Abstract
Biochar (BC), a carbonaceous material derived from biomass pyrolysis, exhibits a wide range of physicochemical properties, including a high cation exchange capacity, porosity, and specific surface area, which make it a highly valuable amendment for soil enhancement and environmental sustainability. As BC has shown strong potential to remediate soils, enhance their fertility, and increase crop productivity, it can successfully be used as a soil remediation factor. Additionally, it can play a critical role in carbon sequestration and climate change mitigation, revealing a high sorption capacity, multifunctionality, and long-term persistence in soils, where it can remain stable for hundreds to thousands of years. The present systematic review aims at presenting the dynamics of BC when incorporated into a soil system, focusing on its pH, water-holding capacity, aeration, microbiota, and carbon and nutrient availability across various case studies, particularly in acid, saline/sodic, and heavy metal-contaminated soils. Given the variability in BC performance, robust, long-term field-based research is essential to validate the current findings and support the development of targeted and sustainable biochar applications.
1. Introduction
Soils are directly and indirectly the basis for the supply of ecosystem services, including food production, which is indispensable for human prosperity [1,2]. According to the Food and Agriculture Organization (FAO), 95% of the world’s food is produced directly or indirectly through soil-based processes [3]. High-quality soil supports nutrient cycling, agricultural production, and biodiversity conservation [4]. However, one-third of this valuable resource has degenerated to a considerable extent because of erosion–desertification, nutrient replenishment, loss of organic carbon, compaction, acidification, and salinization [3].
In addition, a range of anthropogenic activities such as pollution, waste discarding, and land use/land cover (LU/LC) changes further deteriorate the properties of the soil. Population growth, sectoral developments, and shifts in the waste produced, reported during epidemics, have resulted in a dynamic increase in environmental pollution [5]. The degradation of soil has intensified due to the input of rising quantities of pollutants such as polycyclic aromatic hydrocarbons (PAHs), persistent chemicals, heavy metals, and engineered nanomaterials [6,7]. An additional environmental and economic burden enhancing the nutrient imbalance of soils is the ongoing “fertilizer crisis”. The excessive use of chemical fertilizers and the lack of knowledge on their optimal application have worsened soil degradation and caused severe spot and diffuse pollution, which can be critical on a catchment scale [8,9]. Moreover, chemical fertilizers often exhibit low efficiency since nutrients are not in a form directly accessible to plants. Additionally, their production demands high energy input while failing to compensate for the loss of organic matter caused by humus degradation.
An indicative example of the extent of soil degradation nowadays is demonstrated by the European Soil Data Centre (ESDC) Dashboard, where it is presented that 62% of European soil is degraded [10]. According to the above, a number of countries in Central and Southern Europe face threats to the biological functions of their soil, with a peak value of 77.5% in the Netherlands, where nearly 60% of the land is used for agriculture. Additionally, phosphorus deficiency (<20 mg kg−1) is found in large regions of Southern Europe, including Portugal (>64.4%), Spain, Slovenia, and Greece (>53.5%). On the other hand, nitrogen surplus (>50 kg ha−1) is a major issue in Ireland (>79.6%), the Netherlands (>87.4%), and Luxemburg (>85.6). Nutrient imbalance in soils strengthens the link between soil degradation and fertility loss through limited nutrient use efficiency (NUE, PUE, KUE, etc.) by plants [5,11]. Moreover, a recent study reported that unamended drought-tolerant millet plots observed a 37% yield reduction per cm of topsoil loss [12,13].
Beyond direct human activities, climate change can lead to soil erosion, degradation, and desertification, endangering the sustainability of several ecosystems. Therefore, along with farming practices unfavorable to soil conservation, land use/land cover (LU/LC) changes, and pollution, the human-induced climate crisis also aggravates soil quality, afflicting soil fertility and crop productivity. The encapsulated soil organic carbon (SOC) that is liberated with soil erosion can be emitted into the atmosphere in the form of carbon dioxide (CO2), increasing the concentrations of greenhouse gases (GHGs). The aforementioned highlights the necessity for sustainable solutions for soil amendment.
One emerging solution is biochar (BC), a carbon-based material produced by pyrolysis of biomass under anaerobic conditions, which has attracted considerable attention as a soil amendment. Its composition includes elements such as nitrogen (N), potassium (K), and phosphorus (P), which serve as nutrients for plant growth, while its carbon content ameliorates the organic matter concentration of soils. BC is very stable and recalcitrant in soil [6]. Its physicochemical properties are favorable for barren soil mitigation and microbiota increase. These properties place biochar as a promising option to enhance soil health and productivity of degraded soils [14,15].
Although previous reviews have addressed various aspects regarding biochar use in soils, this review attempts to gain insight into the complex dynamics accompanying BC application to soil by reviewing the latest advances in the literature and the emerging developments on former limitations and providing discernment of its multiple benefits and potential. This review aims to move beyond generalizations and offer insight for agricultural and agroforestry managers, as well as policymakers, in adopting good practices and stipulating guidelines regarding the establishment of BC as a sustainable soil amendment.
2. Materials and Methods
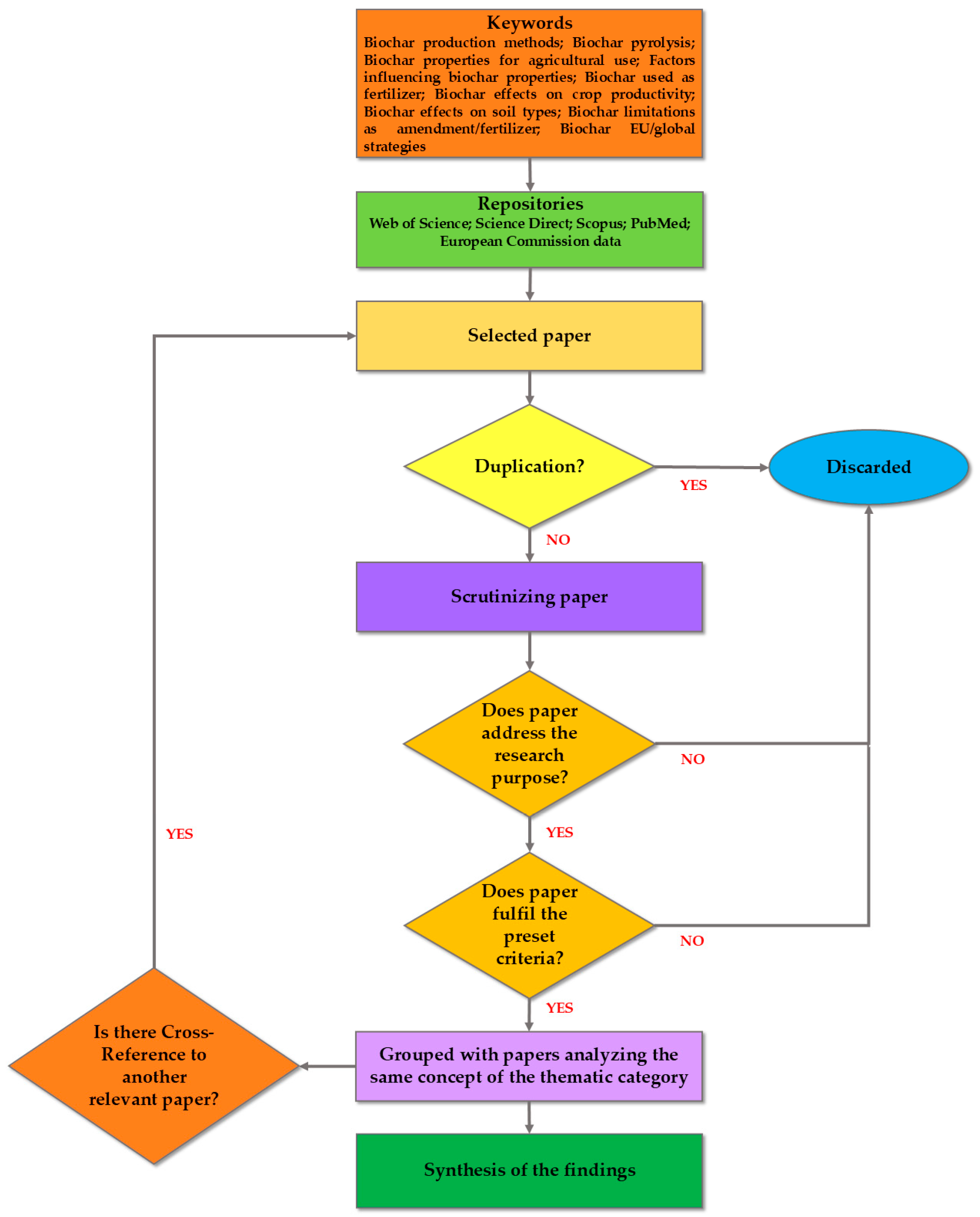
The methodology of this review is based on the criteria of a systematic review, often followed in the waste treatment field and broader environmental research [16,17], developed by Boaz et al. [18]. The review methodology incorporates the following steps: Firstly, the survey of the literature was based on specific criteria to ensure that only relevant and high-quality studies were considered. Research articles were retrieved from trustworthy sources such as the following scientific repositories: Web of Science (https://www.webofscience.com/wos/woscc/smart-search, accessed on 5 February 2025); Science Direct (https://www.sciencedirect.com/, accessed on 5 February 2025); Scopus (https://www.scopus.com/pages/home?display=basic&zone=header&origin=sbrowse#basic, accessed on 6 February 2025); PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 6 February 2025); and European Commission data (https://ec.europa.eu/eurostat/data/database, accessed on 7 February 2025). The advances in the subject matter (i.e., biochar as a soil amendment) were elaborated based on up-to-date studies where BC implementations are discussed. The retrieved studies were then scrutinized, and their methods and references were also checked, to ensure their validity. A large part of the articles employed was obtained through the snowball method, ensuring that they fulfilled the criteria described. A number of articles were discarded during the process, when one of the criteria was not met. Figure 1 depicts the main steps of the review process.
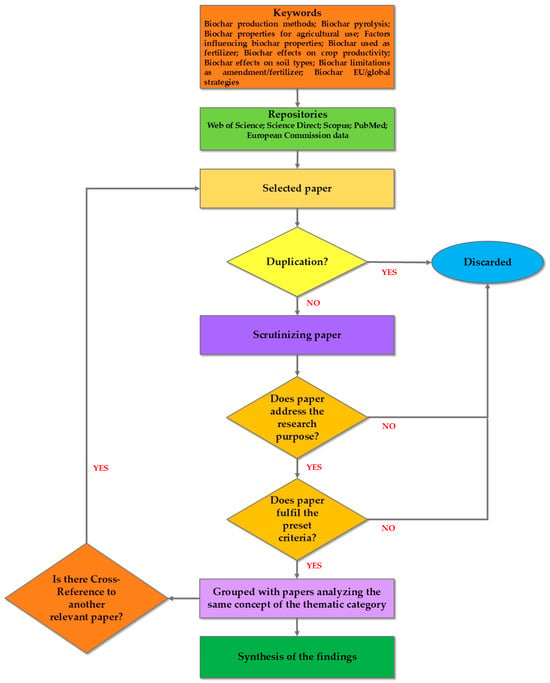
Figure 1.
Indicative flow chart of the systematic review process.
The next step was the determination of the research questions of the review, to organize the thematic axes as paragraphs: “What are the latest environmental applications of BC?” “What are the recent advantages of BC implementation in soil?” “Is there quantifiable evidence that the described BC treatment is beneficial to soil and plants?” “What are the limitations or side effects of BC treatment and what are the reasons behind them?” “What are the emerging mechanisms and interactions that should be taken under consideration when apply BC aiming at a beneficial use?” “What are the policies and global strategies BC fits in?”
The following step was identifying the studies employed. Specifically, 171 peer-reviewed articles were studied. These studies were cross-referenced, and those with similar objectives were combined to form a cohesive synthesis within thematic paragraphs. Any conflicting results were included in each thematic paragraph. The subsequent step was to discuss the main advances towards the sustainable future employment of BC presented in the thematic paragraphs. Verification regarding the deductions of the review was achieved with multiple up-to-date references, and lastly, emphasis was placed on maintaining the relevance of the incorporated information by structuring thematic paragraphs that provide the latest developments in each subject and promote this ability sufficiently.
