | 5113.8 | 12,229.6 | 0.0 | 9055.4 | 87.0 | 26,485.8 |
| 5 | SH-2-24 | 0.0 | 6332.7 | 5992.9 | 0.0 | 5014.7 | 0.0 | 17,340.3 |
| 6 | SH-3-24 | 0.0 | 2649.0 | 5332.9 | 0.0 | 592.1 | 31.0 | 8604.9 |
| 7 | KU-1-23 | 63.7 | 8212.9 | 94,248.2 | 0.0 | 0.0 | 813.1 | 103,337.9 |
| 8 | KU-2-23 | 4.5 | 5648.2 | 96,845.7 | 0.0 | 740.4 | 197.6 | 103,436.5 |
| 9 | KU-3-23 | 13.5 | 3416.3 | 39,862.4 | 0.0 | 572.9 | 382.3 | 44,247.4 |
| 10 | KU-1-24 | 0.0 | 592.6 | 584.5 | 504.1 | 116.5 | 3365.6 | 5163.3 |
| 11 | KU-2-24 | 0.0 | 439.4 | 1560.8 | 1512.3 | 116.5 | 4786.1 | 8415.0 |
| 12 | KU-3-24 | 0.0 | 605.7 | 1406.5 | 0.0 | 174.7 | 4588.4 | 6775.4 |
| 13 | OR-1-23 | 0.0 | 106.6 | 171.1 | 417.9 | 0.0 | 11.2 | 706.7 |
| 14 | OR-2-23 | 13.5 | 688.6 | 1167.9 | 2149.0 | 52.7 | 247.9 | 4319.6 |
| 15 | OR-3-23 | 27.1 | 451.7 | 302.9 | 1790.8 | 64.5 | 460.0 | 3097.0 |
| 16 | OR-1-24 | 0.0 | 309.6 | 396.1 | 129.1 | 2547.0 | 3769.2 | 7150.0 |
| 17 | OR-2-24 | 0.0 | 745.9 | 976.4 | 129.1 | 293.4 | 3033.2 | 5180.0 |
| 18 | OR-3-24 | 0.0 | 2707.2 | 959.1 | 1162.1 | 13.8 | 3516.2 | 8358.3 |
| 19 | SI-1-23 | 54.1 | 1353.4 | 4305.2 | 4022.9 | 1240.7 | 309.1 | 11,285.5 |
| 20 | SI-2-23 | 13.5 | 1487.0 | 3771.3 | 9963.3 | 774.7 | 317.6 | 16,327.4 |
| 21 | SI-3-23 | 0.0 | 635.5 | 2480.4 | 6324.4 | 607.3 | 441.5 | 10,489.1 |
| Ecological Preferences of Phytoplankton Species * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | HAB | TEMP | OXY | HAL | pH | D | AUT-HET | TRO | S Total |
| Charophyta | |||||||||
| Closterium acerosum Ehrenberg ex Ralfs, 1848 | B | – | aer | – | acf | – | – | om | – |
| Closterium acutum Brébisson, 1848 | P-B | – | st-str | – | ind | – | – | m | 2.05 |
| Closterium parvulum Nägeli, 1849 | P-B | – | – | i | ind | – | – | m | 2.0 |
| Cosmarium punctulatum Brébisson, 1856 | P-B | – | – | hb | ind | – | – | m | 1.3 |
| Mougeotia sp. C.Agardh, 1824 | B | – | – | – | – | – | – | – | 1.0 |
| Staurastrum gracile Ralfs ex Ralfs, 1848 | P-B | – | st | i | acf | – | – | m | – |
| Chlorophyta | |||||||||
| Actinastrum aciculare Playfair, 1917 | P | – | – | – | – | – | – | – | – |
| Actinastrum hantzschii Lagerheim, 1882 | P-B | – | st-str | i | – | – | – | – | 2.3 |
| Ankistrodesmus fusiformis Corda, 1838 | P-B | – | st-str | i | – | – | – | e | 2.0 |
| Ankistrodesmus spiralis (W.B.Turner) Lemmermann, 1908 | P | – | – | – | – | – | – | e | 2.1 |
| Ankistrodesmus arcuatus Korshikov, 1953 | P-B | – | st-str | i | – | – | – | – | 2.1 |
| Ankyra ocellata (G.M.Smith) Fott, 1957 | Ep | – | – | oh | – | – | – | – | – |
| Binuclearia lauterbornii (Schmidle) Proshkina-Lavrenko, 1966 | – | – | – | – | – | – | – | – | 1.8 |
| Chlorella vulgaris Beijerinck, 1890 | P-B,pb,S | – | – | hl | – | – | – | e | 3.1 |
| Chlorella vulgaris f. globosa V.M.Andreeva, 1975 | – | – | – | – | – | – | – | – | – |
| Chlorotetraedron incus (Teiling) Komárek & Kovácik, 1985 | P-B | – | st-str | i | – | – | – | – | 1.9 |
| Closteriopsis acicularis (Chodat) J.H.Belcher & Swale, 1962 | P-B | – | st-str | i | – | – | – | e | 1.9 |
| Closteriopsis longissima (Lemmermann) Lemmermann, 1899 | P | – | st-str | i | – | – | – | e | 1.8 |
| Coelastrum microporum Nägeli, 1855 | P-B | – | st-str | i | ind | – | – | e | 2.3 |
| Coenochloris pyrenoidosa Korshikov, 1953 | P | – | – | hl | – | – | – | – | – |
| Crucigenia fenestrata (Schmidle) Schmidle, 1900 | P-B | – | st-str | – | – | – | – | e | 1.8 |
| Crucigenia quadrata Morren, 1830 | P-B | – | st-str | i | acf | – | – | e | 1.9 |
| Desmodesmus armatus (Chodat) E.H.Hegewald, 2000 | P-B | – | st-str | – | – | – | – | e | 1.9 |
| Desmodesmus armatus var. bicaudatus (Guglielmetti) E.H. Hegewald, 2000 | P-B | – | st-str | – | – | – | – | e | 2.2 |
| Desmodesmus communis (E.Hegewald) E.Hegewald, 2000 | P-B | – | st-str | – | – | – | – | e | 2.0 |
| Desmodesmus subspicatus (Chodat) E.Hegewald & A.W.F.Schmidt, 2000 | P | – | – | – | – | – | – | e | – |
| Eudorina elegans Ehrenberg, 1832 | P | – | st-str | i | – | – | – | – | 2.3 |
| Hyaloraphidium contortum Pascher & Korshikov, 1931 | P-B | – | – | i | – | – | – | – | – |
| Kirchneriella lunaris (Kirchner) Möbius, 1894 | P-B | – | st-str | i | – | – | – | e | – |
| Lemmermannia tetrapedia (Kirchner) Lemmermann, 1904 | P-B | – | st-str | i | ind | – | – | e | 2.0 |
| Monactinus simplex (Meyen) Corda, 1839 | P-B | – | st-str | – | – | – | – | – | 2.0 |
| Monoraphidium contortum (Thuret) Komárková-Legnerová, 1969 | P-B | – | st-str | i | – | – | – | – | – |
| Monoraphidium convolutum (Corda) Komárková-Legnerová, 1969 | P-B | – | st-str | – | – | – | – | e | 2.2 |
| Monoraphidium griffithii (Berkeley) Komárková-Legnerová, 1969 | P-B | – | st-str | i | – | – | – | e | 2.5 |
| Monoraphidium minutum (Nägeli) Komárková-Legnerová, 1969 | P-B | – | st-str | i | – | – | – | – | – |
| Monoraphidium pusillum (Printz) Komárková-Legnorová, 1969 | P | – | – | – | – | – | – | – | 1.0 |
| Mucidosphaerium pulchellum (H.C.Wood) C.Bock, Proschold & Krienitz, 2011 | P-B | – | st-str | i | ind | – | – | – | 1.8 |
| Nephrochlamys subsolitaria (G.S.West) Korshikov, 1953 | P | – | – | – | – | – | – | – | – |
| Oocystis borgei J.W.Snow, 1903 | P-B | – | st-str | i | ind | – | – | e | 1.7 |
| Oocystis lacustris Chodat, 1897 | P-B | – | st-str | hl | – | – | – | – | – |
| Oocystis submarina Lagerheim, 1886 | P-B | – | st | i | – | – | – | – | – |
| Pediastrum duplex Meyen, 1829 | P | – | st-str | i | ind | – | – | e | – |
| Phacotus lenticularis (Ehrenberg) Diesing, 1866 | P | – | st | – | – | – | – | – | – |
| Pseudopediastrum boryanum (Turpin) E.Hegewald, 2005 | P-B | – | st-str | i | ind | – | – | e | 2.1 |
| Raphidocelis sigmoidea Hindák, 1977 | P | – | st-str | – | – | – | – | e | 1.5 |
| Scenedesmus semipulcher Hortobágyi, 1960 | P | – | – | – | – | – | – | e | 2.2 |
| Scenedesmus ellipticus Corda, 1835 | P-B, S | – | st-str | – | – | – | – | – | 1.7 |
| Scenedesmus obtusus Meyen, 1829 | P-B | – | st-str | – | – | – | – | e | 1.8 |
| Sphaerocystis planctonica (Korshikov) Bourrelly, 1974 | P | – | – | i | – | – | – | e | 1.0 |
| Stauridium tetras (Ehrenberg) E.Hegewald, 2005 | P-B | – | st-str | i | ind | – | – | om | – |
| Tetradesmus lagerheimii M.J.Wynne & Guiry, 2016 | P-B | – | st-str | i | ind | – | – | e | 2.15 |
| Tetradesmus incrassatulus (Bohlin) M.J.Wynne, 2016 | P-B | – | st-str | – | – | – | – | e | – |
| Tetradesmus obliquus (Turpin) M.J.Wynne, 2016 | P-B, S | – | st-str | i | ind | – | – | ot | 2.4 |
| Tetraedron caudatum (Corda) Hansgirg, 1888 | P-B | – | st-str | i | ind | – | – | e | 2.0 |
