Assessing Microplastic Contamination and Depuration Effectiveness in Farmed Pacific Oysters (Crassostrea gigas)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area—Oyster Farm
2.2. Sample Collection
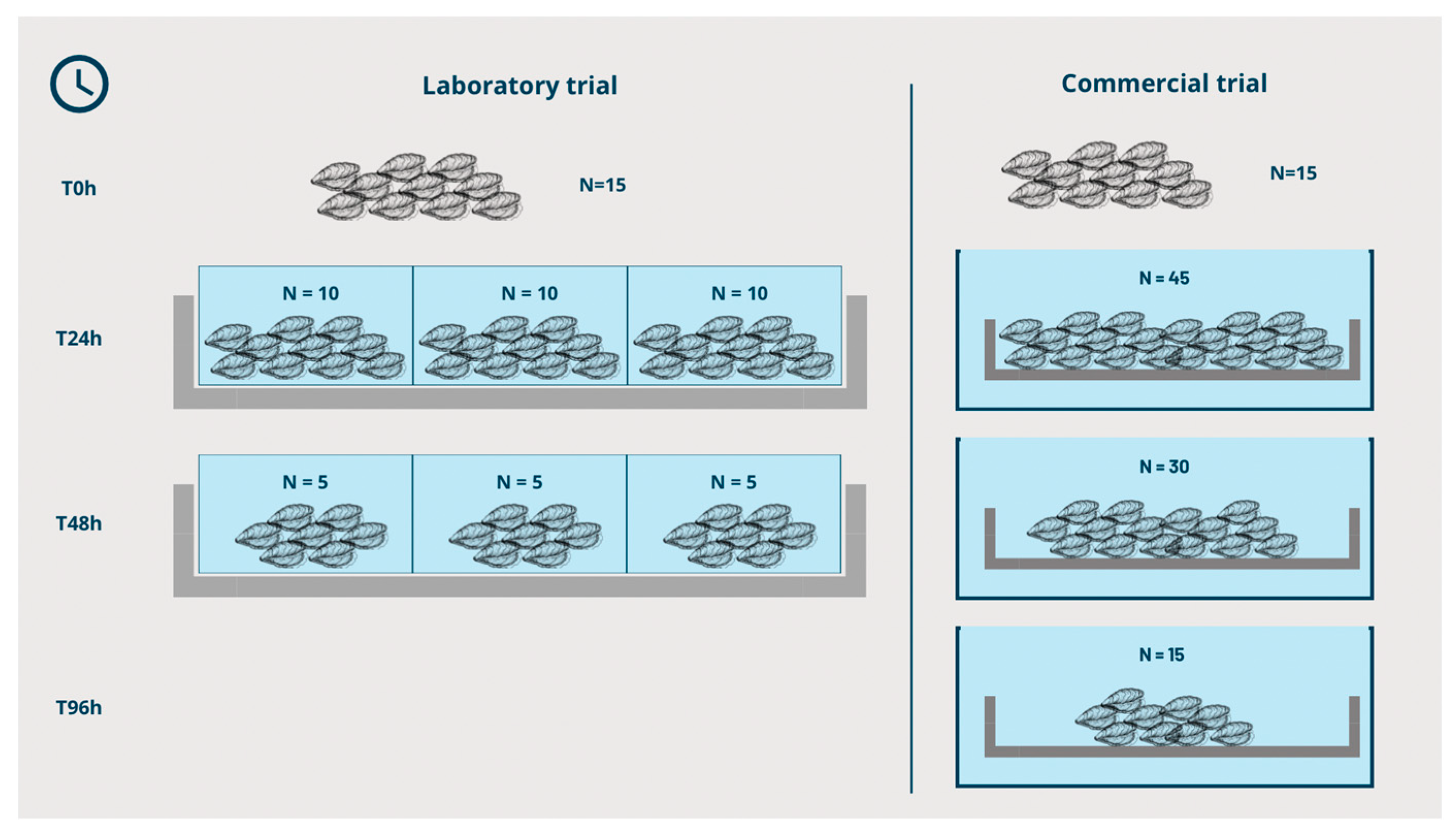
2.3. Depuration Trials
2.3.1. Laboratory Trial
2.3.2. Commercial Depuration
2.4. Sample Preparation and MP Extraction
2.5. MP Characterisation