3. Biochar Meets European and Global Roadmap Targets
3.1. Degraded and Problematic Soils Across Europe
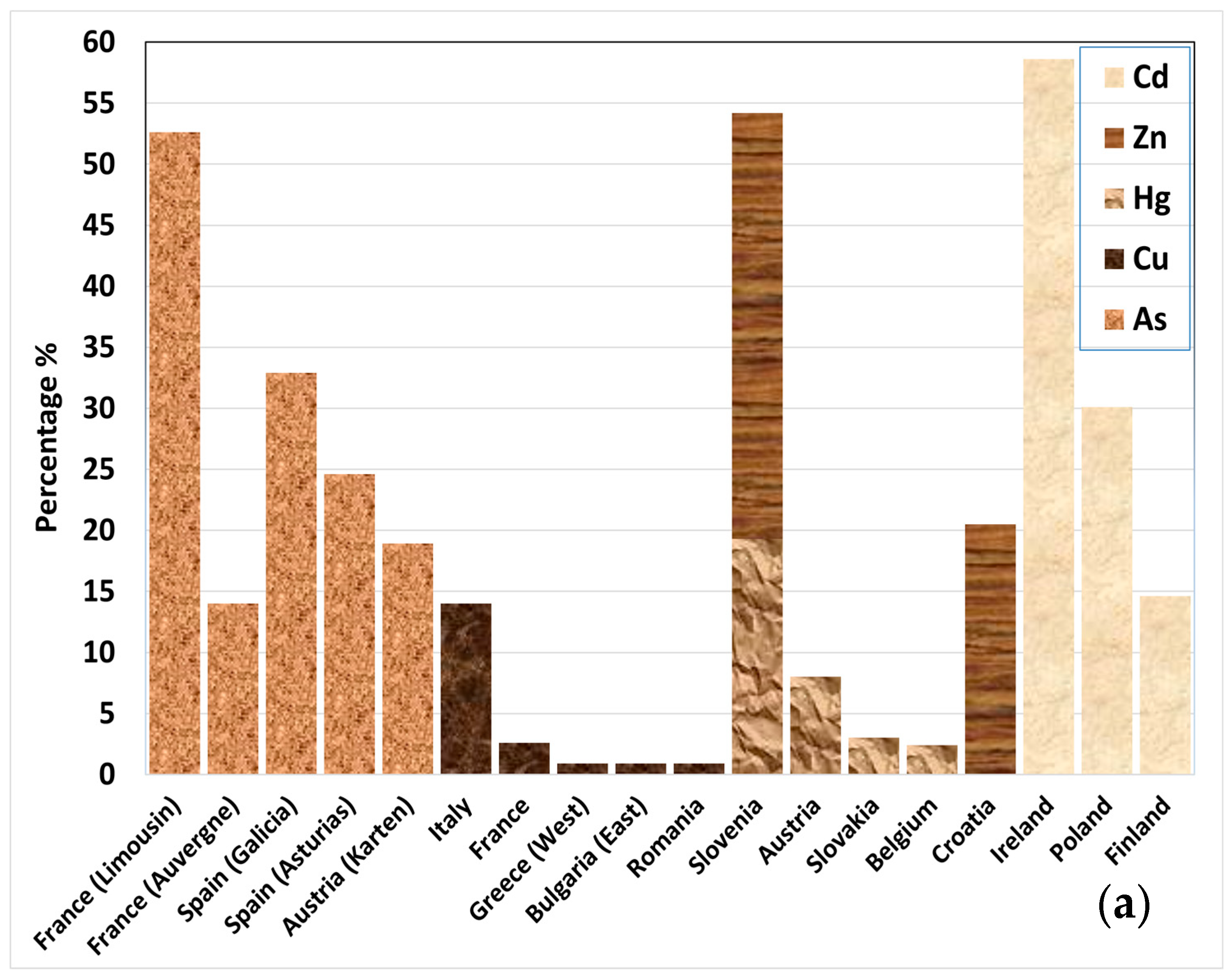
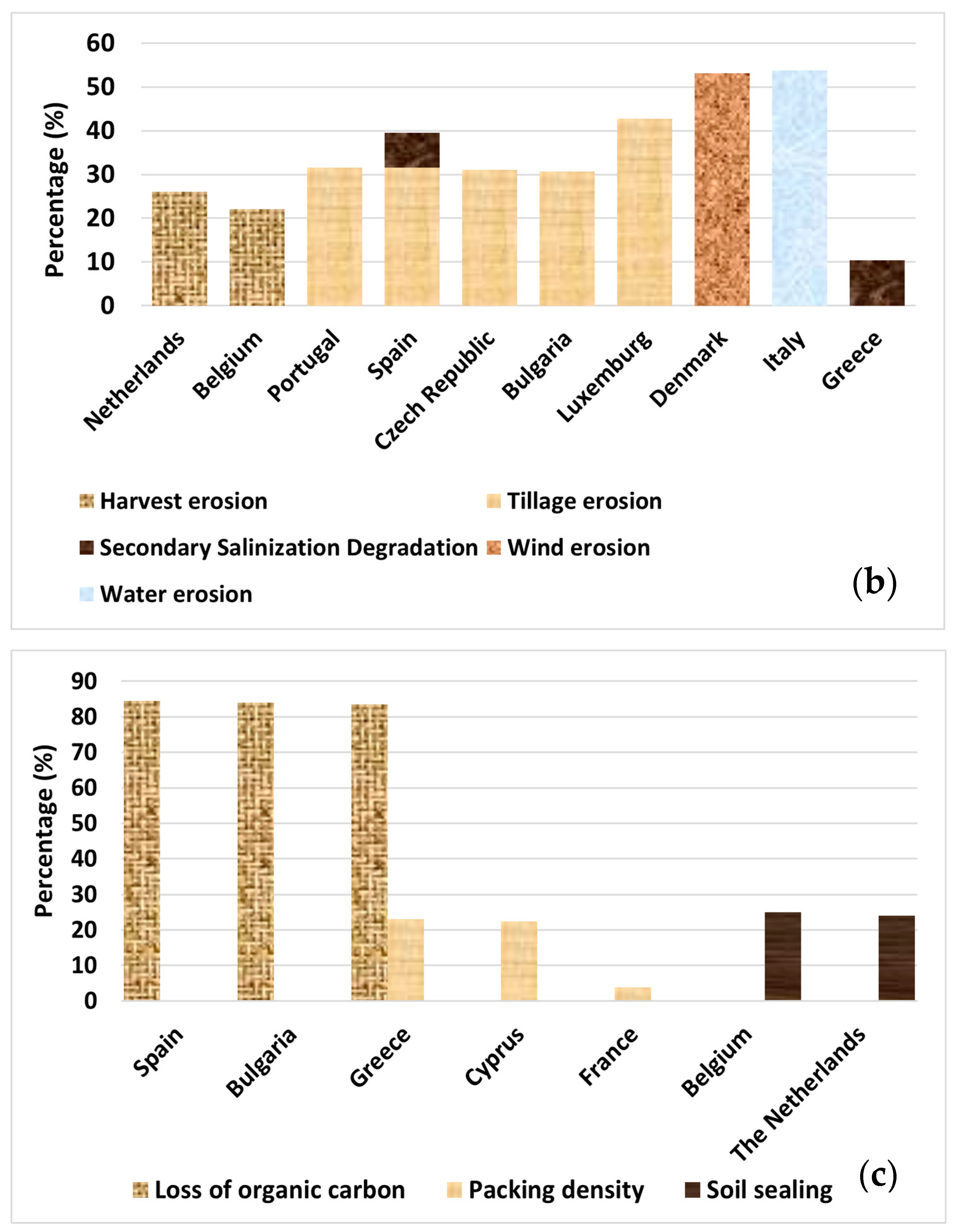
Problematic soils in Europe are defined by their poor physicochemical and biological properties that hinder or prevent plant growth [19]. These soils are often the result of long-term degradation processes, including erosion, pollution, and radical changes in their initial composition, mainly driven by anthropogenic activities. The European Soil Data Centre (ESDAC) monitors heavy metal pollution, nutrient imbalance, (harvest, tillage, wind, and water) erosion, loss of organic carbon, loss of biodiversity, salinization, and (soil) sealing of European land [10,20]. The European areas with problematic soil are depicted in Figure 2 [10,20,21,22,23,24,25,26,27,28,29].
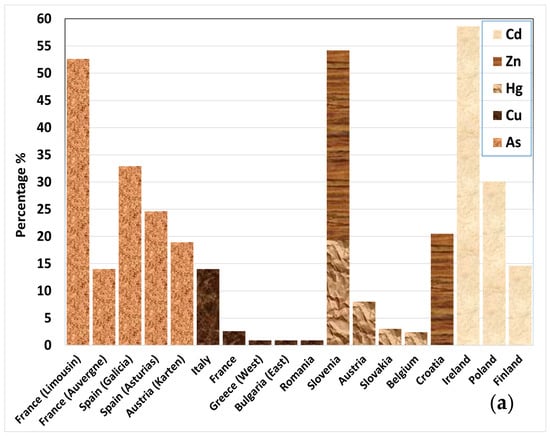
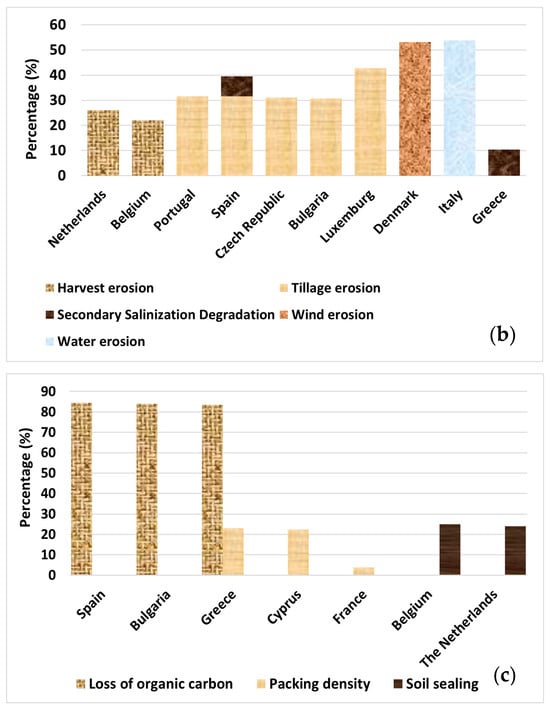
Figure 2.
Percentage (%) of area with (a) probability of arsenic (As) concentration exceeding 45 mg kg−1, higher than 5%; concentration levels exceeding indicative thresholds for copper (Cu; 50 mg kg−1), mercury (Hg; 200 μg kg−1), cadmium (Cd; 0.7 mg kg−1), and zinc (Zn; 65 mg kg−1) (note: thresholds used by EU Soil Observatory, based on EU Sewage Sludge Directive and LUCAS survey for topsoil [10]). (b) Erosion rates of all types exceeding the sustainable baseline of >2 t ha−1 yr−1 and salinization (for areas where >30% is equipped with irrigation). (c) Loss of organic carbon expressed by the variable “the distance to maximum SOC level > 60%”, Packing Density > 1.75 g cm−3, and soil sealing.
Erosion is another critical factor affecting soil health. Using a sustainable baseline of >2 t ha−1 yr−1 for all types of erosion, the ESDAC estimated high erosion rates exceeding 5 t ha−1 yr−1 (>5.1%) in some areas [26,27,28]. Such extensive soil loss not only reduces the quality of land but also changes how water moves through the land and increases vulnerability to desertification. Degradation due to pollution is obvious in Ireland, Slovenia, and France, with elevated levels of heavy metals such as Cd, As, and Cu, followed by Poland and Spain [20,21,22,23]. Overall, it has been found that 89% of agricultural land and 35.7% of forest areas have unhealthy soil, based on degradation processes [20]. These data highlight the urgent need for sustainable soil management strategies across Europe and worldwide.
3.2. Biochar in Global Mitigation Strategies
3.2.1. BC Application Meets the Targets of European and Global Strategies
Soil quality is considered a matter of the utmost importance on the EU agenda. The recent mission “A Soil Deal for Europe” has set the ambitious goal for a radical transition from the current 62% problematic soils to 75% healthy soils “for food, people, nature and climate” by 2030 [30]. Soil mitigation is undoubtedly the linchpin of all individual strategies of the EU (Table 1). The transition to sustainable crop nutrition, soil management, and restoration is not only a European but a global concern. Our planet has lost one-third of its arable land during the past 40 years due to erosion and pollution [19]. Therefore, nutrient imbalances and soil pollution are key targets of global guidelines (Table 1) where BC can play a pivotal role (elaborated in Section 6, Section 7, and Section 9).

Table 1.
European and global strategies in which widespread BC use could substantially contribute.
3.2.2. BC in Agriculture Reduces GHG Emissions
BC could play a pivotal role in a global strategy to achieve the 2030 and 2050 targets, not only by capturing and sequestering carbon, but also by reducing GHG emissions from soils following its application (Table 2).

Table 2.
The positive effect of BC treatment in agriculture on GHG emissions.
In recent studies, as summarized in Table 2, BC application has been associated with a 12–50% decrease in non-CO2 GHG emissions, depending on the soil type and management practices [42]. Moreover, combining BC with inorganic fertilizers [44] results in the lowest CO2 emissions from soil. Raza et al. [46] studied the use of biochar in waste management, particularly in vermicomposting, which led to notably lower emissions of CH4, N2O, and NH3. Overall, these findings underscore biochar’s role as a practical tool for reducing agriculture emissions globally.
4. Biomass Influence on Biochar Characteristics
4.1. Differentiations in Properties
A crucial parameter determined by the target purpose is the type of biomass feedstock used for BC production [48]. As presented in Table 3, both the content and physicochemical properties of BC depend on the raw material used. These properties face alterations induced by pyrolysis temperature [19].
The selection of the raw material requires deep insight into several mechanisms and stresses that soils undergo, the responses of different plants to the same BC, and complex relations among physicochemical and biological variables [42]. For example, BC originating from plant residues (e.g., from rice husk and sugar cane) tends to have high ash content and alkalinity, raising the soil pH and making it suitable for acidic or heavy metal-contaminated soil [49]. In contrast, BC produced from livestock biomass (manure, etc.) contains more functional groups, improving sorption capacity, and is typically more acidic (Table 3). Moreover, corn straw and waterweed biomasses produce BCs with high CEC values, which enhance nutrient retention and microbial activity, particularly in degraded soils [19,42]. Woody feedstocks, on the other hand, like pecan shells or wood pellets, are rich in carbon and appear to have improved water retention [42]. Therefore, optimizing BC properties through appropriate biomass and pyrolysis temperature is essential for maximizing the benefits in polluted soils.

Table 3.
Biomass properties’ effects on the produced BC.
Table 3.