| Tetraedron minimum (A.Braun) Hansgirg, 1889 | P-B | – | st-str | i | alf | – | – | e | 2.1 |
| Tetraedron triangulare Korshikov, 1953 | P-B | – | st-str | i | – | – | – | e | 2.0 |
| Volvox globator Linnaeus, 1758 | P | – | – | – | – | – | – | – | 2.0 |
| Cyanobacteria | |||||||||
| Anathece clathrata (West & G.S.West) Komárek, Kaštovský & Jezberová, 2011 | P-B | – | – | hl | – | – | – | me | 1.8 |
| Aphanizomenon flos-aquae Ralfs ex Bornet & Flahault, 1886 | P-B | – | – | hl | alb | – | – | m | 1.95 |
| Aphanocapsa incerta (Lemmermann) G.Cronberg & Komárek, 1994 | P-B | – | – | i | – | – | – | me | 2.2 |
| Aphanocapsa planctonica Komárek & Anagnostidis, 1995 | P-B | – | – | i | – | – | – | o-e | – |
| Aphanocapsa delicatissima West & G.S.West, 1912 | P-B | – | – | i | – | – | – | m | – |
| Aphanothece elabens (Meneghini) Elenkin, 1936 | P-B | – | – | – | – | – | – | ot | – |
| Chroococcus minimus (Keissler) Lemmermann, 1904 | P-B | – | – | hl | – | – | – | e | – |
| Chroococcus minor (Kützing) Nägeli, 1849 | B,S | – | – | – | – | – | – | e | 1.4 |
| Chroococcus turgidus (Kützing) Nägeli, 1849 | P-B,S | – | aer | hl | alf | – | – | e | 0.8 |
| Chrysosporum bergii (Ostenfeld) E.Zapomelová, O.Skácelová, P.Pumann, R.Kopp & E.Janecek, 2012 | P | – | – | – | – | – | – | e | – |
| Coelomoron pusillum (Van Goor) Komárek, 1988 | P | – | – | – | – | – | – | e | 1.8 |
| Coelosphaerium kuetzingianum Nägeli, 1849 | P | – | – | i | – | – | – | m | 1.6 |
| Dolichospermum sigmoideum (Nygaard) Wacklin, L.Hoffmann & Komárek, 2009 | P | – | – | i | – | – | – | e | 1.7 |
| Dolichospermum spiroides (Klebahn) Wacklin, L.Hoffmann & Komárek, 2009 | P-B | – | st-str | i | – | – | – | e | 1.3 |
| Glaucospira laxissima (G.S.West) Simic, Komárek & Dordevic, 2014 | P | – | st | – | – | – | – | – | – |
| Gomphosphaeria aponina Kützing, 1836 | P-B | – | st-str | hl | alf | sx | – | – | – |
| Leptolyngbya angustissima (West & G.S.West) Anagnostidis & Komárek, 1988 | B,Ep,S | warm | st-str,aer | – | – | – | – | om | – |
| Leptolyngbya tenuis (Gomont) Anagnostidis & Komárek, 1988 | B.S | – | st-str | i | alf | – | – | om | 1.1 |
| Limnococcus limneticus (Lemmermann) Komárková, Jezberová, O.Komárek & Zapomelová, 2010 | P-B | – | – | i | acf | – | – | me | 1.8 |
| Merismopedia elegans A.Braun ex Kützing, 1849 | P-B,Ep | – | – | i | ind | – | – | me | – |
| Merismopedia glauca (Ehrenberg) Kützing, 1845 | P-B | – | – | i | ind | – | – | e | – |
| Merismopedia minima G.Beck, 1897 | B,S | – | aer | – | – | – | – | e | – |
| Merismopedia tenuissima Lemmermann, 1898 | P-B | – | – | hl | – | – | – | e | – |
| Merismopedia tranquilla (Ehrenberg) Trevisan, 1845 | P-B | – | – | i | ind | – | – | – | 2.3 |
| Microcystis aeruginosa (Kützing) Kützing, 1846 | P-B | – | – | hl | acf | – | – | me | 2.2 |
| Microcystis pulverea (H.C.Wood) Forti, 1907 | P-B,S | – | – | i | – | – | – | e | – |
| Microcystis wesenbergii (Komárek) Komárek ex Komárek, 2009 | P-B | – | – | – | – | – | – | – | 2.3 |
| Microcystis ichthyoblabe (G.Kunze) Kützing, 1843 | P | – | – | i | – | – | – | e | – |
| Planktolyngbya contorta (Lemmermann) Anagnostidis & Komárek, 1988 | P-B | – | – | – | alf | – | – | – | – |
| Pseudanabaena limnetica (Lemmermann) Komárek, 1974 | P-B | – | – | – | alf | – | – | e | 2.2 |
| Pseudanabaena mucicola (Naumann & Huber-Pestalozzi) Schwabe, 1964 | B,Ep | – | – | i | – | – | – | e | 2.1 |
| Rhabdoderma lineare Schmidle & Lauterborn, 1900 | P | – | – | hb | – | – | – | – | – |
| Romeria leopoliensis (Raciborski) Koczwara, 1932 | P | – | st | – | – | – | – | e | 1.5 |
| Snowella atomus Komárek & Hindák, 1988 | P | – | – | – | – | – | – | me | – |
| Spirulina laxa G.M.Smith, 1916 | P | – | st | – | alf | – | – | e | 3.6 |
| Synechocystis aquatilis Sauvageau, 1892 | P-B | warm | – | – | alb | – | – | – | – |
| Woronichinia naegeliana (Unger) Elenkin, 1933 | P | – | st | – | – | – | – | e | 1.8 |
| Dinoflagellata | |||||||||
| Ceratium hirundinella (O.F.Müller) Dujardin, 1841 | P | – | st-str | i | – | – | – | e | 1.3 |
| Peridiniopsis quadridens (F.Stein) Bourrelly, 1968 | P | – | – | – | – | – | – | – | 1.4 |
| Peridinium cinctum (O.F.Müller) Ehrenberg, 1832 | P-B | – | st-str | i | – | – | – | – | 1.4 |
| Euglenophyta | |||||||||
| Euglena viridis (O.F.Müller) Ehrenberg, 1830 | P-B,S | eterm | st-str | mh | ind | – | – | – | 1.5 |
| Lepocinclis acus (O.F.Müller) B.Marin & Melkonian, 2003 | P | eterm | st | i | ind | – | – | – | 2.4 |
| Lepocinclis oxyuris (Schmarda) B.Marin & Melkonian, 2003 | P-B | – | st-str | mh | ind | – | – | – | 2.3 |
| Monomorphina pyrum (Ehrenberg) Mereschkowsky, 1877 | P-B | eterm | st-str | mh | ind | – | – | – | – |
| Phacus longicauda (Ehrenberg) Dujardin, 1841 | P-B | – | st | i | ind | – | – | – | 2.8 |
| Trachelomonas hispida (Perty) F.Stein, 1878 | P-B | eterm | st-str | i | acf | – | – | – | 2.2 |
| Trachelomonas oblonga Lemmermann, 1899 | P | eterm | st-str | i | – | – | – | – | 2.4 |
| Trachelomonas oblonga var. attenuata Playfair, 1915 | – | – | – | – | – | – | – | – | 2.4 |
| Trachelomonas volvocina (Ehrenberg) Ehrenberg, 1834 | P-B | eterm | st-str | i | ind | – | – | – | 2.0 |
| Heterokontophyta | |||||||||
| Amphora ovalis (Kützing) Kützing, 1844 | B | temp | st-str | i | alf | sx | ate | e | 1.5 |
| Asterionella formosa Hassall, 1850 | P | temp | st-str | i | alf | sx | ate | me | 1.35 |
| Aulacoseira granulata (Ehrenberg) Simonsen, 1979 | P-B | temp | st-str | i | alf | es | ate | e | 2.0 |
| Aulacoseira italica (Ehrenberg) Simonsen, 1979 | P-B | cool | st-str | i | ind | es | ate | me | 1.45 |
| Cocconeis placentula Ehrenberg, 1838 | P-B | temp | st-str | i | alf | es | ate | me | 1.35 |
| Cymbella cistula (Ehrenberg) O.Kirchner, 1878 | B | – | st-str | i | alf | sx | ats | e | 1.2 |
| Cymbella tumida (Brébisson) Van Heurck, 1880 | B | temp | st-str | i | alf | sx | ats | me | 2.2 |
| Cymbopleura lata (Grunow ex Cleve) Krammer, 2003 | B | – | – | i | ind | – | – | ot | 1.0 |
| Diatoma vulgaris Bory, 1824 | P-B | temp | st-str | i | alf | – | – | – | 2.4 |
| Dinobryon divergens O.E.Imhof, 1887 | P-B | – | st-str | i | ind | – | – | – | 1.2 |
| Dinobryon sertularia Ehrenberg, 1834 | P-B | – | – | i | – | – | – | – | 1.3 |
| Epithemia turgida (Ehrenberg) Kützing, 1844 | B | temp | st-str | i | alf | – | – | – | 1.1 |
| Epithemia adnata (Kützing) Brébisson, 1838 | B | temp | st-str | i | alb | – | – | – | 1.2 |