Biomass properties’ effects on the produced BC.
| Biomass Source | Key Properties | Pyrolysis Temperature | Effects on BC |
|---|---|---|---|
| Tea Waste | High nitrogen (N) content; beneficial for promoting soil fertility. | 300 and 500 °C | N content: 2.46–2.61% [48] |
| Sugar Cane | Elevated ash content. | 600 °C | Ash content: 15% [5] |
| Rice Husk | High ash content. | 400 °C | Ash content: 49% [5] |
| Corn Straw | Cation exchange capacity (CEC) improves with pyrolysis temperature. | 300 °C | CEC at 300 °C: 183 cmol kg−1 [19] |
| 700 °C | CEC at 700 °C: 210 cmol kg−1 [19] | ||
| Waterweeds | High CEC; suitable for improving soil nutrient retention and fertility. | 500 °C | CEC: 509 cmol kg−1 [7,19] |
| Wood Chips | High nitrogen (N) content and low CEC, which limits cation retaining ability. | 550 °C | N content: 480.3 g kg−1; CEC: 9 cmol kg−1 [19] |
| Wood Pellets | High carbon (C) content; long-lasting benefits to soils. | 500 °C | Carbon content: 800 g kg−1 [19] |
| Pecan Shells (Pyrolyzed) | High carbon (C) content; suitable for improving soil organic matter and water retention. | 700 °C | Carbon content: 834 g kg−1 [42] |
| Sewage Sludge (SSB) | Contamination concerns (pathogens, heavy metals, PAHs) limit widespread use without proper treatment. | High temperature and time reduce PAHs | Can help in neutralizing acidic soils; improving EC; and increasing microbial and enzymatic activity [50,51,52,53] |
| Co-pyrolyzed SSB + Ash | Increased bioavailable phosphorus (P); enhanced surface area; ideal for agricultural applications; P recovery; SS recycling. | 600 °C | P bioavailability reached 92 wt%; potential as P and K slow-release fertilizer; improved adsorption properties compared to SSB alone [51,54] |
| Pyrolyzed SSB + Carrier Gas | Carrier gas during pyrolysis and cooling phase eliminates micropollutants (below their detection limits) except Cu and Zn. | 650 °C | The produced SSB adheres to the guidelines set by the International Biochar Initiative (IBI) [55] |
4.2. Sewage Sludge Biochar (SSB)
Agricultural remnants have been thoroughly discussed as BC feedstocks. The use of sewage sludge biochar (SSB) as a soil amendment has recently attracted attention. In both Europe and the US, treated sewage sludge is utilized in the construction material industry, agriculture (reuse), and landfills (disposal) [50,56,57]. SSB, or alternatively biosolid-derived biochar (BDB), has been employed as a cement replacement material [58]. Utilizing SSB as a source of phosphorus (P) for soil revitalization would be an upgraded step in waste management in the frame of the circular economy.
Studies on SSB’s impact on soil and crops still present inconsistent findings. Although large-amount SSB application was associated with inhibitory effects on corn plant growth in alkaline soil due to high background nutrient levels, a moderate rate of 1500 kg hm−2 was found to be significantly beneficial [59]. This rate broadens the usage of SSB for soil amendment and crop enhancement, minimizing health risks from corn consumption. Notably, crop productivity increased by over 50% compared to the fertilized control, while the immobilization of inorganic P in SSB was worth noting [59]. There are studies that report significantly decreased metal (Cd, Zn, Cu, and Pb) bioavailability in soil and an increase in crop yield after SSB application [60,61]. However, the heavy metals from the SS are retained in the SSB, and this is still the main concern regarding its large-scale usage. Schlederer et al., 2024 [55], deduced that specific treatments (i.e., pyrolysis temperature of 650 °C and the use of a carrier gas during the phases of pyrolysis and cooling) can be used to produce BC which conforms to the limits set by the International Biochar Initiative (IBI) and the European Biochar Certificate (EBC) regarding all micropollutants, except for Cu and Zn. The main methods in the literature for SSB production are pretreatment, high-temperature pyrolysis with a carrier gas, co-pyrolysis, and BC fabrication [52,55]. Therefore, scaling up SSB’s application as a soil amendment prerequires optimized and standardized production methods [62]. As a solid waste rich in P, SS constitutes an important renewable resource. The product of pyrolysis of SS, SSB, exhibits reduced water-extractable P, with slow release [63]. It is worth mentioning that BC application in medium- to fine-textured soils was reported to increase the available P content of the soil by 324%, with more pronounced effects at higher application rates (>20 t ha−1) [63]. In addition, BC application was found to improve the crop productivity of maize and wheat by more than 50% compared to unamended controls [63]. Therefore, SSB is a promising slow-release P fertilizer which provides long-term benefits for soil health and productivity.
5. Production Methods of Biochar
BC can also be produced by gasification, pyrolysis (slow, fast), or hydrothermal carbonization [64]. Gasification is a thermochemical process that favors syngas production, while a small percentage (usually around 10%) of BC is formed. Among them, pyrolysis is the most widely used process. Table 4 presents the latest production methods, grouping their main characteristics.

Table 4.
Production methods of biochar.
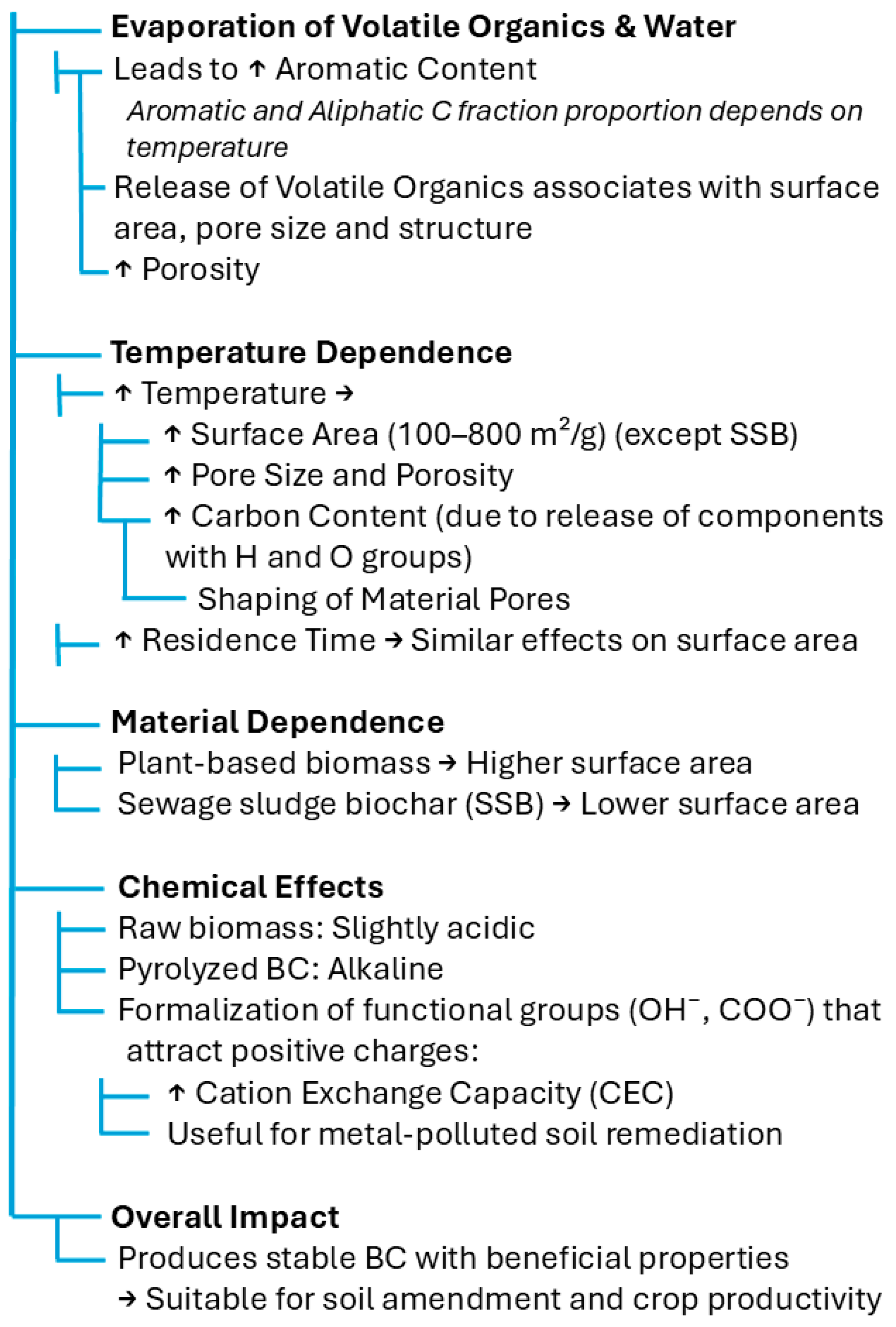
Both the temperature and duration of pyrolysis are determined based on the target purpose [68]. BC accumulates approximately half of the carbon content of its original feedstock. Due to its high content of aromatic carbon, it is recalcitrant to microbial decomposition, unlike uncharred biomass. The fact that BC is a refractory material makes it persist in soil for long periods (e.g., 1000–10,000 years), serving as an organic carbon sink [74], as presented in Figure 3.
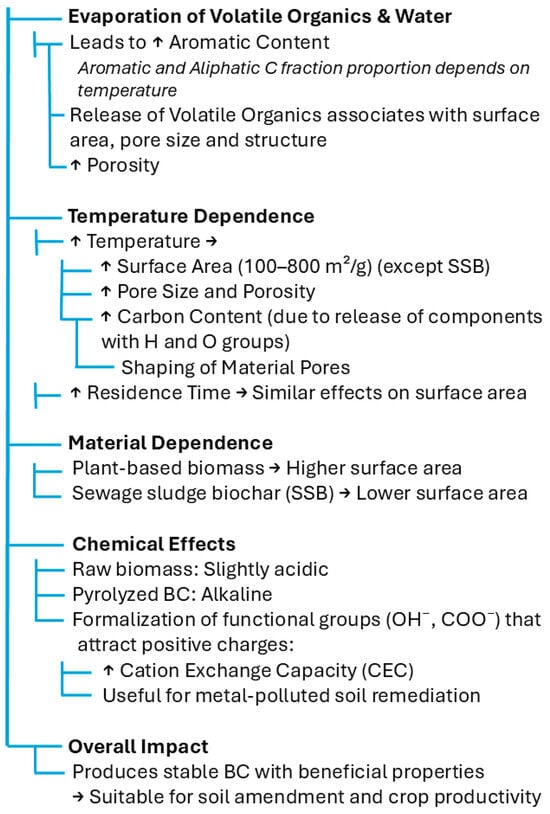
Figure 3.
Effect of pyrolysis on BC properties [6,75,76,77,78].
The potential soil type trend after BC treatment was another unclear parameter in the literature. Purakayastha et al. [79] investigated the effect of three BC types on three different soil orders: Inceptisol (alkaline soil with low SOM), Mollisol (alkaline soil with high SOM), and Alfisol (acidic soil with SOM). They carried out an incubation experiment for 290 days to acquire long-term results. No trend was found for pyrolysis temperature across soil type and biomass regarding the priming effect and nutrient availability, whereas the pH and EC were ameliorated. After a thorough review of the literature, the authors deduced that, in most cases, BC treatment raises the soil pH by between 0.1 and 2.0 units, for a wide variety of soil types with different native pH values [79].
6. Biochar and Soil Revitalization
According to Joseph et al. [42], BC undergoes three main transformation stages after its incorporation into soil: the dissolution stage (1–3 weeks), the stage of reactive surface development (1–6 months), and the aging stage. During these phases, BC contributes significantly to soil improvement in both physical (Figure 4) [5,80] and biological (Figure 5) aspects.
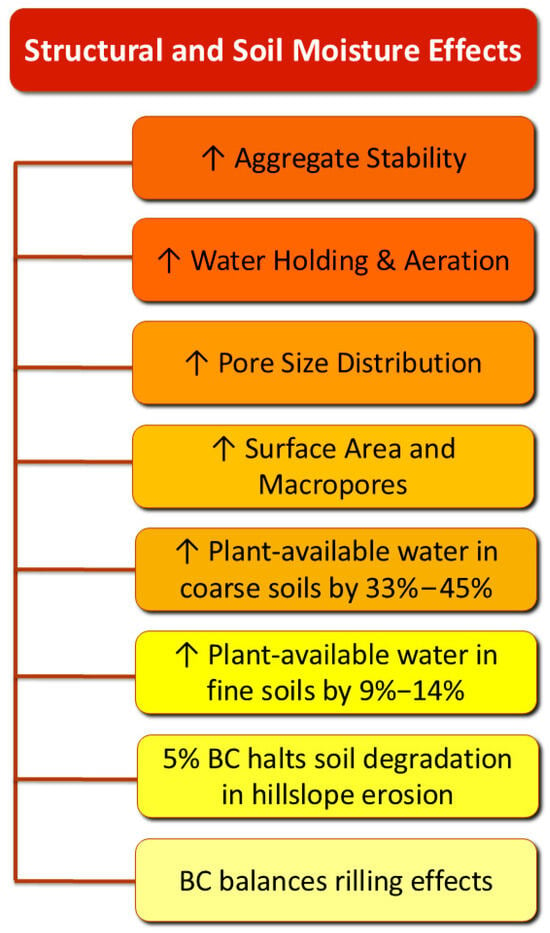
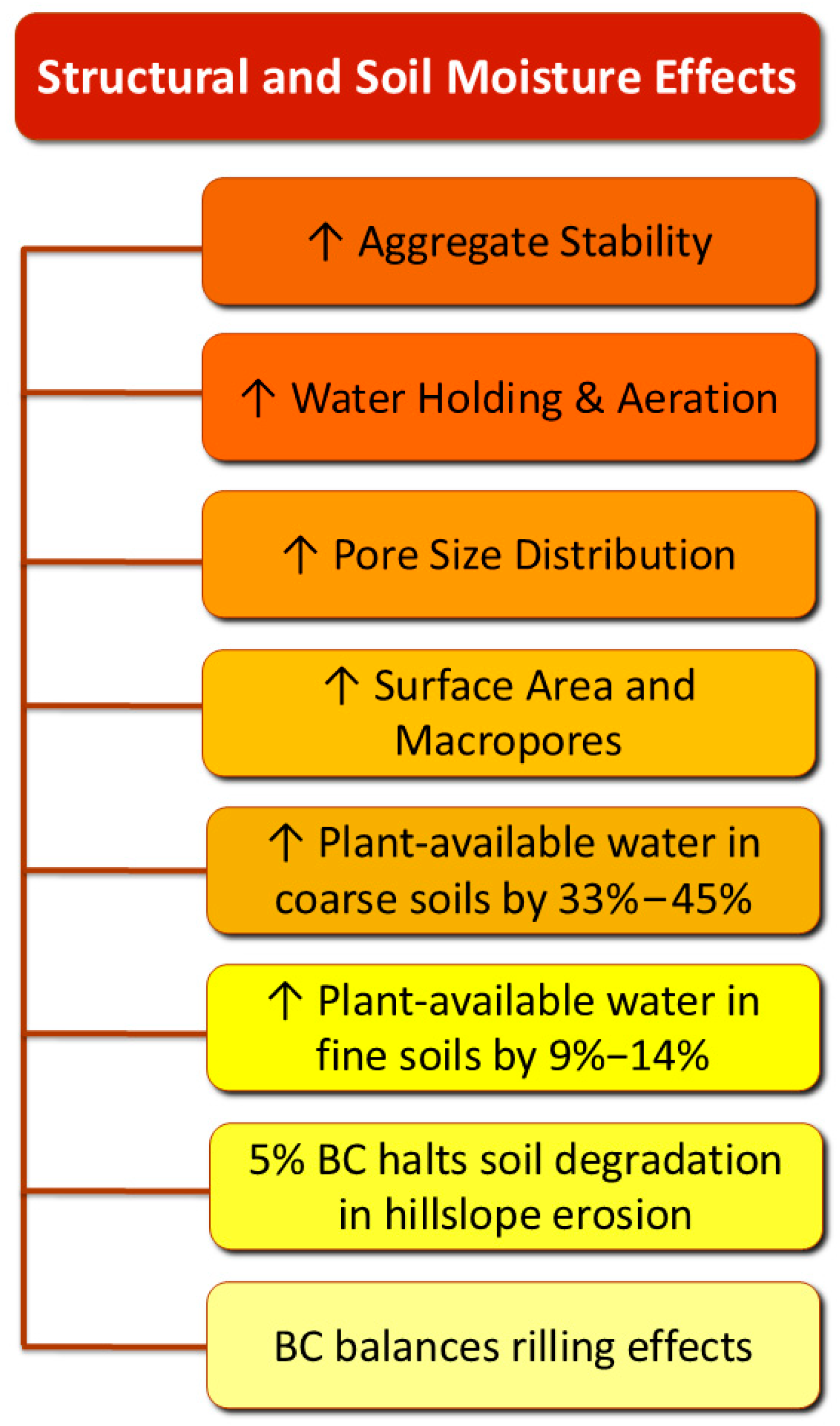
Figure 4.
Structural and moisture effects of BC treatment on soils.
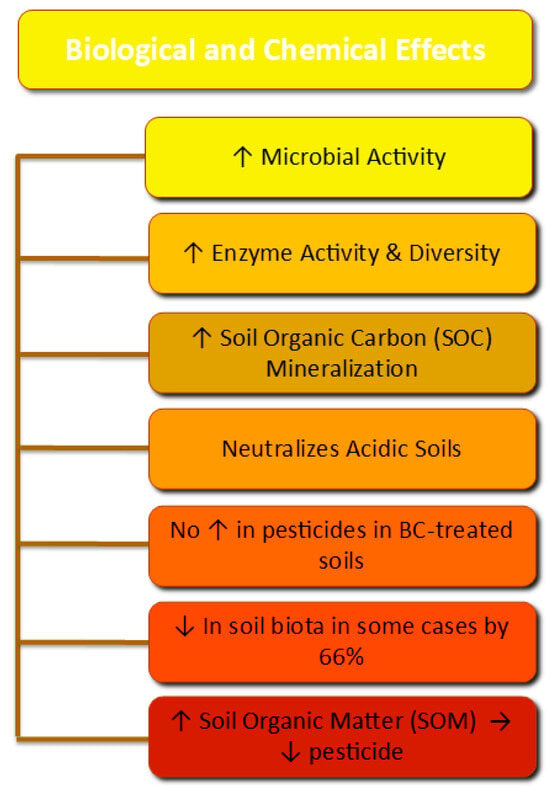
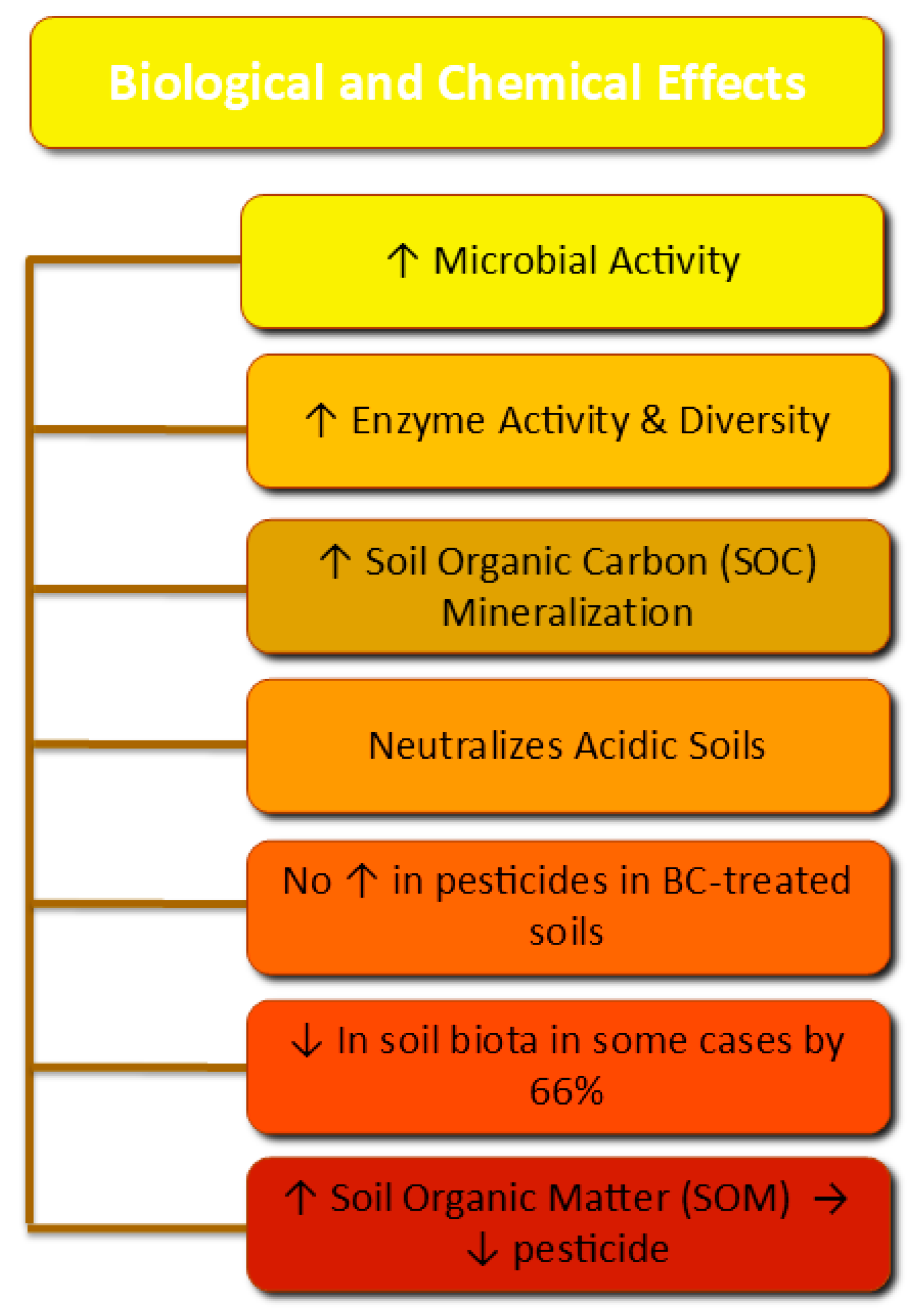
Figure 5.
Biological and chemical effects of BC treatment on soils.
Its high specific surface area and porous structure, especially the abundance of macropores, not only aid water retention but also create favorable microhabitats for soil microbiota [81].
Biologically, BC plays a key role in stimulating microbial activity. The macroporous structure supports heterotrophic microorganisms by facilitating access to organic matter, thereby enhancing SOC mineralization [82]. Additionally, the application of alkaline BC facilitates the neutralization of acidic soils, thereby enhancing soil enzymatic activity and fostering a more diverse and active microbial community [81,83].
The positive effects of BC are more pronounced in coarse-textured soils (sandy soils), where it significantly enhances soil properties compared to its application in finer-textured soils (loamy or clay soils). El-Naggar et al. [84] treated soils with three types of BC. The CEC of the sandy soil increased by 906%, 180%, and 130%, whereas the only amelioration for the sandy loam soil was by 13% for the paddy straw BC. The former indicates that the application of BC enhanced the availability of cations in the sandy soil, based on the following mechanism: The untreated sandy soil exhibits a low pH. At a low soil pH, most of the cation exchange capacity comes from pH-dependent charges located on the edges of 2:1-type clay minerals and organic matter [84]. These sites hold onto H+ (and Al3+) more strongly in acidic conditions, meaning fewer base cations (Ca2+, Mg2+, K+) are present. After BC application, the pH of the sandy soil increases, and more sites are deprotonated, allowing Ca2+, Mg2+, K+, etc., to bind. Thus, the pH-dependent charges contribute more to the CEC [84]. A slight rise in the pH was reported in the sandy loam soil after the application of BC, attributed to the relatively high buffering capacity. Therefore, BC treatment did not cause significant alterations in exchangeable cations in the sandy loam soil [84]. These different responses to the same BC type highlight that the selection of BC should consider the specific site traits.
Meta-analyses concluded that the plant-available water content in coarse-textured soils increased by 33–45%, while in fine soils, the increase was only 9–14% [85,86]. Elevated soil organic matter (SOM) was identified as an indicator of reduced pesticide concentrations due to its enhanced sorption capacity [87]. In the same vein were the results for herbicide concentrations after BC amendment. For example, in BC-amended soils, the dissipation of fenoxaprop-ethyl was slower, resulting in the formation of fewer metabolites. However, these metabolites degraded significantly faster than in untreated soils [88]. In cases of hillslope erosion, a high rate (5%) of BC was reported to halt the aggravation of soil characteristics, as the alterations due to riling were balanced by BC application to the soil [89].
6.1. Biochar Effects on Saline and Sodic Soils
Among various stress conditions, soil salinity and sodicity pose important stresses to soil fertility in arable croplands [90]. BC properties are beneficial to salt-affected soils, especially in terms of cationic nutrients (e.g., K, Ca, Mg, Zn, Mn).
As shown in Table 5, BC treatment affects physicochemical processes, resulting in the alleviation of salinization and sodification, hence ameliorating soil properties. More specifically, in salt-stressed fields, BC reduced exchangeable Na+, Cl−, and electrical conductivity, leading to improved crop performance [90]. It also reduced the negative effects of high sodium, increasing K availability for plants to grow [90,91]. Thus, these results highlight the potential of biochar to mitigate the adverse effects of salinity and sodicity by enhancing soil health and supporting long-term agricultural productivity.

Table 5.
BC’s effect on salt-stressed soils.
6.2. Biochar for Remediation of Heavy Metal-Contaminated Soils
BC has shown strong potential in remediation of heavy metal-contaminated soils, primarily due to its high cation exchange capacity (CEC), functional groups on the surface, and porous structure.
As presented in Table 6, BC effectively immobilizes cationic contaminants (e.g., Cd, Zn, Pb) [6] and contributes to reduction in fertilizer runoff. The pyrolysis temperature and feedstock type significantly influence BC performance, which can achieve metal reductions up to 90% [6].

Table 6.
BC’s effect on heavy metal soil reclamation.
Moreover, synergistic effects, such as BC combined with earthworms or macrobiota, have been shown to enhance the metal removal efficiency and promote soil microbial health [97,98]. Overall, these findings underline biochar as a sustainable solution for the remediation of heavy metal-polluted soils.
7. Biochar’s Effect on Soil Fertility
7.1. Fresh BC
It is of utmost importance that BC provides nutrients in forms directly available for plant uptake, as happens with K, which is conserved unaffected in BC from the raw biomass [49]. Table 7 summarizes the latest findings regarding BC complex impacts on soil fertility.

Table 7.
BC treatment effects on soil fertility.