| Fragilaria capucina Desmazières, 1830 | P-B | temp | st-str | i | ind | – | – | – | – |
| Gyrosigma acuminatum (Kützing) Rabenhorst, 1853 | B | temp | st-str | i | alf | – | – | – | – |
| Melosira inflexa (Roth) Guiry, 2019 | P-B | eterm | str | mh | alf | – | – | – | 2.0 |
| Navicula cincta (Ehrenberg) Ralfs, 1861 | B | temp | st-str | hl | alf | – | – | – | – |
| Navicula cryptocephala Kützing, 1844 | P-B | temp | st-str | i | ind | – | – | – | 2.4 |
| Navicula minima Grunow, 1880 | P-B | temp | st-str | hl | alf | – | hce | e | 1.0 |
| Navicula radiosa Kützing, 1844 | B | temp | st-str | i | ind | sx | – | – | – |
| Navicula rhynchocephala Kützing, 1844 | B | temp | st-str | hl | alf | – | – | – | 1.3 |
| Navicula tripunctata (O.F.Müller) Bory, 1822 | P-B | temp | st-str | i | alf | es | – | e | – |
| Nitzschia acicularis (Kützing) W.Smith, 1853 | P-B | temp | st | i | alf | es | ats | om | 1.4 |
| Nitzschia linearis W.Smith, 1853 | B | temp | st-str | i | alf | – | – | – | – |
| Nitzschia palea (Kützing) W.Smith, 1856 | P-B | temp | st-str | i | ind | – | – | – | 2.0 |
| Nitzschia longissima (Brébisson ex Kützing) Grunow, 1862 | – | – | – | mh | alf | – | – | – | – |
| Pseudokephyrion entzii W.Conrad, 1939 | – | – | – | – | – | – | – | – | 1.5 |
| Sellaphora pupula (Kützing) Mereschkovsky, 1902 | B | eterm | st-str | hl | ind | sx | ate | me | 1.9 |
| Staurosira construens Ehrenberg, 1843 | P-B | temp | st-str | i | alf | – | – | – | 1.0 |
| Staurosira subsalina (Hustedt) Lange-Bertalot, 2004 | P-B | – | st-str | hl | alf | – | – | – | – |
| Stephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal & Kociolek, 2022 | P-B | temp | st-str | hl | alf | sp | hne | e | 2.8 |
| Stephanodiscus hantzschii Grunow, 1880 | P | temp | st-str | i | alf | sx | – | – | – |
| Tryblionella hantzschiana Grunow, 1862 | B | – | st-str | hl | alf | – | ate | e | 2.6 |
| Ulnaria ulna (Nitzsch) Compère, 2001 | P-B | temp | st-str | i | alf | es | ate | e | 2.4 |
| Ulnaria ulna var. spathulifera (Grunow) Aboal, 2003 | B | – | st-str | i | alf | – | ats | e | 1.7 |
| Abbreviation, Station Number and Sampling Year * | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group of indicators | BA-1-24 | BA-2-24 | BA-3-24 | SH-1-24 | SH-2-24 | SH-3-24 | KU-1-23 | KU-2-23 | KU-3-23 | KU-1-24 | KU-2-24 | KU-3-24 | OR-1-23 | OR-2-23 | OR-3-23 | OR-1-24 | OR-2-24 | OR- 3-24 | SI- 1-23 | SI- 2-23 | SI- 3-23 |
| Habitat | |||||||||||||||||||||
| Ep | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B | 1 | 3 | 3 | 3 | 3 | 5 | 5 | 2 | 6 | 1 | 2 | 2 | 0 | 3 | 3 | 4 | 5 | 3 | 1 | 2 | 2 |
| P-B | 6 | 6 | 4 | 32 | 27 | 28 | 23 | 23 | 24 | 16 | 17 | 16 | 9 | 22 | 21 | 18 | 17 | 24 | 21 | 23 | 27 |
| P | 3 | 3 | 3 | 6 | 6 | 6 | 5 | 6 | 4 | 10 | 6 | 5 | 8 | 9 | 7 | 9 | 9 | 9 | 10 | 10 | 7 |
| total | 10 | 12 | 11 | 41 | 36 | 39 | 33 | 31 | 34 | 27 | 25 | 23 | 17 | 34 | 31 | 31 | 31 | 36 | 32 | 35 | 36 |
| Temperature | |||||||||||||||||||||
| cool | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| temp | 0 | 2 | 2 | 1 | 0 | 3 | 6 | 2 | 7 | 4 | 6 | 4 | 2 | 7 | 8 | 6 | 5 | 7 | 1 | 2 | 4 |
| eterm | 1 | 2 | 1 | 3 | 3 | 4 | 0 | 1 | 3 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 0 | 1 | 3 | 1 | 2 |
| warm | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| total | 2 | 4 | 3 | 5 | 4 | 8 | 6 | 3 | 10 | 7 | 9 | 6 | 2 | 10 | 10 | 7 | 6 | 9 | 4 | 4 | 7 |
| Oxygen | |||||||||||||||||||||
| aer | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| str | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| st-str | 4 | 6 | 6 | 26 | 22 | 23 | 17 | 13 | 18 | 14 | 15 | 14 | 9 | 20 | 20 | 16 | 18 | 21 | 15 | 20 | 20 |
| st | 0 | 0 | 0 | 2 | 2 | 3 | 3 | 4 | 3 | 4 | 3 | 3 | 4 | 2 | 3 | 5 | 3 | 3 | 5 | 4 | 2 |
| total | 5 | 7 | 6 | 28 | 25 | 27 | 23 | 18 | 22 | 18 | 18 | 18 | 13 | 23 | 23 | 21 | 22 | 24 | 20 | 24 | 24 |
| Salinity | |||||||||||||||||||||
| hb | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| i | 4 | 4 | 3 | 24 | 20 | 20 | 14 | 18 | 19 | 18 | 17 | 14 | 10 | 20 | 18 | 19 | 19 | 20 | 16 | 19 | 24 |
| hl | 1 | 3 | 3 | 6 | 6 | 6 | 7 | 3 | 8 | 2 | 3 | 3 | 0 | 4 | 5 | 4 | 3 | 7 | 5 | 4 | 5 |
| mh | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| oh | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| total | 6 | 8 | 7 | 32 | 26 | 27 | 22 | 22 | 29 | 20 | 20 | 17 | 11 | 26 | 24 | 23 | 23 | 29 | 22 | 24 | 31 |
| pH | |||||||||||||||||||||
| acf | 0 | 0 | 0 | 3 | 3 | 2 | 2 | 4 | 3 | 2 | 2 | 1 | 0 | 3 | 2 | 0 | 0 | 2 | 3 | 2 | 3 |
| ind | 3 | 3 | 3 | 10 | 8 | 9 | 6 | 7 | 10 | 6 | 6 | 6 | 3 | 7 | 4 | 5 | 3 | 6 | 4 | 6 | 9 |
| alf | 1 | 3 | 1 | 6 | 3 | 5 | 10 | 2 | 9 | 3 | 5 | 4 | 2 | 7 | 8 | 6 | 8 | 10 | 3 | 3 | 5 |
| alb | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 |
| total | 5 | 7 | 5 | 20 | 15 | 17 | 18 | 13 | 23 | 12 | 14 | 11 | 5 | 18 | 16 | 12 | 12 | 20 | 11 | 12 | 19 |
| Watanabe | |||||||||||||||||||||
| sx | 0 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 1 | 1 |
| es | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 2 | 2 | 1 | 2 | 4 | 4 | 2 | 2 | 2 | 0 | 0 | 2 |
| sp | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| total | 1 | 2 | 1 | 3 | 1 | 2 | 5 | 2 | 5 | 4 | 3 | 2 | 2 | 6 | 7 | 3 | 3 | 4 | 1 | 2 | 4 |
| Autotrophy-Heterotrophy | |||||||||||||||||||||
| ats | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 0 | 0 | 0 |
| ate | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 4 | 1 | 1 | 1 | 1 | 0 | 3 |
| hne | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| hce | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| total | 1 | 2 | 1 | 1 | 0 | 1 | 3 | 2 | 4 | 3 | 3 | 2 | 2 | 6 | 7 | 3 | 3 | 3 | 2 | 1 | 4 |
| Trophic State | |||||||||||||||||||||
| ot | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 1 |
| om | 0 | 0 | 0 | 2 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 1 | 0 | 2 | 0 |
| m | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 3 | 4 | 0 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 1 |
| me | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 4 | 4 | 3 | 3 | 1 | 1 | 4 | 6 | 0 | 0 | 1 | 3 | 3 | 6 |
| e | 3 | 3 | 3 | 19 | 13 | 14 | 14 | 11 | 11 | 11 | 8 | 8 | 8 | 15 | 12 | 14 | 15 | 18 | 14 | 13 | 16 |
| o-e | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| total | 6 | 7 | 5 | 25 | 20 | 21 | 24 | 22 | 22 | 16 | 14 | 13 | 11 | 24 | 22 | 19 | 20 | 23 | 22 | 22 | 24 |
| Class of Water Quality | |||||||||||||||||||||
| Class 2 | 3 | 3 | 2 | 2 | 2 | 5 | 5 | 4 | 6 | 5 | 4 | 4 | 3 | 10 | 8 | 4 | 6 | 7 | 8 | 11 | 11 |