As presented in Table 7, BC application in soil may affect the decomposition of native SOC, depending on the type of BC raw material. The accurate way in which BC treatment affects SOC mineralization remains unsettled in the literature, as the positive or negative effect of BC on SOC mineralization could shift over time if management or climate conditions alter [108]. There has been an attempt to address this uncertainty in the literature with updated models such as MIMICS-BC, which depicted the authors’ observations regarding the SOM response after BC treatment [109]. The effect of BC on native SOC decomposition varies, probably because of their different carbon stability, CEC, and pore structure. BC with a high cellulose content, BC produced at low temperatures (<400 °C), and fresh BC, as well as BC with a reduced surface area, a lower aromatic content, an elevated ash content, more hydrophobic surfaces, and a higher O/C ratio, may achieve faster mineralization and a prominent (positive) priming effect [74]. It must be underlined that most of the current literature investigates BC effects on temperate soils, followed by tropical soils. The response to BC employment in boreal environments is rather undocumented [6].
The total nitrogen (TN) in BC varies depending on the raw material and the pyrolysis temperature. The typical TN content ranges between 0.1 and 9.5% across different feedstocks and temperatures [112]. BC’s contribution is not to add N into soil but to improve N retention in soil by reducing leaching and gaseous loss. Moreover, it improves phosphorus availability by reducing the leaching process in the soil [113]. The impact of BC application on other nutrients can be inconsistent; however, it has been found that BC conserves K in a plant-available form from raw biomass [49,113]. Therefore, selecting feedstocks or altering the raw biomass via physicochemical/biological approaches can lead to BC with desirable properties. The engineered or modified BC regulates the stability of macroaggregates and soil inorganic N transformation, thereby enhancing plant responses under N deficiency (or even N toxicity) in the soil. BC affects biological N fixation by enhancing enzymatic activity that stimulates the conversion of N2O to N2, hence reducing denitrification and N2O release [114,115]. As a result, BC ameliorates the nutrient use efficiency of plants.
BC, in contrast to hydrochar, generally enhances germination and seedling growth [116]. BC mitigates root growth inhibition in acidic soils [117]. BC amendment alleviated heavy metal phytotoxicity (Medicago sativa) in polymetallic-contaminated soil [96]. It has been reported that BC amendments reduce Al phytotoxicity via liming and adsorption. They also reduce Cd and Zn phytotoxicity and downregulate antioxidant enzyme transcript levels (MnSOD, CAT, and GR expression) by lowering reactive oxygen species (ROS) [118]. ROS are produced in plants during various processes associated with abiotic stress [119]. Furthermore, BC influences soil microorganisms, depending on the feedstock, pyrolysis temperature, and soil type. It has been found that wood chips and mechanically deboned meat (MDM) BC affect microbial diversity and enzyme activity differently in different soil types, such as in Cambisols (typically found in temperate and boreal regions) and Luvisols (typically found in temperate regions) [120]. A concern of extended use is the localized accumulation of organic contaminants, although BC reduces their bioavailability. Since BC’s impacts on soil and microbiota are multifactorial, site-specific trials are recommended before extended BC application [121].
Overall, BC has a beneficial impact on physio-morphological and functional plant traits such as height, leaf area and number of leaves, total biomass, root and shoot weight, crop yield, pigments such as chlorophylls and flavonoids, nutrient uptake, and soil properties like pH, water-holding capacity, CEC, and soil microbiota activity [111].
7.2. Aged BC
Aged biochar in soil BC as a soil conditioner is not a brand-new concept. Its origins can be traced back to ancient Amazonian cultures that converted bio-waste into black, carbon-rich soil with perpetual fertility, known as Terra Preta [122]. The key component of Terra Preta, which provides long-term persistence of organic matter and enduring fertility, is black carbon. Crop productivity on Terra Preta soils is almost double that on adjacent unamended soils, providing evidence that BC aging does not hinder soil fertility at least for several centuries [123]. Table 8 shows that aged BC generally has positive effects on ecosystem stability and diversity, through long-term soil health, nutrient cycling, and microbial interactions. However, some studies noted a potential toxic effect in tropical soils. Therefore, its use must be tailored to the specific soil conditions.

Table 8.
Aged BC positive effects and concerns.
8. Biochar–Earthworm Interactions in Soil System
Understanding the interaction between biochar and earthworms is essential for evaluating the long-term behavior and ecological impact of BC in soil systems. Earthworms are soil macrofauna well-known as “soil engineers” since their activities promote soil health [128]. Moreover, they are employed in vermicomposting, an eco-friendly process whereby earthworms biodegrade organic waste, converting it into compost. On the one hand, integrating BC into vermicomposting can significantly upgrade the overall quality of the product (by expediting organic matter degradation, humus formation, etc.) [129]. On the other hand, the effect of BC on earthworm populations is debatable. There are contradictory findings in the literature regarding earthworm responses to BC amendments. Table 9 summarizes the main negative and positive effects reported in the latest literature.

Table 9.
Negative and positive/neutral effects of BC on earthworm populations.
Avoidance behavior tests are usually employed to assess whether BC addition into soil is beneficial or risky to the earthworm population [133]. This variability in effects is probably due to the differentiated toxicity of different BC types, determined by feedstock composition and pyrolysis temperature. Specifically, BC produced from plant residues or at lower pyrolysis temperatures usually presents lower toxicity than that derived from livestock waste or produced at higher pyrolysis temperatures. However, as shown in Table 9, SSB produced at 300 °C had a stronger negative impact on Eisenia fetida survival than SSB produced at 700 °C. This result is likely attributed to the high dose (10%) used in the experiment. Moreover, earthworms can only ingest small BC particles (e.g., <0.5 mm); therefore, smaller particles are responsible for stress [135]. Overall, field-based long-term research is considered the most secure practice in evaluating earthworm responses to BC applications.
9. Discussion
9.1. BC as a Soil Amendment
Soil quality and fertility are closely linked to food chains and sustainable ecosystems, directly impacting food safety and accessibility. In this context, BC amendments could play a pivotal role in agroforestry and smallholdings by utilizing local available feedstocks, such as wood ash, poultry litter and manure, across regions from SE Asia to Sub-Saharan Africa. Chemical fertilizers, compost, and microbial fertilizers were employed to increase the humus content, aiming at facilitating the development of fruit tree root systems and nutrient uptake. They underlined that both SOM and nutrients of the slope plots followed the same trend. Increased porosity and SOM supplement along with mitigation of soil agrochemical contamination could be efficiently addressed by BC amendments. However, the required alterations in biomass for achieving soil remediation cannot be accomplished in the short term [141]. Aged BC has proven beneficial to the soil by stimulating nutrient availability and expediting nutrient cycling in the long term [102].
BC improves the water-holding capacity by reducing bulk density and enhances the nutrient holding of coarse-textured soils such as sandy soils, since these soils usually drain too fast and have low fertility [142]. Moreover, BC application in sandy soils increases tensile strength and enhances water infiltration [142]. Fine-textured, clayey soils may benefit in terms of an aeration improvement and a decrease in compaction; however, the efficiency of BC in these soils is less remarkable [142]. In loamy soils, the effects are generally moderate, since these soils are already balanced. In soils with low organic matter, BC provides a habitat for microbes and helps in the retention of nutrients, fostering fertility. The benefits are smaller in soils with high organic matter since these soils already support microbial activity and nutrient cycling [143]. BC (usually alkaline) can raise pH of acidic soil and improve nutrient availability, decreasing Al/Mn toxicity, which is a result of high solubilization and competition of Al and Mn with essential nutrients in acidic soils [144]. Podzolic soil, for example, is an acidic, sandy, and barren soil type that forms in cool, humid regions. A significant difference was reported with 2% biochar amendment in all the soil mixtures that were examined by Saha et al. [145], while BC treatment in newly converted boreal Podzols can significantly improve soil fertility and agricultural productivity [146]. In alkaline soils, additional liming may either not promote soil quality or cause negative effects [147]. Sandy soils or acidic soils can benefit considerably from BC with a high ash content (or low carbon content), boosting plant productivity [148]. BC improves water retention in dry soils and can improve aeration and microbial activity in waterlogged soil. In degraded soils, BC stimulates biological activity more noticeably. In the case of soil subject to erosion, incorporating BC into the topsoil boosts soil structure and stability and suppresses surface runoff, hence minimizing soil erosion rates [149]. Moreover, co-utilization of BC with organic additives such as compost or manure has been reported to enhance soil aggregation and water-holding capacity by creating a soil matrix less vulnerable to erosion [150]. The former also promotes BC efficiency and productivity in tropical weathered soil [151]. The efficiency of BC depends not only on BC characteristics but also on different soil types and the physicochemical composition and genesis of the soil. BC is particularly beneficial to soils with low water-holding capacity, such as sandy soils, acidic or saline soils, podzolic soils of boreal regions, and weathered tropical soils. In these cases, BC significantly improves soil revitalization and crop yields.
9.2. BC’s Side Benefits in Agriculture
Soil treated with BC presented enduring elevated susceptibility to erosion, greater than the corresponding runoff loss [152]. BC contributes to climate-resilient and sustainable agriculture also by its ability to achieve long-term carbon dioxide removal (CDR) [153]. It exhibits an elevated technology readiness level as a climate change mitigation alternative and has the potential to be deployed for nationwide climate objectives, combined with conventional site-specific benefits. In the same frame, the European Green Deal and the derived strategies intend to diminish P losses and fertilizer usage in agriculture [154]. P is a limiting factor for plant growth, but excessive P fertilization aggravates ecosystems [155]. A surplus of P is a characteristic mainly of the Northern EU and UK, whereas bioavailable P depletion is pronounced in the Mediterranean zone [140]. The evaluation of the current soil degradation and soil compaction extent in the European territory renders concern [155,156]. Nevertheless, soil degradation is not only about biodiversity loss or Europe. It is a global matter of major economic importance. Sartori et al. [157] estimated an average increase in soil erosion rates of between 30 and 66% under different climate–economic scenarios over the period 2015–2070. Subsequently, they used the projected land productivity losses as inputs into an economic global simulation model. The latter projected a global economic recession reaching USD 625 billion by the year 2070. Vulnerable regions are expected to face a serious challenge in terms of food security and food accessibility. Global primary agricultural production could lose more than 350 million tons under the worst-case scenario by the year 2070 [157]. Therefore, soil remediation and fertility enhancement should constitute a top priority, and BC could substantially contribute to this cause.
9.3. Advanced Uses of BC
Due to its properties, BC has great potential to be established for soil remediation and soil fertility enhancement. Its positive effects are enhanced when the soil type, feedstock and target application are well aligned. BC should be exploited more efficiently, with novel directions that fit in with and enhance the cyclic economy model. For instance, according to the EU, about 80% of the plastic waste in agriculture is due to plastic mulch films [158]. To a considerable extent, they end up as microplastics on land. Co-pyrolysis of livestock feedstock with waste plastic mulch films (plastichars) promotes and stabilizes soil enzyme activities without harming earthworms. These plastichars presented superior enzyme binding capacities compared to the livestock-based BC [159] while also preventing microplastic pollution in agricultural fields. Additionally, BC composites produced from feedstock–plastic blends have a higher carbon content [160]. It has been also documented that BC can accelerate the composting process and lead to a superior end-product [79]. In addition, BC can indirectly enrich soil through manure amelioration and can be used as a supplement in livestock feed [161].
Moreover, BC production exhibits economic feasibility and reduces the footprint of both the production and use of chemical fertilizers. For example, co-composting with organic materials has been put forward as one of the most efficient BC application practices for barren soils [79]. To maximize the economic benefit, the cost of BC application should be minimized by identifying a valid minimum BC application rate. In addition, the slow release of nutrients by BC has recently been employed for the synthesis of eco-friendly BC-based fertilizers aiming to overcome nutrient excess in soils and nutrient loss from chemical fertilizers [162]. However, before the policy is stipulated in detail, further research should be conducted on application rates, long-term effects, and potential limitations reported in the literature on BC treatment.
9.4. Future Perspectives
It is necessary that agricultural and waste research, and BC research, exploits technological affordances. Hyperspectral imaging (HSI) is a simple though cutting-edge technology based on the electromagnetic spectrum that can acquire spectral and spatial information for material identification in environmental monitoring. Sanchez-Hernandez et al. [159] employed an extended visible/near-infrared (EVNIR; 600–1700 nm) mobile hyperspectral imager with quartz–tungsten–halogen (QTH) light sources that produce visible and NIR outputs. They captured the temperatures at which gases were produced during pyrolysis. After employing statistics (principal component analysis; PCA), they managed to classify the BC samples (i.e., fresh BC, BC from non-disturbed soils and the burrow walls and plastichars). The affordances of HSI in BC research should be further investigated since HSI could technologically upgrade and facilitate BC identification and monitoring.
According to Wang et al. (2024) [163] and Rex et al., 2023 [164], BC production is still in the phase of “pilot scale production to primary demonstration” (TRL 2–5), which is less mature than bio-oil production (TRL 6–9). Therefore, efforts could focus on developing engineered BC with advanced properties and on optimizing methods to maximize its effectiveness in improving soil health and plant growth. Machine learning (ML) could play a significant role in this attempt, addressing the complexity of these interactions. Recently, ML algorithms, such as ANNs and Random Forests have been used to predict the yield and composition of BC products under different feedstock inputs and pyrolysis conditions, as well as to determine the optimized rate of BC for capturing pollutants from water bodies [165,166]. Random forest has been also employed to predict biomarkers of the BC response. The model provided insights into how BC amendment affected the soil microbial community [167]. Moreover, the RBF_SVR model revealed the behavior of gas diffusion in BC-amended soil [168]. So far, this review has mostly focused on predicting the adsorption efficiency of BC for water quality improvement [166,169]. It is suggested that the models presented in this manuscript can be trained with sequential datasets and incorporate multiple factors (e.g., soil type and properties; BC types and properties; plant species, properties, and fertilizing needs; environmental conditions; application rate; aged BC; etc.) for the evaluation of tailored BC usage scenarios on stressed soils. Another suggestion in the literature is that BC dynamics should be incorporated into Earth System Models (ESMs) in a way that is sufficiently fast and explicit, where machine learning could assist to suppress the computational load [87].