| Class 3 | 5 | 6 | 5 | 24 | 21 | 22 | 13 | 13 | 13 | 13 | 11 | 9 | 8 | 11 | 10 | 13 | 14 | 16 | 16 | 16 | 21 |
| Class 4 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 3 | 2 | 1 |
| Class 5 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| total | 8 | 9 | 7 | 28 | 25 | 29 | 20 | 19 | 22 | 18 | 15 | 13 | 11 | 22 | 20 | 19 | 21 | 25 | 27 | 29 | 33 |
References
- Fan, X.; Zhang, Q.; Melack, J.M.; Tang, H.; Yuan, S.; Jia, Y.; Xue, C.; Song, Y. Floodplain lakes: Linking hydrology to ecology and conservation. Earth-Sci. Rev. 2024, 258, 104967. [Google Scholar]
- Shiel, R.; Green, J.; Nielsen, D. Floodplain biodiversity: Why are there so many species? Hydrobiologia 1998, 387, 39–46. [Google Scholar] [CrossRef]
- Museth, J.; Johnsen, S.; Walseng, B.; Hanssen, O.; Erikstad, L. Managing biodiversity of floodplains in relation to climate change. Int. J. Clim. Change Strateg. Manag. 2011, 3, 402–415. [Google Scholar] [CrossRef]
- Silvano, R.; Ramires, M.; Zuanon, J. Effects of fish management of fish communities in the floodplain lakes of a Brazilian Amazonian Reserve. Ecol. Freshw. Fish 2009, 18, 156–166. [Google Scholar] [CrossRef]
- Ferencz, B.; Toporowska, M.; Dawidek, J. Role of Hydrology in Cyanobacterial Blooms in the Floodplain Lakes. Water 2023, 15, 1547. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Wang, S.; Jia, J.; Ha, X.; Lu, Y. Floodplain Lake response to climate-nutrient-hydrological pressure revealed through phytoplankton community succession over the past century. J. Hydrol. 2023, 623, 129838. [Google Scholar] [CrossRef]
- Souhardya, S.; Kumar, U.; Iqbal, M.; Kabir, I.; Billah, M.; Das, C.; Barman, S.; Shawlin, J.; Ahmed, I.; Das, J. Phytoplankton in contrasting ecosystem of southeastern coast of Bangladesh: Effects of seasonality and environmental factors. Environ. Monit. Assess. 2025, 197, 260. [Google Scholar] [CrossRef]
- Barinova, S. Database of Ecological Indicators of Freshwater Algae and Cyanobacteria. Ecol. Divers. 2025, 2, 10003. [Google Scholar] [CrossRef]
- Sahoo, D.; Bera, S.; Pal, P.; Tamili, D.; Sarkar, B.; Madhu, N.; Ghorai, S. Plankton act as a key indicator of the health and stability of aquatic ecosystems: A review. BOESD 2024, e03, 18. [Google Scholar] [CrossRef]
- Khamzina, S.S.; Sharipova, Z.M.; Omarova, G.M. Water Resources of the Pavlodar Region, Their Protection and Rational Use: Textbook; Innovative Eurasian University: Pavlodar, Kazakhstan, 2013; p. 248. (In Russian) [Google Scholar]
- Burlibaev, M.Z.; Kuts, S.I.; Faschevsky, K.O.; Tsaregorodtseva, A.G.; Shenberger, I.V.; Burlibayeva, D.M.; Aytureev, A.M. Flooding of the Irtysh Floodplain as the Main Factor of Sustainable Development of the River Ecosystem; Kaganat: Almaty, Kazakhstan, 2014; p. 396. (In Russian) [Google Scholar]
- Skabichevsky, A.P. On the Changes in Phytoplankton of the Irtysh River from Lake Zaysan to Omsk. In Proceedings of the VII Scientific Conference, Tomsk, Russia, 16–23 November 1957; Volume 3, pp. 31–33. (In Russian). [Google Scholar]
- Kozlyatkin, A.L.; Danilova, O.K. Flora and Fauna of the Middle Irtysh under Conditions of Regulated Flow. In Proceedings of the Scientific-Practical Conference “Environmental Protection and Nature Management of the Irtysh Region”, Ust-Kamenogorsk, Kazakhstan, 28–30 March 1990; Part 2. pp. 154–157. (In Russian). [Google Scholar]
- Barsukova, N.; Bazhenova, O. Phytoplankton and the Ecological State of the Tributaries of the Middle Irtysh; Lap Lambert Academic Publishing GmbH & Co. KG: Saarbrucken, Germany, 2012; 160p. (In Russian) [Google Scholar]
- Bazhenova, O.P.; Gulchenko, Y.I. Indicator Significance of Certain Phytoplankton Species of the Middle Irtysh River as Indicators of Water Pollution. Vestn. Omsk. State Agrar. Univ. 2016, 1, 82–92. (In Russian) [Google Scholar]
- Moldrakhman, A.S.; Mazhibaeva, Z.O.; Dolgopolova, S.Y.; Kozhizhanova, B.A.; Suleimenova, A.M. Hydrochemical Studies and Phytoplankton of Saline Lakes in the Pavlodar Region. Bull. Sci. Kazakh Agric. Tech. Uni. Named S. Seifullin 2022, 1, 145–152. (In Russian) [Google Scholar] [CrossRef]
- Bazhenova, O.P. Main Directions of Changes in Phytoplankton of the Upper and Middle Irtysh under Anthropogenic Impact. In Nature and Environmental Management at the Turn of the 21st Century; Courier: Omsk, Russia, 1999; pp. 134–136. (In Russian) [Google Scholar]
- Bazhenova, O.P. Phytoplankton of the Upper and Middle Irtysh under Conditions of Regulated Flow; FSBEI HPE Omsk State Agrarian University Publishing House: Omsk, Russia, 2005; 248p. (In Russian) [Google Scholar]
- Bazhenova, O.P. Assessment of Long-Term Changes in the Ecosystems of the Upper and Middle Irtysh Based on Phytoplankton Development Indicators. Sib. Ecol. J. 2006, 6, 785–790. (In Russian) [Google Scholar]
- Bazhenova, O.P.; Barsukova, N.N.; Gulchenko, Y.I. Summer Phytoplankton of the Irtysh River in the Section Pavlodar (Republic of Kazakhstan)–Omsk (Russian Federation). Vestn. Omsk. State Agrar. Univ. 2017, 3, 42–50. (In Russian) [Google Scholar]
- Bazhenova, O.P. Long-Term Dynamics of Phytoplankton in the Irtysh River Basin (State and Trends). Ph.D. Thesis, Biological Faculty, Moscow State University, Moscow, Russia, 2005; 318p. (In Russian). [Google Scholar]
- Bazhenova, O.P. Algal Bloom in the Bukhtarma Reservoir as a First Sign of Eutrophication. Ekologiya 1980, 5, 89–90. (In Russian) [Google Scholar]
- Bazhenova, O.P. Changes in the Diatom Flora of the Bukhtarma Reservoir. In Proceedings of the International Conference “Diatoms as Indicators of Environmental and Climate Changes”, Irkutsk, Russia, 16–20 March 1993; pp. 3–4. (In Russian). [Google Scholar]
- Bazhenova, O.P.; Genkal, S.I.; Shahoval, V.Y.; Bragina, Y.A. Centric Diatoms (Centrophyceae) of the Bukhtarma Reservoir (Irtysh River, Kazakhstan). Algologia 2013, 23, 308–317. (In Russian) [Google Scholar] [CrossRef]
- Argynbayeva, Y.M.; Barinova, S.S.; Ormanova, G.Z. Characteristics of the Modern Taxonomic Composition of the Phytoplankton in the Kazakhstan Part of the Irtysh River. Exp. Biol. 2024, 2, 102–114. [Google Scholar] [CrossRef]
- Toleuzhanova, A.; Ubaskin, A.; Akhmetov, K.; Erzhanov, N.; Lunkov, A.; Minakov, A.; Abylkhasymov, T. Phytoplankton of Saline Lakes in the Pavlodar Region. Bull. L.N. Gumilyov Eurasian Natl. Univ. Ser. Biol. Sci. 2019, 3, 73–80. (In Russian) [Google Scholar]
- Noskov, A.A. Phytoplankton of the Bukhtarma Reservoir in the First Years of Its Filling. In Biological Foundations of Fisheries in the Water Bodies of Central Asia and Kazakhstan; Nauka: Alma-Ata, Kazakhstan, 1966; pp. 295–296. (In Russian) [Google Scholar]