The abundance of soil- and crop-beneficial properties is the strong point of BC [49]. BC is a multifunctional material that has the potential to address agricultural challenges. Efforts should be geared towards the combination of BC types with sustainable management practices such as agroforestry, diversification of crops, deployment of climate-tolerant cultivars, and tree-based farming systems which favor bio-amelioration and carbon sequestration [170,171]. A paradigm shift in the production model, incorporating tailored BC utilization, seems imperative, aiming to secure biodiversity and resource conservation and enhance productivity.
10. Conclusions
Biochar presents a broad range of advantageous properties that make it a promising tool for enhancing soil health and addressing environmental challenges. Its ability to supply cationic nutrients (e.g., K, Ca, Mg, Zn, Mn), increase the CEC, enhance the specific surface area, and significantly increase the porosity and water-holding capacity contributes significantly to soil fertility. BC also raises the pH of acidic soils and favors microbiota, as it serves as a shelter and a nutrient sink. Therefore, BC enhances microbial abundance and soil enzymatic activity and improves soil properties. In addition, BC persists in soil for hundreds to thousands of years. Aged BC continues to support microbial activity and diversity selectively and fosters beneficial species growth. It supports the symbiotic microbe–plant relationship and promotes ecosystem resilience.
Furthermore, BC has been used for soil restoration. BC is a promising material in the removal of heavy metals from contaminated soil, favorably affecting plant growth. It acts as an effective amendment in problem soils. BC’s effect on soil reclamation and fertility is determined by multiple factors such as raw biomass quality (composition and contaminant content), pyrolysis characteristics (mostly temperature), and soil attributes. Long-lasting positive effects on earthworm activity have been reported with tailored BC application; additionally, it facilitates distribution of BC particles into the topsoil and promotes transportation of aggregates to lower soil layers. Advanced BC composites such as plastichars have attracted attention due to their elevated properties and ecological side benefits.
State-of-the-art technologies and modeling approaches, such as machine learning, hyperspectral imaging (HSI), and Earth System Models (ESMs), could contribute to a better understanding of BC dynamics. A current limitation is that most of the studies are conducted at the laboratory scale and are short-term. This fact raises the uncertainty of the claims, as underlined by numerous researchers. Consequently, robust, long-term field research is essential before shaping global biochar-related policies. The current literature indicates BC as a promising tool for sustainability. Although it is sometimes referred to as “black gold,” its widespread adsorption requires a careful and evidence-based approach that accounts for both promises and limitations. With continued research, BC has the potential to contribute meaningfully to soil regeneration and the circular economy.
Author Contributions
Investigation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, E.G., E.A.I., P.K., and I.K.K.; visualization, E.A.I.; supervision, I.K.K.; project administration, I.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in the context of the project “Improving soil fertility by combining earthworms and biochars to produce high nutritional and agronomic value products. Implementation of technology at a pilot stage—BIOCOM” (Μ16ΣΥΝ2-00101) by the Rural Development Programme (RDP) 2014–2020 and co-financed by the European Agricultural Fund for Rural Development (EAFRD) and the Greek state.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Telo de Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Wang, X. Managing Land Carrying Capacity: Key to Achieving Sustainable Production Systems for Food Security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- FAO. Soils, Where Food Begins. Outcome Document of the Global Symposium on Soils for Nutrition 26–29 July 2022; FAO: Rome, Italy, 2023; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/0fa64150-221d-4d8e-9260-d76eb09eed6a/content (accessed on 18 June 2025).
- Dong, X.; Martin, J.B.; Cohen, M.J.; Tu, T. Bedrock Mediates Responses of Ecosystem Productivity to Climate Variability. Commun. Earth Environ. 2023, 4, 114. [Google Scholar] [CrossRef]
- Çelebi, H.; Bahadır, T.; Şimşek, İ.; Tulun, Ş. Turning Waste into Soil Conditioner with a Sustainable Innovative Approach: Biochar. Eng. Proc. 2023, 56, 149. [Google Scholar]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Nurhidayati, N.; Ansari, A.S.; Sholihah, A.; Chiangmai, P.N. Vermicompost and rice husk biochar interaction ameliorates nutrient uptake and yield of green lettuce under soilless culture. J. Hort. Res. 2022, 30, 55–66. [Google Scholar] [CrossRef]
- Thornton, P.; Dinesh, D.; Cramer, L.; Loboguerrero, A.M.; Campbell, B. Agriculture in a changing climate: Keeping our cool in the face of the hothouse. Outlook Agric. 2018, 47, 283–290. [Google Scholar] [CrossRef]
- European Soil Data Centre (ESDAC). Euro Soil Degradation Dashboard. Soil Degradation in the Member States. 2024. Available online: https://esdac.jrc.ec.europa.eu/esdacviewer/euso-dashboard/ (accessed on 13 January 2025).
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; Sujayananad, G.K.; Vadivel, R.; Das, T.K.; et al. Nitrogen use efficiency-a key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 18, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, T.; Ikazaki, K.; Shinjo, H.; Tanaka, U.; Fatondji, D.; Funakawa, S. Pearl millet yield reduction by soil erosion and its recovery potential through fertilizer application on an Arenosol in the Sahel. Soil Tillage Res. 2025, 246, 106324. [Google Scholar] [CrossRef]
- Liu, B.; Tang, C.-S.; Pan, X.-H.; Cheng, Q.; Xu, J.-J.; Lv, C. Mitigating rainfall induced soil erosion through bio-approach: From laboratory test to field trail. J. Eng. Geol. 2025, 344, 107842. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.; Ren, Y.; Zhu, X.; Si, S.; Kou, B.; Zhang, Z.; Wang, J.; Shen, B. Remediation of polycyclic aromatic hydrocarbons polluted soil by biochar loaded humic acid activating persulfate: Performance, process and mechanisms. Bioresour. Technol. 2024, 399, 130633. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Dimitriadou, S.; Kokkinos, P.A.; Kyzas, G.Z.; Kalavrouziotis, I.K. Fit-for-purpose WWTP unmanned aerial systems: A game changer towards an integrated and sustainable management strategy. Sci. Total Environ. 2024, 949, 174966. [Google Scholar] [PubMed]
- Gill, J.C.; Malamud, B.D. Anthropogenic processes, natural hazards, and interactions in a multi-hazard framework. Earth-Sci. Rev. 2017, 166, 246–269. [Google Scholar] [CrossRef]
- Boaz, A.; Ashby, D.; Young, K. Systematic Reviews: What Have They Got to Offer Evidence Based Policy and Practice? ESRC UK Centre for Evidence Based Policy and Practice; Working Paper 2, Queen Mary; University of London: London, UK, 2002; pp. 1–26. Available online: https://emilkirkegaard.dk/en/wp-content/uploads/Should-I-do-a-systematic-review.pdf (accessed on 5 February 2024).
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manage. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- European Soil Data Centre (ESDAC). Soil Compaction. 2024. Available online: https://esdac.jrc.ec.europa.eu/themes/soil-compaction1 (accessed on 13 January 2025).
- Fendrich, A.N.; Van Eynde, E.; Stasinopoulos, D.M.; Rigby, R.A.; Mezquita, F.Y.; Panagos, P. Modeling arsenic in European topsoils with a coupled semiparametric (GAMLSS-RF) model for censored data. Environ. Int. 2024, 185, 108544. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernandez-Ugalde, O.; Borelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in topsoils of the European Union–An analysis based on LUCAS topsoil database. Sci. Total Environ. 2024, 912, 168710. [Google Scholar] [CrossRef]
- Ballabio, C.; Jiskra, M.; Osterwalder, S.; Borrelli, P.; Montanarella, L.; Panagos, P. A spatial assessment of mercury content in the European Union topsoil. Sci. Total Environ. 2021, 769, 144755. [Google Scholar] [CrossRef]
- Van Eynde, E.; Fendrich, A.N.; Ballabio, C.; Panagos, P. Spatial assessment of topsoil zinc concentrations in Europe. Sci. Total Environ. 2023, 892, 164512. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Poesen, J. Soil loss due to crop harvesting in the European Union: A first estimation of an underrated geomorphic process. Sci. Total Environ. 2019, 664, 487–498. [Google Scholar] [CrossRef]
- Borrelli, P.; Panagos, P.; Alewell, C.; Ballabio, C.; de Oliveira Fagundes, H.; Haregeweyn, N.; Lugato, E.; Maerker, M.; Poesen, J.; Vanmaercke, M.; et al. Policy implications of multiple concurrent soil erosion processes in European farmland. Nat. Sustain. 2022, 6, 103. [Google Scholar] [CrossRef]
- De Rosa, D.; Ballabio, C.; Lugato, E.; Fasiolo, M.; Jones, A.; Panagos, P. Soil organic carbon stocks in European croplands and grasslands: How much have we lost in the past decade? Glob. Change Biol. 2024, 30, e16992. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S.; Henrich, V.; Frenken, K.; Burke, J. FAO Global Map of Irrigation Areas—Version 5.0—Area equipped for irrigation expressed as percentage of total area. In Update of the Digital Global Map of Irrigation Areas to Version 5; Rheinische Friedrich-Wilhelms-Universität: Bonn, Germany; FAO: Rome, Italy, 2013. [Google Scholar]
- European Commission (EC). EU Mission: A Soil Deal for Europe. 2020. Available online: https://research-and-innovation.ec.europa.eu/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe/eu-missions-horizon-europe/soil-deal-europe_en?wt-search=yes (accessed on 14 January 2025).
- European Commission (EC). The European Green Deal. Striving to Be the First Climate-Neutral Continent. 2019. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 14 January 2025).
- European Commission (EC). The Common Agricultural Policy at a Glance. 2023. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-glance_en#cap2023-27 (accessed on 14 January 2025).
- European Commission (EC). Farm to Fork Strategy. 2020. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en#F2F-Publications (accessed on 14 January 2025).
- European Commission (EC). Zero Pollution Action Plan. Towards Zero Pollution for Air, Water and Soil. 2021. Available online: https://environment.ec.europa.eu/strategy/zero-pollution-action-plan_en (accessed on 14 January 2025).
- European Commission (EC). Circular Economy Action Plan. 2020. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 14 January 2025).
- FAO. The International Code of Conduct for the Sustainable Use and Management of Fertilizers; FAO: Rome, Italy, 2019; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/a8edc4fb-1614-426b-b21c-440fff19c46b/content (accessed on 14 January 2025).
- FAO-CFS. Voluntary Guidelines on the Responsible Governance of Tenure of Land, Fisheries and Forests in the Context of National Food Security. 2012. Available online: https://www.fao.org/cfs/cfs-old/home/activities/vggt/en/ (accessed on 14 January 2025).
- Net Zero; University of Oxford. What Is Net-Zero? 2024. Available online: https://netzeroclimate.org/what-is-net-zero-2/ (accessed on 14 January 2025).
- United Nations Framework Convention on Climate Change (UNFCCC). COP29 UN Climate Conference Agrees to Triple Finance to Developing Countries, Protecting Lives and Livelihoods. In Proceedings of the COP29 UN Climate Conference Agrees to Triple Finance to Developing Countries, Protecting Lives and Livelihoods, Baku, Azerbaijan, 11–22 November 2024; Available online: https://unfccc.int/cop29 (accessed on 14 January 2025).
- Organization for Economic Co-Operation and Development (OECD). Production Growth to Slow in Step with Population, While Geopolitical Tensions, Climate Change, Animal and Plant Diseases and Price Volatility of Critical Farming Inputs Pose Long-Term Uncertainty. 2023. Available online: https://www.oecd.org/en/about/news/press-releases/2023/07/production-growth-to-slow-in-step-with-population-while-geopolitical-tensions-climate-change-animal-and-plant-diseases-and-price-volatility-of-critical-farming-inputs-pose-long-term-uncertainty.html (accessed on 14 January 2025).