- Tsaregorodtseva, A.G.; Yesimova, D.D. On the Formation of Floodplain Water Bodies and Watercourses of the State Nature Reserve “Floodplain of the Irtysh River”. Hydrometeorol. Ecol. 2014, 3, 179–184. (In Russian) [Google Scholar]
- Beisembayeva, M.A.; Bazarbekov, K.U. The effect of environmental releases on the hydrological regime of the Irtysh river within the territory of Pavlodar region of the republic of Kazakhstan. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2015, 17, 781–786. (In Russian) [Google Scholar]
- Baisholanova, S.S. Agroclimatic Resources of Pavlodar Region: Scientific and Applied Reference Book; Baisholanov, S.S., Ed.; Astana Publishing: Astana, Kazakhstan, 2017; 127p. (In Russian) [Google Scholar]
- Baisholanov, S.; Akshalov, K.; Mukanov, Y.; Zhumabek, B.; Karakulov, E. Agro-Climatic Zoning of the Territory of Northern Kazakhstan for Zoning of Agricultural Crops Under Conditions of Climate Change. Climate 2025, 13, 3. [Google Scholar] [CrossRef]
- Erokhina, O.G.; Kusainova, M.M.; Sokolov, A.A.; Pachikin, K.M. Soils of Kazakhstan; Natural Conditions and Resources: Almaty, Kazakhstan, 2006; Volume 1, 316p. (In Russian) [Google Scholar]
- Philonec, P.P.; Omarov, T.P.; Muravlev, G.G. Lakes of Kazakhstan; Nauka: Almaty, Kazakhstan, 1995; 237p. (In Russian) [Google Scholar]
- Ministry of Environment and Water Resources of the Republic of Kazakhstan. Order of April 4, 2014 No. 104- Ö “On approval of the Rules for the Preparation of Biological Justification for the Use of Wildlife”; Registered in the Ministry of Justice of the Republic of Kazakhstan on April 10, 2014 No. 9307. Available online: https://adilet.zan.kz/rus/docs/V1400009307 (accessed on 28 August 2025). (In Russian)
- Semenov, A.D. Manual for Chemical Analysis of Surface Waters of Land; Gidrometeoizdat: Leningrad, Russia, 1977; pp. 156–163. (In Russian) [Google Scholar]
- Fomin, G.S. Water: Control of Chemical, Bacterial, and Radiation Safety According to International Standards; NPO “Alternativa”: Moscow, Russia, 1995; 618p. (In Russian) [Google Scholar]
- Abakumova, V.A. Manual on Methods of Hydrobiological Analysis of Surface Waters and Bottom Sediments; Gidrometeoizdat: Leningrad, Russia, 1983; 240p. (In Russian) [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota: Chroococcales. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Jena, Germany, 1999; 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota: Oscillatoriales. In Süßwasserflora von Mitteleuropa; Elsevier GmbH: Munich, Germany, 2005; Volume 19, 759p. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen), Ordnung: Chlorococcales. In Das Phytoplankton des Süßwassers (Die Binnengewässer); Huber-Pestalozzi, G., Ed.; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1983; Volume 16, pp. 1–445. (In German) [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Naviculaceae. In Süßwasserflora von Mitteleuropa; Gustav Fischer: Jena, Germany, 1986; Volume 2, 860p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Bacillariaceae, Epithemiaceae, Surirellaceae. In Süßwasserflora von Mitteleuropa; Gustav Fischer: New York, NY, USA, 1988; Volume 2, 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Achnanthaceae. Critical Additions to Navicula (Lineolatae) and Gomphonema. Complete Bibliography; In Süßwasserflora von Mitteleuropa; Gustav Fischer: Stuttgart, Germany, 1991; Volume 2, 437p. [Google Scholar]
- Popovsky, J.; Pfiester, L.A. Dinophyceae (Dinoflagellata). In Süßwasserflora von Mitteleuropa; Gustav Fischer: Jena, Germany, 1990; Volume 6, 272p. [Google Scholar]
- Williams, D.M.; Round, F.E. Revision of the Genus Synedra Ehrenb. Diatom Res. 1986, 1, 313–339. [Google Scholar] [CrossRef]
- Williams, D.M.; Round, F.E. Revision of the Genus Fragilaria. Diatom Res. 1987, 2, 267–288. [Google Scholar] [CrossRef]
- Aboal, M.; Silva, P.C. Validation of New Combinations. Diatom Res. 2004, 19, 361. [Google Scholar] [CrossRef]
- Tsarenko, P.M.; Wasser, S.P.; Nevo, E. Algae of Ukraine: Diversity, Nomenclature, Taxonomy, Ecology and Geography; Vol. 3: Chlorophyta; A.R.A. Gantner K.G.: Ruggell, Liechtenstein, 2006. [Google Scholar]
- Gollerbakh, M.M.; Kossinskaya, E.K.; Polyansky, V.I. Blue-Green Algae. In Guide to Freshwater Algae of the USSR, Issue 2; Soviet Science: Moscow, Russia, 1953; 652p. (In Russian) [Google Scholar]
- Popova, T.G. Euglenophyta. In Guide to Freshwater Algae of the USSR.; Soviet Science: Moscow, Russia, 1955; 282p. (In Russian) [Google Scholar]
- Dedussenko-Shchegoleva, I.T.; Gollerbakh, M.M. Yellow-Green Algae. In Guide to Freshwater Algae of the USSR.; Publishing House of the USSR Academy of Sciences: Moscow, Russia; Saint Petersburg, Russia, 1962; 272p. (In Russian) [Google Scholar]
- Palamar-Mordvintseva, G.M. Green Algae, Class Conjugates. Order Desmidiales (2). Chlorophyta: Conjugatophyceae. Desmidiales (2). In Guide to Freshwater Algae of the USSR.; Nauka: Saint Petersburg, Russia, 1982; Volume 11, 620p. (In Russian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2013; Available online: http://www.algaebase.org (accessed on 24 January 2025).
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Krupa, E.G.; Argynbayeva, Y.M.; Povetkin, R.D.; Ormanova, G.Z. Methodology for Simplified Calculation of Morphometric Characteristics of Unicellular Algae; Certificate of Copyright Registration No. 57018; National Institute of Intellectual Property of the Ministry of Justice of the Republic of Kazakhstan: Almaty, Kazakhstan, 2025. [Google Scholar]
- Megarran, E. Ecological Diversity and Its Measurement; Mir: Moscow, Russia, 1998; 184p. [Google Scholar]
- Clarke, K.; Gorley, R. PRIMER Version 7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; 192p. [Google Scholar]
- Love, J.; Selke, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, A.J.; Ly, A.; Gronau, Q.F.; Smira, M.; Epskamp, S. Graphical Statistical Software for Common Statistical Designs. J. Stat. Softw. 2019, 88, 1–17. Available online: https://pure.uva.nl/ws/files/43961937/v88i02.pdf#page=7.63 (accessed on 5 August 2025). [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012; 496p. [Google Scholar]
- Moreau, E.D.; Lescord, G. Trophic State: A Useful Scale for Classifying Lakes Based on Biological Productivity; University of Florida IFAS Extension: Gainesville, FL, USA, 2025; Available online: https://edis.ifas.ufl.edu/publication/FA268 (accessed on 2 September 2025).
- Carlson, R.E. A Trophic State Index for Lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Kitaev, S.P. Fundamentals of Limnology for Hydrobiologists and Ichthyologists; Karelian Research Centre of RAS: Petrozavodsk, Russia, 2007; 395p. (In Russian) [Google Scholar]
- Dokulil, M.; Donabaum, K.; Teubner, K. Modifications in Phytoplankton Size Structure by Environmental Constraints Induced by Regime Shifts in an Urban Lake. Hydrobiologia 2007, 578, 59–63. [Google Scholar] [CrossRef][Green Version]
- Abonyi, A.; Kiss, K.T.; Hidas, A.; Borics, G.; Varbíró, G.; Ács, É. Cell Size Decrease and Altered Size Structure of Phytoplankton Constrain Ecosystem Functioning in the Middle Danube River over Multiple Decades. Ecosystems 2020, 23, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Kazhydromet. Available online: https://www.kazhydromet.kz/ru/post/2671 (accessed on 9 September 2025).
- Barinova, S. On the Classification of Water Quality from an Ecological Point of View. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555581. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherland J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Stević, F.; Mihaljević, M.; Špoljarić, D. Changes of Phytoplankton Functional Groups in a Floodplain Lake Associated with Hydrological Perturbations. Hydrobiologia 2013, 709, 143–158. [Google Scholar] [CrossRef]
- Barinova, S.S.; Belous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Perspectives; University of Haifa Publisher: Haifa, Israel; Kyiv, Ukraine, 2019; 367p. (In Russian) [Google Scholar]
- Umanskaya, M.V.; Gorbunov, M.; Tarasova, N.G. Cyanobacterial Blooms in Freshwater Continental Water Bodies: A Review. Izv. Samara Sci. Cent. Russ. Acad. Sci. 2023, 25, 182–194. (In Russian) [Google Scholar] [CrossRef]
- Carvalho, L.; McDonald, C.; de Hoyos, C.; Mischke, U.; Phillips, G.; Borics, G.; Poikane, S.; Skjelbred, B.; Lyche Solheim, A.; Van Wichelen, J.; et al. Sustaining Recreational Quality of European Lakes: Minimizing the Health Risks from Algal Blooms through Phosphorus Control. J. Appl. Ecol. 2013, 50, 315–323. [Google Scholar] [CrossRef]
- Maileht, K.; Nôges, T.; Nôges, P.; Ott, I.; Mischke, U.; Carvalho, L.; Dudley, B. Water Colour, Phosphorus and Alkalinity Are the Major Determinants of the Dominant Phytoplankton Species in European Lakes. Hydrobiologia 2013, 704, 115–126. [Google Scholar] [CrossRef]
- Vuorio, K.; Järvinen, M.; Kotamäki, N. Phosphorus Thresholds for Bloom-Forming Cyanobacterial Taxa in Boreal Lakes. Hydrobiologia 2020, 847, 4389–4400. [Google Scholar] [CrossRef]
- Herrero, A.; Muro-Pastor, A.M.; Flores, E. Nitrogen Control in Cyanobacteria. J. Bacteriol. 2001, 183, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Inabe, K.; Miichi, A.; Matsuda, M.; Yoshida, T.; Kato, Y.; Hidese, R.; Kondo, A.; Hasunuma, T. Nitrogen Availability Affects the Metabolic Profile in Cyanobacteria. Metabolites 2021, 11, 867. [Google Scholar] [CrossRef]
- Lehman, P.W.; Mayr, S.; Liu, L.; Tang, A. Tidal Day Organic and Inorganic Material Flux of Ponds in the Liberty Island Freshwater Tidal Wetland. SpringerPlus. 2015, 4, 273. [Google Scholar] [CrossRef]
- Felisberto, S.; Leandrini, J.; Rodrigues, L. Effects of Nutrients Enrichment on Algal Communities: An Experimental in Mesocosms Approach. Acta Limnol. Bras. 2011, 23, 128–137. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a Functional Classification of the Freshwater Phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Bruno, S.; McLaughlin, J. The Nutrition of the Freshwater Dinoflagellate Ceratium hirundinella. J. Eukaryot. Microbiol. 1977, 24, 548–552. [Google Scholar] [CrossRef]
- Zarei Darki, B.; Krakhmalnyi, A.F. Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi. Diversity 2019, 11, 137. [Google Scholar] [CrossRef]
- Pham, T.L.; Tran, T.H.Y.; Tran, T.T. Factors affecting the seasonal succession of phytoplankton functional groups in a tropical floodplain reservoir in Vietnam. Aqua-Water Infrastruct. Ecosyst. Soc. 2022, 71, 401–414. [Google Scholar] [CrossRef]
- Hao, X.; Shi, X.; Zhao, S.; Yu, H.; Kang, R.; Han, Y.; Sun, Y.; Wang, S. Impacts of temperature and nutrient dynamics on phytoplankton in a lake: A case study of Wuliangsuhai Lake, China. Sustainability 2024, 16, 11195. [Google Scholar] [CrossRef]
- Gayazova, A.O.; Abdullaev, S.M. The Impact of Climate Variability on Phytoplankton Communities of the Shershnevskoye Drinking Reservoir (Southern Urals). Vestnik VGU. Series: Geography. Geoecology 2019, 2, 53–64. [Google Scholar] [CrossRef]
- Tarasova, N.G. Phytoplankton of Lake Molochka (Samara Region): Composition and seasonal dynamics. Samar. Luka Probl. Reg. Glob. Ecol. 2009, 18, 160–166. [Google Scholar]