- Lefebvre, D.; Fawzy, S.; Aquije, C.A.; Osman, A.I.; Draper, K.T.; Trabold, T.A. Biomass residue to carbon dioxide removal: Quantifying the global impact of biochar. Biochar 2023, 5, 65. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Z.; Wang, J.; Guo, S.; Li, Z.; Lin, H.; Huang, Y.; Han, Z.; Kuzyakov, Y.; Zou, J. Benefits and limitations of biochar for climate-smart agriculture: A review and case study from China. Biochar 2023, 5, 77. [Google Scholar] [CrossRef]
- Khan, R.; Abbas, A.; Farooque, A.A.; Abbas, F.; Wang, X. Mitigation of Greenhouse Gas Emissions from Agricultural Fields through Bioresource Management. Sustainability 2022, 14, 5666. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, Y.; Zhang, X.; Xu, X.; Xiong, Z. Biochar single application and reapplication decreased soil greenhouse gas and nitrogen oxide emissions from rice-wheat rotation: A three-year field observation. Geoderma 2023, 435, 116498. [Google Scholar] [CrossRef]
- Raza, S.T.; Rong, L.; Rene, E.R.; Ali, Z.; Iqbal, H.; Sahito, Z.A.; Chen, Z. Effects of vermicompost preparation and application on waste recycling, NH3, and N2O emissions: A systematic review on vermicomposting. Environ. Technol. Innov. 2024, 35, 103722. [Google Scholar] [CrossRef]
- Khaliq, M.A.; Alsudays, I.M.; Alhaithloul, H.A.S.; Rizwan, M.; Yong, J.W.H.; Rahman, S.U.; Sagir, M.; Bashir, S.; Ali, H.; Zuo, H. Biochar impacts on carbon dioxide, methane emission, and cadmium accumulation in rice from Cd-contaminated soils; A meta-analysis. Ecotoxicol. Environ. Saf. 2024, 274, 116204. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M. Feedstock type, pyrolysis temperature and acid modification effects on physiochemical attributes of biochar and soil quality. Arab. J. Geosci. 2022, 15, 305. [Google Scholar] [CrossRef]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Steele, J.C.; Meng, X.Z.; Venkatesan, A.K.; Halden, R.U. Comparative meta-analysis of organic contaminants in sewage sludge from the United States and China. Sci. Total Environ. 2022, 821, 153423. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef]
- Dudnikova, T.; Wong, M.H.; Minkina, T.; Sushkova, S.; Bauer, T.; Khroniuk, O.; Barbashev, A.; Shuvaev, E.; Nemtseva, A.; Kravchenko, E. Effects of pyrolysis conditions on sewage sludge-biochar properties and potential risks based on PAH contents. Environ. Res. 2025, 266, 120444. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liang, S.; Zou, Z.; Xu, X.; Yang, F.; Quan, J.; Li, X.; Duan, H.; Yu, W.; Yang, J. Enhanced phosphorus bioavailability of biochar derived from sewage sludge co-pyrolyzed with K, Ca-rich biomass ash. Water Res. 2025, 271, 122901. [Google Scholar] [CrossRef]
- Schlederer, F.; Martin-Hernandez, E.; Vaneeckhaute, C. Ensuring safety standards in sewage sludge-derived biochar: Impact of pyrolysis process temperature and carrier gas on micropollutant removal. J. Environ. Manag. 2024, 352, 119964. [Google Scholar] [CrossRef] [PubMed]
- Levidow, L.; Raman, S. Metamorphosing waste as a resource: Scaling waste management by ecomodernist means. Geoforum 2019, 98, 108–122. [Google Scholar] [CrossRef]
- Yu, G.; Xie, S.; Ma, J.; Shang, X.; Wang, Y.; Yu, C.; You, F.; Tang, X.; Levatti, H.U.; Pan, L.; et al. Influence of Sewage Sludge Biochar on the Microbial Environment, Chinese Cabbage Growth, and Heavy Metals Availability of Soil. In Biochar—An Imperative Amendment for Soil and the Environment; IntechOpen: Rijeka, Croatia, 2018; Available online: https://www.intechopen.com/chapters/64481 (accessed on 14 January 2025).
- Kumar, R.; Whelan, A.; Cannon, P.; Reeves, L.; Antunes, E. Transforming contaminated biosolids into biochar for a sustainable cement replacement material. Biomass Conv. Bioref. 2025, 15, 18083–18095. [Google Scholar] [CrossRef]
- Xie, S.; Yu, G.; Jiang, R.; Ma, J.; Shang, X.; Wang, G.; Wang, Y.; Yang, Y.; Li, C. Moderate sewage sludge biochar application on alkaline soil for corn growth: A field study. Biochar 2021, 3, 135–147. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Zhang, J.; Li, H.; Wu, J. Effects of biosolid biochar on crop production and metal accumulation through a rice-wheat rotation system in fields. Environ. Pollut. Bioavailab. 2023, 35, 2240016. [Google Scholar] [CrossRef]
- Faria, W.M.; Figueiredo, C.C.; de Coser, T.R.; Vale, A.T.; Schneider, B.G. Is sewage sludge biochar capable of replacing inorganic fertilizers for corn production? Evidence from a two-year field experiment. Arch. Agron. Soil Sci. 2017, 64, 505–519. [Google Scholar] [CrossRef]
- Mcintyre, H.; Li, S. From Waste to Resource: Evaluating the Impact of Biosolid-Derived Biochar on Agriculture and the Environment. Biomass 2024, 4, 809–825. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.L.; Viana, R.S.R.; Melo, L.C.A.; de Figueiredo, C.C. Changes in phosphorus due to pyrolysis and in the soil-plant system amended with sewage sludge biochar compared to conventional P fertilizers: A global meta-analysis. Chemosphere 2025, 371, 144055. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Visser, E.D.; Seroka, N.S.; Khotseng, L. Recent Advances in Biochar: Synthesis Techniques, Properties, Applications, and Hydrogen Production. Processes 2024, 12, 1111. [Google Scholar] [CrossRef]
- González, W.A.; Pérez, J.F. CFD analysis and characterization of biochar produced via fixed-bed gasification of fallen leaf pellets. Energy 2019, 186, 115904. [Google Scholar] [CrossRef]
- Bona, D.; Bertoldi, D.; Borgonovo, G.; Mazzini, S.; Ravasi, S.; Silvestri, S.; Zaccone, C.; Giannetta, B.; Tambone, F. Evaluating the potential of hydrochar as a soil amendment. Waste Manag. 2023, 159, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Li, S.M.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sanchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Ahmad, Z.; Naveed, M. Low pyrolysis temperature biochar improves growth and nutrient availability of Maize on Typic Calciargid. Commun. Soil Sci. Plant Anal. 2016, 47, 41–51. [Google Scholar] [CrossRef]
- Rambhatla, N.; Panicker, T.F.; Mishra, R.K.; Manjeshwar, S.K.; Sharma, A. Biomass pyrolysis for biochar production: Study of kinetics parameters and effect of temperature on biochar yield and its physicochemical properties. Results Eng. 2025, 25, 103679. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.; Rinklebe, J.; Ok, Y.S. Biochar composition dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Development of biochar-based green functional materials using organic acids for environmental applications. J. Clean. Prod. 2020, 244, 118841. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelfattah, A.M.; Tharwat, R.M.; Nabil, G.M. Adsorption of negatively charged food tartrazine and sunset yellow dyes onto positively charged triethylenetetramine biochar: Optimization, kinetics and thermodynamic study. J. Mol. Liq. 2020, 318, 114297. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Dey, S.; Pande, P.; Kumari, S.; Bhowmik, A. Biochar aided priming of carbon and nutrient availability in three soil orders of India. Sci. Rep. 2024, 14, 8420. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Niazi, N.K.; Rizwan, M.; Al-Wabel, M.I.; Usman, A.R.; Moon, D.H.; Lee, S.S. Effect of corn residue biochar on the hydraulic properties of sandy loam soil. Sustainability 2017, 9, 266. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, Y.; Tan, X.; Li, L.; Cao, S.; Dou, J.; Chen, R.; Hu, X.; Qiu, Z.; Li, M.; et al. Sludge-based biochar preparation: Pyrolysis and co-pyrolysis methods, improvements, and environmental applications. Fuel 2024, 373, 132265. [Google Scholar] [CrossRef]
- Buss, W.; Shepherd, J.G.; Heal, K.V.; Mašek, O. Spatial and temporal microscale pH change at the soil-biochar interface. Geoderma 2018, 331, 50–52. [Google Scholar] [CrossRef]
- Mutushev, A.; Nuraly, A.; Kaya, A.; Mukhunov, D. Dev elopment and application of microcapsules based on rice husk and metallurgical sludge to improve soil fertility. Sci. Rep. 2025, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinkleben, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties –New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Wang, J.; Norgaard, T.; Pugliese, L.; Carvalho, P.N.; Wu, S. Global meta-analysis and machine learning reveal the critical role of soil properties in influencing biochar-pesticide interactions. Environ. Int. 2024, 193, 109131. [Google Scholar] [CrossRef]
- Jing, X.; Wang, T.; Yang, J.; Wang, Y.; Xu, H. Effects of biochar on the fate and toxicity of herbicide fenoxaprop-ethyl in soil. R. Soc. Open Sci. 2018, 5, 171875. [Google Scholar] [CrossRef] [PubMed]
- Bagarello, V.; Conte, P.; Ferro, V.; Iovino, M.; Librici, C.; Nicosia, A.; Palmeri, V.; Pampalone, V.; Zanna, F. How Rilling and Biochar Addition Affect Hydraulic Properties of a Clay-Loam Soil. Eur. J. Soil. Sci. 2025, 76, e70034. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Yang, F.; Xing, Y.; Huang, Y.; Xu, L.; Liu, Z.; Holtzman, R.; Kan, I.; Li, Y.; Zhang, L.; et al. Use of biochar to manage soil salts and water: Effects and mechanisms. CATENA 2022, 211, 106018. [Google Scholar] [CrossRef]
- Manasa, M.R.K.; Katukuri, N.R.; Nair, S.S.D.; Haojie, Y.; Yang, Z.; Guo, R.B. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737. [Google Scholar] [CrossRef]
- Jia, A.; Song, X.; Li, S.; Liu, Z.; Liu, X.; Han, Z.; Gao, H.; Gao, Q.; Zha, Y.; Liu, Y.; et al. Biochar enhances soil hydrological function by improving the pore structure of saline soil. Agric. Water Manag. 2024, 306, 109170. [Google Scholar] [CrossRef]
- Kharel, G.; Sacko, O.; Feng, X.; Morris, J.R.; Phillips, C.L.; Trippe, K.; Kumar, S.; Lee, J.W. Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Chem. Engin. 2019, 7, 16410–16418. [Google Scholar] [CrossRef]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Environ. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Helaoui, S.; Boughattas, I.; Mkhinini, M.; Chebbi, L.; Elkribi-Boukhris, S.; Alphonse, V.; Liver, A.; Banni, M.; Bousserrhine, N. Biochar amendment alleviates heavy metal phytotoxicity of Medicago sativa grown in polymetallic contaminated soil: Evaluation of metal uptake, plant response and soil properties. Plant Stress 2023, 10, 100212. [Google Scholar] [CrossRef]
- Yuan, R.; Li, W.; Salam, M.; Li, H. Nano-biochar reduces sustainable remediation of cadmium-contaminated soil more than micro-biochar: Evidence from cadmium removal and Eisenia foetida toxicity. Environ. Pollut. 2025, 366, 125479. [Google Scholar] [CrossRef] [PubMed]
- Boughattas, I.; Zitouni, N.; Mkhinini, M.; Missawi, O.; Helaoui, S.; Hattab, S.; Mokni, M.; Bousserrhine, N.; Banni, M. Combined toxicity of Cd and 2,4-dichlorophenoxyacetic acid on the earthworm Eisenia andrei under biochar amendment. Environ. Sci. Pollut. Res. 2023, 30, 34915–34931. [Google Scholar] [CrossRef]
- Liang, W.Y.; Wang, G.H.; Peng, C.; Tan, J.Q.; Wan, J.; Wu, Y.H. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zi, Y.; Luo, Q.; Li, H.; Wu, G.; Li, M.; Duan, C.; Liu, C. Biochemical Combination Benefits Remediation of Soil Lead and Cadmium Pollution: Evidence from Sunflowers, Earthworms, and Soil Microorganisms. Soil Sediment Contam. 2025, 34, 1–22. [Google Scholar] [CrossRef]
- Chew, J.; Joseph, S.; Chen, G.; Zhang, Y.; Zhu, L.; Liu, M.; Taherymoosavi, S.; Munroe, P.; Mitchell, D.R.G.; Pan, G.; et al. Biochar-based fertiliser enhances nutrient uptake and transport in rice seedlings. Sci. Total Environ. 2022, 826, 154174. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Shyam, S.; Zhang, M.; Guerriero, G. Nano-biochar interactions with contaminants in the rhizosphere and their implications for plant-soil dynamics. Soil Environ. Health 2024, 2, 100095. [Google Scholar] [CrossRef]
- Ndoung, O.C.N.; de Souza, L.P.; Fachini, J.; Leão, T.P.; Sandri, D.; de Figueiredo, C.C. Dynamics of potassium released from sewage sludge biochar fertilizers in soil. J. Environ. Manag. 2023, 346, 119057. [Google Scholar] [CrossRef]
- Dimitriadou, S.; Nikolakopoulos, K.G. Evapotranspiration Trends and Interactions in Light of the Anthropogenic Footprint and the Climate Crisis: A Review. Hydrology 2021, 8, 163. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, Z.; Song, H.; Li, K.; Lu, H.; Liu, Y.; Cheng, F. Biochar induced changes of soil dissolved organic matter: The release and adsorption of dissolved organic matter by biochar and soil. Sci. Total Environ. 2021, 783, 147091. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Pal Singh, B. Interactive Priming of Biochar and Labile Organic Matter Mineralization in a Smectite-Rich Soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Wang, Y.; Lu, H.; He, L.; Yang, S. Negative priming effect of three kinds of biochar on the mineralization of native soil organic carbon. Land Degrad. Dev. 2018, 29, 3985–3994. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Q.; Wang, X.; Wang, Y.-P.; Ciais, P.; Zhang, H.; Goll, D.S.; Zhu, L.; Zhao, Z.; Guo, Z.; et al. Modeling biochar effects on soil organic carbon on croplands in a microbial decomposition model (MIMICS-BC_v1.0). Geosci. Model Dev. 2024, 17, 4871–4890. [Google Scholar] [CrossRef]