- Tijdens, M.; Hoogveld, H.L.; Kamst-van Agterveld, M.P.; Simis, S.G.H.; Gons, H.J.; Laanbroek, H.J. Population dynamics and diversity of viruses, bacteria and phytoplankton in a shallow eutrophic lake. Microb. Ecol. 2008, 56, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Krivina, E.S.; Tarasova, N.G. Phytoplankton of an urbanized reservoir (on the example of Lake Vosmerka, Tolyatti, Samara Region). II. Quantitative development, dominant species and water quality assessment. Bull. Samara Sci. Cent. Russ. Acad. Sci. 2015, 17, 203–209. [Google Scholar]
- Fernández, R.; Alcocer, J.; Oseguera, L.A.; Zuñiga-Ramos, C.A.; Vilaclara, G. Phytoplankton communities’ seasonal fluctuation in two neighboring tropical high-mountain lakes. Plants 2024, 13, 3021. [Google Scholar] [CrossRef] [PubMed]
- Paltsev, A.; Bergström, A.-K.; Vuorio, K.; Creed, I.F.; Hessen, D.O.; Kortelainen, P.; Vuorenmaa, J.; de Wit, H.A.; Lau, D.C.P.; Vrede, T.; et al. Phytoplankton biomass in northern lakes reveals a complex response to global change. Sci. Total Environ. 2024, 940, 173570. [Google Scholar] [CrossRef]
- Watanabe, T. Biological indicator for the assessment of organic water pollution. Jpn. J. Water Pollut. Res. 1986, 19, 7–11, (In Japanese with English Summary). [Google Scholar]
- Sládeček, V. System of water quality from the biological point of view. Arch. Hydrobiol. 1973, 7, 1–218. [Google Scholar]
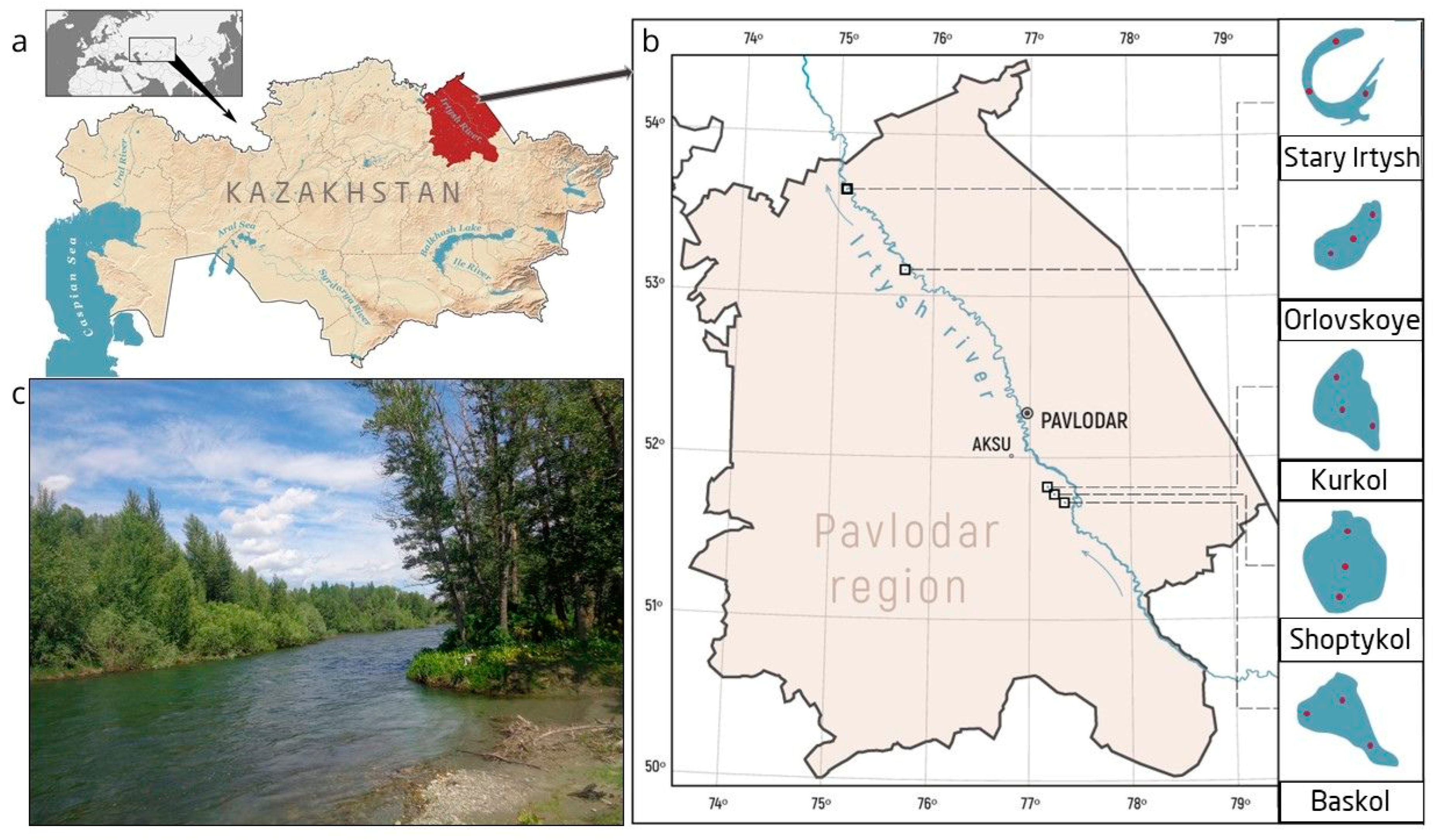












| Variable | Baskol | Shoptykol | Kurkol | Orlvoskoye | Stary Irtysh |
|---|---|---|---|---|---|
| Abbreviation | BA | SH | KU | OR | SI |
| Coordinates | N 51.726238 E 77.353618 | N 51.470083 E 77.144481 | N 51.826111 E 77.177222 | N 53.154167 E 75.733611 | N 53.641389 E 75.115556 |
| Area, km2 | 1.03 | 1.68 | 1.13 | 0.82 | 0.42 |
| Altitude above the sea level, m | 117 | 114 | 113 | 85 | 82 |
| Depth, m | 1.5–1.7 | 1.5–2.2 | 1.5–2.0 | 2.0 | 1.5 |
| Transparency, m | 1.0–1.3 | 0.3–0.5 | 2.0 | 2.0 | 0.3 |
| Bottom sediments | black and grey silt | grey silt | black silt | black sludge with a smell of hydrogen sulphide | black silt |
| Aquatic and coastal vegetation | reed | pondweed, cattails, reed | cattails, reed | reed, elodea, pondweed, hornwort | reed, water lily |
| Variable | Baskol | Shoptykol | Kurkol | Orlovskoye | Stary Irtysh | ||
|---|---|---|---|---|---|---|---|
| 2024 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | |
| Temperature, °C | 23.5 ± 0.15 | 19.7 ± 0.35 | 21.5 ± 0.23 | 20.5 ± 0.11 | 22.2 ± 0.30 | 13.3 ± 0.10 | 23.4 ± 0.56 |
| O2, mg L−1 | 8.54 ± 0.79 | 7.98 ± 0.15 | 5.49 ± 1.14 | 10.94 ± 0.38 | 12.61 ± 0.86 | 9.62 ± 0.58 | 12.56 ± 0.40 |
| pH | 6.74 ± 1.68 | 8.53 ± 0.08 | 8.30 ± 0.08 | 8.46 ± 0.008 | 8.59 ± 0.45 | 7.43 ± 0.08 | 7.99 ± 0.02 |
| Na+ + K+, mg L−1 | 67.50 ± 1.32 | 75.33 ± 7.29 | 28.83 ± 5.71 | 24.17 ± 1.01 | 43.00 ± 2.25 | 22.17 ± 1.64 | 23.3 ± 1.83 |
| Ca, mg L−1 | 40.08 ± 1.15 | 33.73 ± 1.46 | 30.06 ± 0.58 | 27.72 ± 0.84 | 22.71 ± 3.54 | 18.71 ± 0.67 | 26.05 ± 1.16 |
| Mg, mg L−1 | 22.29 ± 0.40 | 15.00 ± 1.07 | 11.15 ± 0.54 | 7.09 ± 0.54 | 13.98 ± 0.61 | 6.69 ± 0.35 | 12.56 ± 1.07 |
| HCO3, mg L−1 | 207.47 ± 1.76 | 256.29 ± 18.64 | 138.31 ± 5.38 | 136.28 ± 4.07 | 91.46 ± 25.39 | 101.70 ± 2.03 | 138.31 ± 4.07 |
| SO4, mg L−1 | 99.97 ± 0.83 | 29.30 ± 1.12 | 27.00 ± 4.79 | 23.87 ± 0.19 | 10.00 ± 7.21 | 23.76 ± 0.45 | 29.63 ± 0.76 |
| Cl, mg L−1 | 39.72 ± 0.61 | 39.73 ± 0.61 | 18.40 ± 0.00 | 6.62 ± 0.47 | 41.48 ± 1.74 | 7.57 ± 0.59 | 13.48 ± 0.00 |
| Hardness, mg L−1 | 3.80 ± 0.00 | 2.93 ± 0.03 | 2.38 ± 0.09 | 1.93 ± 0.03 | 2.25 ± 0.21 | 1.47 ± 0.03 | 2.40 ± 0.003 |
| TDS, mg L−1 | 479.03 ± 3.30 | 449.37 ± 26.07 | 253.70 ± 10.56 | 225.75 ± 4.60 | 268.93 ± 18.45 | 180.58 ± 4.29 | 243.36 ± 4.39 |
| PI, mgO L−1 | 22.62 ± 0.63 | 15.21 ± 0.41 | 18.58 ± 0.40 | 11.09 ± 0.48 | 13.33 ± 0.23 | 9.99 ± 0.27 | 8.62 ± 0.82 |
| N-NO3, mg L−1 | 0.250 ± 0.159 | 0.200 ± 0.135 | 0.615 ± 0.276 | 0.201 ± 0.142 | 0.010 ± 0.010 | 0.201 ± 0.111 | 0.0003 ± 0.0003 |
| N-NO2, mg L−1 | 0.038 ± 0.003 | 0.040 ± 0.009 | 0.009 ± 0.004 | 0.018 ± 0.009 | 0.065 ± 0.011 | 0.032 ± 0.010 | 0.060 ± 0.018 |
| N-NH4, mg L−1 | 0.957 ± 0.338 | 0.469 ± 0.084 | 0.088 ± 0.021 | 0.212 ± 0.021 | 1.084 ± 0.077 | 0.160 ± 0.005 | 0.898 ± 0.180 |
| Nitrogen Sum, mg L−1 | 1.245 ± 0.347 | 0.708 ± 0.221 | 0.711 ± 0.300 | 0.431 ± 0.156 | 1.159 ± 0.071 | 0.394 ± 0.099 | 0.959 ± 0.189 |
| PO4, mg L−1 | 0.210 ± 0.006 | 0.025 ± 0.006 | 0.088 ± 0.023 | 0.019 ± 0.002 | 0.027 ± 0.003 | 0.01 ± 0.00 | 0.038 ± 0.006 |
| P-PO4, mg L−1 | 0.068 ± 0.002 | 0.008 ± 0.002 | 0.029 ± 0.008 | 0.006 ± 0.001 | 0.009 ± 0.001 | 0.003 ± 0.000 | 0.012 ± 0.002 |
| Phylum | Kurkol | Orlovskoye | Stary Irtysh |
|---|---|---|---|
| Abundance, thou. cells L−1 | |||
| Charophyta | 7.7 ± 2.2 | 10.0 ± 5.7 | 16.6 ± 12 |
| Chlorophyta | 6181.1 ± 1364.8 | 1151.1 ± 536.5 | 2083.8 ± 225.3 |
| Cyanobacteria | 162,546.6 ± 46,675.8 | 2584.4 ± 1315.5 | 16,045.5 ± 79.8 |
| Dinoflagellata | 0.0 ± 0.0 | 40.5 ± 14.7 | 173.3 ± 46.6 |
| Euglenophyta | 11.6 ± 6.7 | 11.6 ± 1.6 | 60.0 ± 30.7 |
| Heterokontophyta | 213.8 ± 57.7 | 190.5 ± 92.1 | 266.6 ± 78.1 |
| Total | 168,961.1 ± 48,076.8 | 3984.4 ± 1922.1 | 18,646.1 ± 348.3 |
| Biomass, mg L−1 | |||
| Charophyta | 0.0 ± 0.0 | 0.01 ± 0.01 | 0.02 ± 0.02 |
| Chlorophyta | 5.87 ± 1.48 | 0.42 ± 0.17 | 1.16 ± 0.26 |
| Cyanobacteria | 76.88 ± 18.53 | 0.55 ± 0.31 | 3.52 ± 0.54 |
| Dinoflagellata | 0.00 ± 0.00 | 1.45 ± 0.53 | 6.77 ± 1.73 |
| Euglenophyta | 0.44 ± 0.22 | 0.04 ± 0.02 | 0.87 ± 0.19 |
| Heterokontophyta | 0.46 ± 0.18 | 0.24 ± 0.13 | 0.40 ± 0.04 |
| Total | 83.67 ± 19.71 | 2.71 ± 1.06 | 12.70 ± 1.83 |
| Phylum | Baskol | Shoptykol | Kurkol | Orlovskoye |
|---|---|---|---|---|
| Abundance, thou. cells L−1 | ||||
| Charophyta | 1.7 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Chlorophyta | 23,130.0 ± 13,699.5 | 5503.3 ± 1030.9 | 476.1 ± 33.7 | 940.5 ± 240.8 |
| Cyanobacteria | 1396.1 ± 348.1 | 20,341.6 ± 5550.3 | 1175.0 ± 340.0 | 4061.6 ± 423.5 |
| Dinoflagellata | 0.0 ± 0.0 | 0.0 ± 0.0 | 4.4 ± 2.9 | 6.1 ± 4.4 |
| Euglenophyta | 0.0 ± 0.0 | 137.7 ± 66.9 | 7.7 ± 1.1 | 25.0 ± 12.5 |
| Heterokontophyta | 83.8 ± 40.7 | 20.0 ± 11.5 | 1638.3 ± 239.1 | 278.3 ± 40.5 |
| Total | 24,611.7 ± 13,610.3 | 26,002.7 ± 6508.8 | 3301.6 ± 583.5 | 5311.6 ± 621.5 |
| Biomass, mg L−1 | ||||
| Charophyta | 0.04 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Chlorophyta | 50.24 ± 30.04 | 4.70 ± 1.08 | 0.55 ± 0.05 | 1.25 ± 0.74 |
| Cyanobacteria | 1.50 ± 0.44 | 7.85 ± 2.20 | 1.18 ± 0.30 | 0.78 ± 0.19 |
| Dinoflagellata | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.67 ± 0.44 | 0.47 ± 0.34 |
| Euglenophyta | 0.00 ± 0.00 | 4.89 ± 2.44 | 0.14 ± 0.02 | 0.95 ± 0.80 |
| Heterokontophyta | 0.45 ± 0.30 | 0.04 ± 0.03 | 4.25 ± 0.44 | 3.44 ± 0.22 |
| Total | 52.22 ± 30.09 | 17.48 ± 5.16 | 6.78 ± 0.94 | 6.90 ± 0.93 |
| Lake * | Year | Species Name | Share, % | Lake * | Year | Species Name | Share, % | ||
|---|---|---|---|---|---|---|---|---|---|
| Abundance | Biomass | Abundance | Biomass | ||||||
| BA | 2024 | V. globator | 92.5 | 95.2 | OR | 2024 | C. kuetzingianum | 11.0 | 0.4 |
| SH | 2024 | M. aeruginosa | 13.3 | 27.3 | M. pulverea | 21.7 | 0.8 | ||
| M. pulverea | 16.5 | 1.5 | F. capucina | 1.5 | 14.1 | ||||
| P. longicauda | 0.1 | 19.9 | U. ulna | 0.5 | 14.9 | ||||
| KU | 2023 | M. aeruginosa | 50.8 | 81.6 | SI | 2023 | A. clathrata | 10.9 | 0.2 |
| M. pulverea | 21.1 | 0.3 | Ap. flos-aquae | 10.8 | 0.3 | ||||
| 2024 | A. formosa | 18.8 | 15.2 | M. aeruginosa | 10.1 | 11.8 | |||
| D. sertularia | 30.3 | 45.8 | M. pulverea | 13.4 | 0.1 | ||||
| OR | 2023 | M. pulchellum | 13.9 | 3.5 | C. hirundinella | 0.4 | 22.6 | ||
| S. atomus | 19.4 | 1.5 | P.cinctum | 0.3 | 29.4 | ||||
| C. hirundinella | 1.0 | 53.6 | |||||||
| Lake | Year | Average Number of Species | Shannon-Ab | Shannon-Bi | Average Mass (Volume) of the Cell, mg 10−6 |
|---|---|---|---|---|---|
| Baskol | 2024 | 11.6 ± 0.3 | 0.84 ± 0.38 | 0.62 ± 0.30 | 2.05 ± 0.08 |
| Shoptykol | 2024 | 42.6 ± 2.0 | 3.99 ± 0.09 | 3.29 ± 0.18 | 0.66 ± 0.04 |
| Kurkol | 2023 | 33.3 ± 1.2 | 2.39 ± 0.18 | 1.26 ± 0.20 | 0.51 ± 0.05 |
| 2024 | 25.6 ± 1.4 | 3.24 ± 0.06 | 2.66 ± 0.06 | 2.11 ± 0.18 | |
| Orlovskoye | 2023 | 27.6 ± 5.3 | 3.42 ± 0.30 | 2.53 ± 0.27 | 0.81 ± 0.14 |
| 2024 | 34.6 ± 2.1 | 3.92 ± 0.06 | 3.36 ± 0.24 | 1.32 ± 0.19 | |
| Stary Irtysh | 2023 | 36.3 ± 1.2 | 3.86 ± 0.09 | 3.11 ± 0.13 | 0.68 ± 0.09 |
| Variable | Baskol | Shoptykol | Kurkol | Orlovskoye | Stary Irtysh | ||
|---|---|---|---|---|---|---|---|
| 2024 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | |
| P-PO4, mg L−1 | 0.068 ± 0.002 | 0.008 ± 0.002 | 0.029 ± 0.008 | 0.006 ± 0.001 | 0.009 ± 0.001 | 0.003 ± 0.000 | 0.012 ± 0.002 |
| Class of water quality | 3 | 2 | 2 | 2 | 2 | 1 | 2 |
| Trophic state | hypereutrophic | eutrophic | eutrophic | meso-trophic | eutrophic | oligo-trophic | eutrophic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, E.; Argynbayeva, Y.; Barinova, S.; Romanova, S. The Role of Phytoplankton in the Assessment of the Ecological State of the Floodplain Lakes of the Irtysh River, Kazakhstan. Environments 2025, 12, 322. https://doi.org/10.3390/environments12090322
Krupa E, Argynbayeva Y, Barinova S, Romanova S. The Role of Phytoplankton in the Assessment of the Ecological State of the Floodplain Lakes of the Irtysh River, Kazakhstan. Environments. 2025; 12(9):322. https://doi.org/10.3390/environments12090322
Chicago/Turabian StyleKrupa, Elena, Yerkezhan Argynbayeva, Sophia Barinova, and Sophia Romanova. 2025. "The Role of Phytoplankton in the Assessment of the Ecological State of the Floodplain Lakes of the Irtysh River, Kazakhstan" Environments 12, no. 9: 322. https://doi.org/10.3390/environments12090322
APA StyleKrupa, E., Argynbayeva, Y., Barinova, S., & Romanova, S. (2025). The Role of Phytoplankton in the Assessment of the Ecological State of the Floodplain Lakes of the Irtysh River, Kazakhstan. Environments, 12(9), 322. https://doi.org/10.3390/environments12090322