- Weng, Z.H.; Van Zwieten, L.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat. Clim. Change 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Upadhyay, V.; Choudhary, K.K.; Agrawal, S.B. Use of biochar as a sustainable agronomic tool, its limitations and impact on environment: A review. Discov. Agric. 2024, 2, 20. [Google Scholar] [CrossRef]
- de Oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valor. 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Khan, Z.; Yang, X.J.; Fu, Y.; Joseph, S.; Khan, M.N.; Khan, A.; Alam, I.; Shen, H. Engineered biochar improves nitrogen use efficiency via stabilizing soil water-stable macroaggregates and enhancing nitrogen transformation. Biochar 2023, 5, 52. [Google Scholar] [CrossRef]
- Khan, Z.; Zhang, K.; Khan, M.N.; Bi, J.; Zhu, K.; Luo, L.; Hu, L. How Biochar Affects Nitrogen Assimilation and Dynamics by Interacting Soil and Plant Enzymatic Activities: Quantitative Assessment of 2 Years Potted Study in a Rapeseed-Soil System. Front. Plant Sci. 2022, 10, 853449. [Google Scholar] [CrossRef]
- Sun, J.; Benavente, V.; Jansson, S.; Mašek, O. Comparative characterisation and phytotoxicity assessment of biochar and hydrochar derived from municipal wastewater microalgae biomass. Bioresour. Technol. 2023, 386, 129567. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B.; Chen, M. Novel Alleviation Mechanisms of Aluminum Phytotoxicity via Released Biosilicon from Rice Straw-Derived Biochars. Sci. Rep. 2016, 6, 29346. [Google Scholar] [CrossRef]
- Kang, X.; Geng, N.; Li, X.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; Lou, Y. Biochar Alleviates Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Foxtail Millet (Setaria italica L.) Cultivated in Cd- and Zn-Contaminated Soil. Front. Plant Sci. 2022, 13, 782963. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kracmarova-Farren, M.; Alexova, E.; Kodatova, A.; Mercl, F.; Szakova, J.; Tlustos, P.; Demnerova, K.; Stiborova, H. Biochar-induced changes in soil microbial communities: A comparison of two feedstocks and pyrolysis temperatures. Environ. Microbiome 2024, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kaushik, R.; Pandit, M.K.; Lee, Y.-H. Biochar-Induced Microbial Shifts: Advancing Soil Sustainability. Sustainability 2025, 17, 1748. [Google Scholar] [CrossRef]
- De Gisi, S.; Petta, L.; Wendland, C. History and Technology of Terra Preta Sanitation. Sustainability 2014, 6, 1328–1345. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Xu, G.; Nan, J. Multilevel investigation of the ecotoxicological effects of sewage sludge biochar on the earthworm Eisenia fetida. Chemosphere 2024, 360, 142455. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, W.; Yue, S.; Adeel, M.; Qiao, Y. Role of biochar and Eisenia fetida on metal bioavailability and biochar effects on earthworm fitness. Environ. Pollut. 2020, 263, 114586. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, J.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Videgain-Marco, M.; Marco-Montori, P.; Martí-Dalmau, C.; Jaizme-Vega, M.C.; Manyà-Cervelló, J.J.; García-Ramos, F.J. The effects of biochar on indigenous arbuscular mycorrhizae fungi from agroenvironments. Plants 2021, 10, 950. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Earthworms as Soil Ecosystem Engineers. In Earthworms and Ecological Processes; Kooch, Y., Kuzyakov, Y., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Jin, L.; Azhar, Z.; Luo, M.; Gong, X. Synergistic effects of bamboo biochar and ammonia oxidizing bacteria on nitrogen transformation and microbial dynamics during vermicomposting of green waste and chicken manure. Process Saf. Environ. Prot. 2025, 193, 115–124. [Google Scholar] [CrossRef]
- Shi, Z.M.; Wen, M.; Zhao, Y.H.; Wang, C.Y. Vermitoxicity of aged biochar and exploring potential damage factors. Environ. Int. 2023, 172, 107787. [Google Scholar] [CrossRef]
- Prodana, M.; Silva, C.; Gravato, C.; Verheijen, F.G.A.; Keizer, J.J.; Soares, A.M.V.M.; Loureiro, S.; Bastos, A.C. Influence of biochar particle size on biota responses. Ecotox. Environ. Safe. 2019, 1, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.P.; Otomo, P.V. High rates of biochar soil amendment cause increased incidences of neurotoxic and oxidative stress in Eisenia fetida (Oligochaeta) exposed to glyphosate. Appl. Sci. 2022, 12, 2381. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Ríos, J.M.; Attademo, A.M.; Malcevschi, A.; Cares, X.A. Assessing biochar impact on earthworms: Implications for soil quality promotion. J. Hazard. Mater. 2019, 366, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.K.; Benslim, H.; Elmi, A.A.; Husk, R. Earthworm populations are stable in temperate agricultural soils receiving wood-based biochar. Pedosphere 2021, 31, 398–404. [Google Scholar] [CrossRef]
- Sun, F.F.; Chen, J.F.; Chen, F.Y.; Wang, X.; Liu, K.; Yang, Y.W.; Tang, M.Z. Influence of biochar remediation on in Pb-contaminated soils. Chemosphere 2022, 295, 133954. [Google Scholar] [CrossRef]
- Garbuz, S.; Mackay, A.; Camps-Arbestain, M.; DeVantier, B.; Minor, M. Biochar amendment improves soil physico-chemical properties and alters root biomass and the soil food web in grazed pastures. Agric. Ecosyst. Environ. 2021, 319, 107517. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Lebrun, M.; Vedere, C.; Nobile, C.; Guidet, J.; Kervroedan, L.; Aubertin, M.L.; Rumpel, C.; Faucon, M.P.; et al. Positive or neutral effects of biochar-compost mixtures on earthworm communities in a temperate cropping system. Appl. Soil Ecol. 2023, 182, 104684. [Google Scholar] [CrossRef]
- Fonte, S.J.; Hsieh, M.; Mueller, N.D. Earthworms contribute significantly to global food production. Nat. Commun. 2023, 14, 5713. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.D.; Hu, T.S.; Mahmoud, A.; Li, J.; Zhu, R.; Jiao, X.Y.; Jing, P.R. A quantitative review of the effects of biochar application on rice yield and nitrogen use efficiency in paddy fields: A meta-analysis. Sci. Total Environ. 2022, 830, 154792. [Google Scholar] [CrossRef]
- Noronha, F.R.; Manikandan, S.K.; Nair, V. Role of coconut shell biochar and earthworm (Eudrilus euginea) in bioremediation and palak spinach (Spinacia oleracea L.) growth in cadmium-contaminated soil. J. Environ. Manag. 2022, 302, 114057. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and Its Broad Impacts in Soil Quality and Fertility, Nutrient Leaching and Crop Productivity: A Review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Zhao, X.Q.; Shen, R.F. Molecular mechanisms of plant adaptation to acid soils: A review. Pedosphere 2023, 33, 14–22. [Google Scholar] [CrossRef]
- Saha, R.; Galagedara, L.; Thomas, R.; Nadeem, M.; Hawboldt, K. Investigating the Influence of Biochar Amendment on the Physicochemical Properties of Podzolic Soil. Agriculture 2020, 10, 471. [Google Scholar] [CrossRef]
- Abedin, J.; Unc, A. The rate dependent efficacy of biochar for crop yield and nutrition on Podzols newly converted from boreal forests. Field Crops Res. 2023, 303, 109121. [Google Scholar] [CrossRef]
- Shyam, S.; Ahmed, S.; Joshi, S.J.; Sarma, H. Biochar as a Soil amendment: Implications for soil health, carbon sequestration, and climate resilience. Discov. Soil 2025, 2, 18. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A review on biochar’s effect on soil properties and crop growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Sharma, P.; Ali, S.; Biswas, J.K. Application of biochar for soil erosion control and environmental management: Implications for achieving sustainable development goals. Discov. Soil 2025, 2, 36. [Google Scholar] [CrossRef]
- Basak, B.B.; Sarkar, B.; Saha, A.; Sarkar, A.; Mandal, S.; Biswas, J.K.; Wang, H.; Bolan, N.S. Revamping highly weathered soils in the tropics with biochar application: What we know and what is needed. Sci. Total Environ. 2022, 822, 153461. [Google Scholar] [CrossRef]
- Rubio, V.; Núñez, A.; Berger, A.; van Es, H. Biomass inputs drive agronomic management impacts on soil health. Agric. Environ. 2025, 378, 109316. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Yang, M.; Zhang, F. Exploring biochar addition impacts on soil erosion under natural rainfall: A study based on four years of field observations on the Loess Plateau. Soil Tillage Res. 2024, 236, 105935. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Lehmann, J.; Berruti, F.; Giudicianni, P.; Sanei, H.; Masek, O. Biochar is a long-lived form of carbon removal, making evidence-based CDR projects possible. Biochar 2024, 6, 81. [Google Scholar] [CrossRef]
- Panagos, P.; Köningner, J.; Ballabio, C.; Liakos, L.; Muntwyler, A.; Borrelli, P.; Lugato, E. Improving the phosphorus budget of European agricultural soils. Sci. Total Environ. 2022, 853, 158706. [Google Scholar] [CrossRef] [PubMed]
- Orgiazzi, A.; Panagos, P.; Yigini, Y.; Dunbar, M.B.; Gardi, C.; Montanarella, L.; Ballabio, C. A knowledge-based approach to estimating the magnitude and spatial patterns of potential threats to soil biodiversity. Sci. Total Environ. 2016, 545–546, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Ferrari, E.; M’Barek, R.; Philippidis, G.; Boysen-Urban, K.; Borelli, P.; Montanarella, L.; Panagos, P. Remaining Loyal to Our Soil: A Prospective Integrated Assessment of Soil Erosion on Global Food Security. Ecol. Econ. 2024, 219, 108103. [Google Scholar] [CrossRef]
- EIP-AGRI. Inspirational Ideas: Biodegradable Mulch Films to Reduce Plastic Footprint. 2020. Available online: https://ec.europa.eu/eip/agriculture/en/news/inspirational-ideas-biodegradable-mulch-films.html (accessed on 2 February 2025).
- Sanchez-Hernandez, J.C.; Ro, K.S.; Szogi, A.A.; Chang, K.; Park, B. Earthworms increase the potential for enzymatic bio-activation of biochars made from co-pyrolyzing animal manures and plastic wastes. J. Hazard. Mater. 2021, 408, 124405. [Google Scholar] [CrossRef]
- Sajdak, M.; Muzyka, R. Use of plastic waste as a fuel in the co-pyrolysis of biomass. Part I: The effect of the addition of plastic waste on the process and products. J. Anal. Appl. Pyrolysis 2014, 107, 267–275. [Google Scholar] [CrossRef]
- Bilotto, F.; Christie-Whitehead, K.M.; Barnes, N.; Harrison, M.T. Operationalising net-zero with biochar: Black gold or red herring? Trends Food Sci. Technol. 2024, 150, 104579. [Google Scholar] [CrossRef]
- Rafique, M.I.; Al-Wabel, M.; Al-Farraj, A.S.; Ahmad, M.; Aouak, T.; Al-Swabi, H.A.; Awad Mousa, M. Incorporation of biochar and semi-interpenetrating biopolymer to synthesize new slow release fertilizers and their impact on soil moisture and nutrients availability. Sci. Rep. 2025, 15, 9563. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.-S.; Lee, D.-J. Machine learning applications for biochar studies: A mini-review. Bioresour. Technol. 2024, 394, 130291. [Google Scholar] [CrossRef] [PubMed]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Sai Bharadwaj, A.V.S.L. Agricultural Biomass Waste to Biochar: A Review on Biochar Applications Using Machine Learning Approach and Circular Economy. ChemEngineering 2023, 7, 50. [Google Scholar] [CrossRef]
- Nighojkar, A.; Pandey, S.; Naebe, M.; Kandasubramanian, B.; Soboyejo, W.W.; Plappally, A.; Wang, X. Using machine learning to predict the efficiency of biochar in pesticide remediation. Npj Sustain. Agric. 2023, 1, 1. [Google Scholar] [CrossRef]
- Oral, B.; Coşgun, A.; Günay, M.E.; Yıldırım, R. Machine learning-based exploration of biochar for environmental management and remediation. J. Environ. Manag. 2024, 360, 121162. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Lu, T.; Qian, H.; Liu, Y. Machine learning models reveal how biochar amendment affects soil microbial communities. Biochar 2023, 5, 89. [Google Scholar] [CrossRef]
- Onyekwena, C.C.; Xue, Q.; Li, Q.; Wan, Y.; Feng, S.; Umeobi, H.I.; Liu, H.; Chen, B. Support vector machine regression to predict gas diffusion coefficient of biochar-amended soil. Appl. Soft Comput. 2022, 127, 109345. [Google Scholar] [CrossRef]
- Chen, M.W.; Chang, M.S.; Mao, Y.; Hu, S.; Kung, C.C. Machine learning in the evaluation and prediction models of biochar application: A review. Sci. Prog. 2023, 106, 368504221148842. [Google Scholar] [CrossRef]
- Dagar, J.C.; Gupta, S.R.; Gaur, A. Tree-based farming systems for improving productivity and ecosystem services in saline environments of dry regions: An overview. Farming Syst. 2023, 1, 100003. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Grote, U.; Neubacher, F.; Rahut, D.B.; Do, M.N.; Paudel, G.P. Security risks from climate change and environmental degradation: Implications for sustainable land use transformation in the Global South. Cur. Opin. Environ. Sustain. 2023, 63, 101322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).