2.6. Contamination Control
2.7. Data Analysis
3. Results
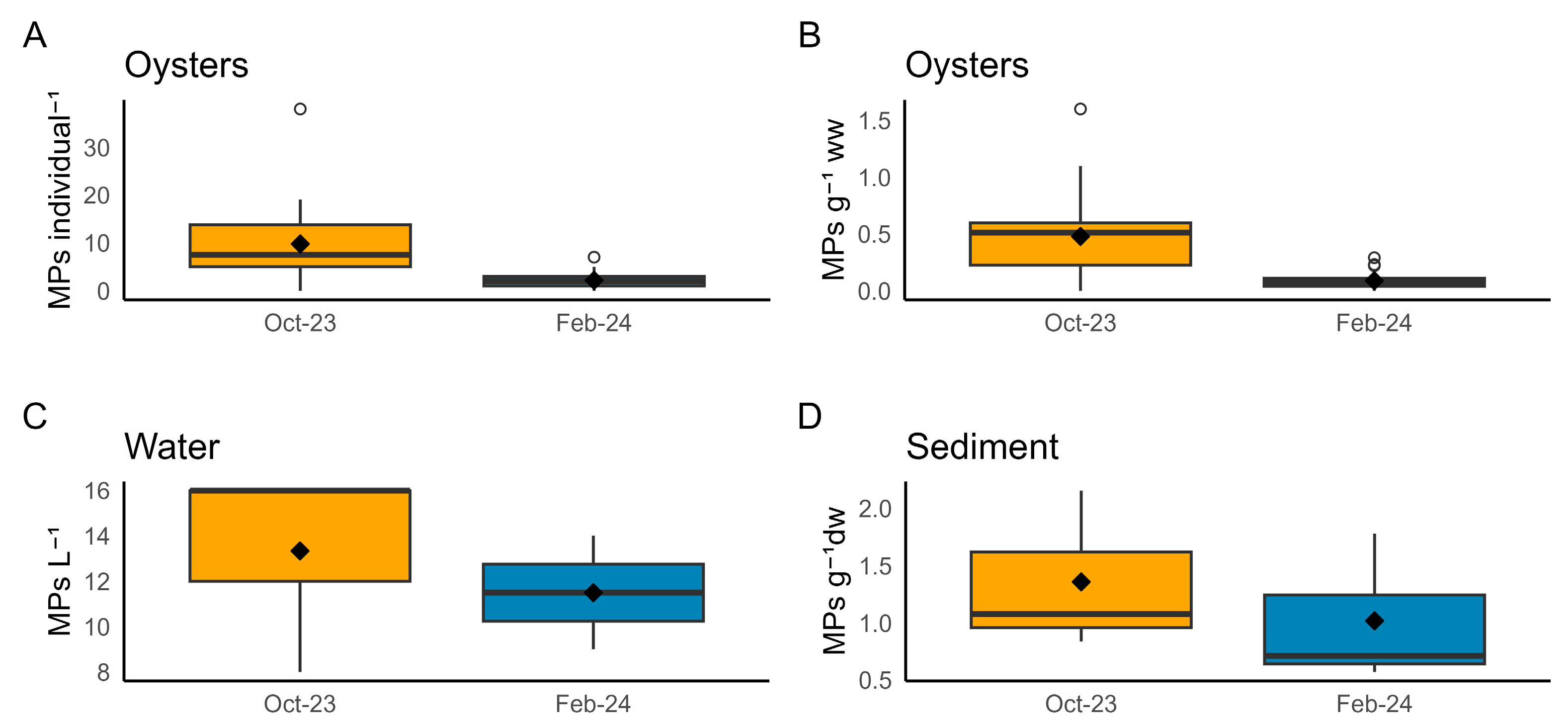
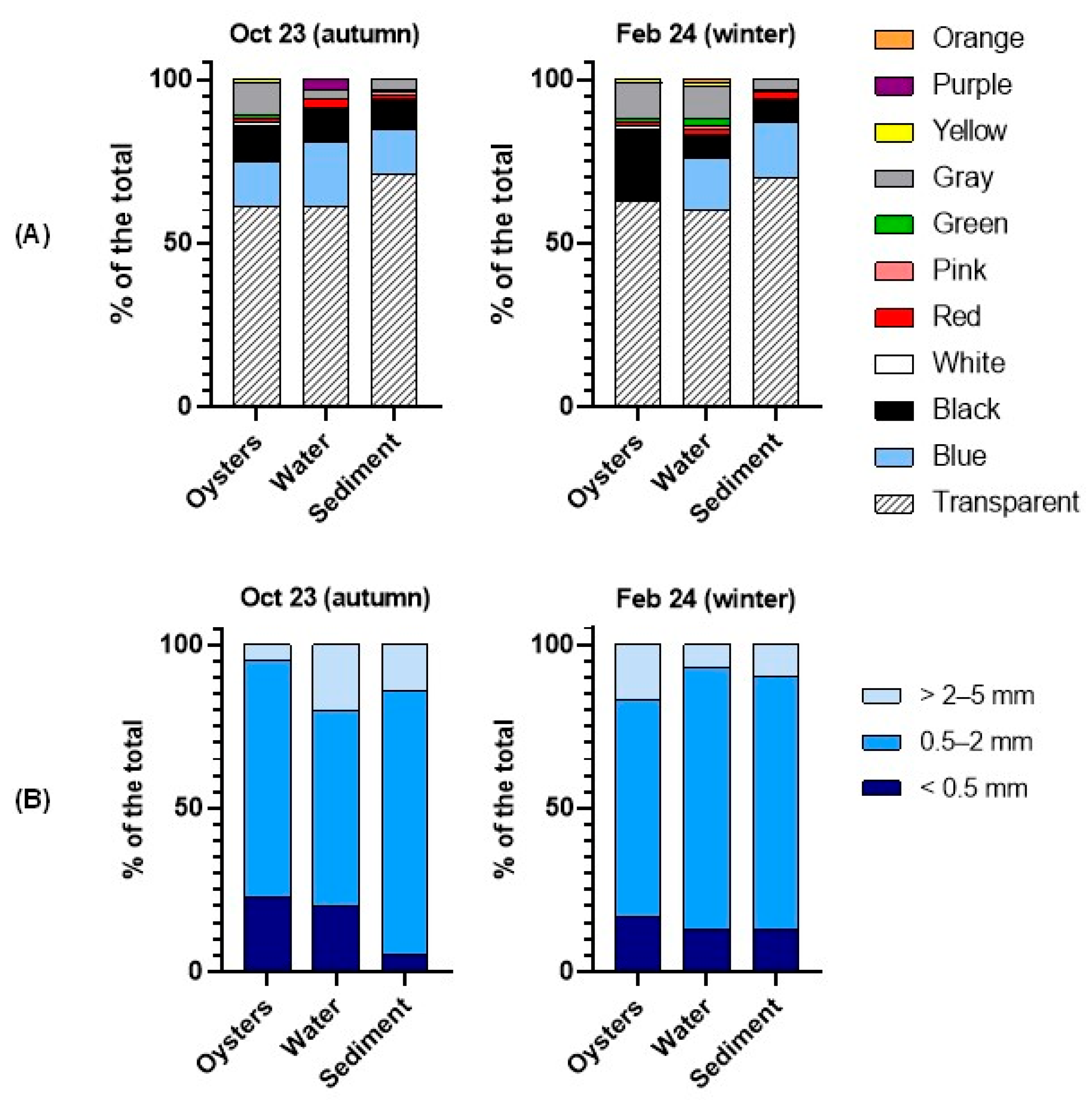
3.1. Microplastics in Oysters and Environmental Samples
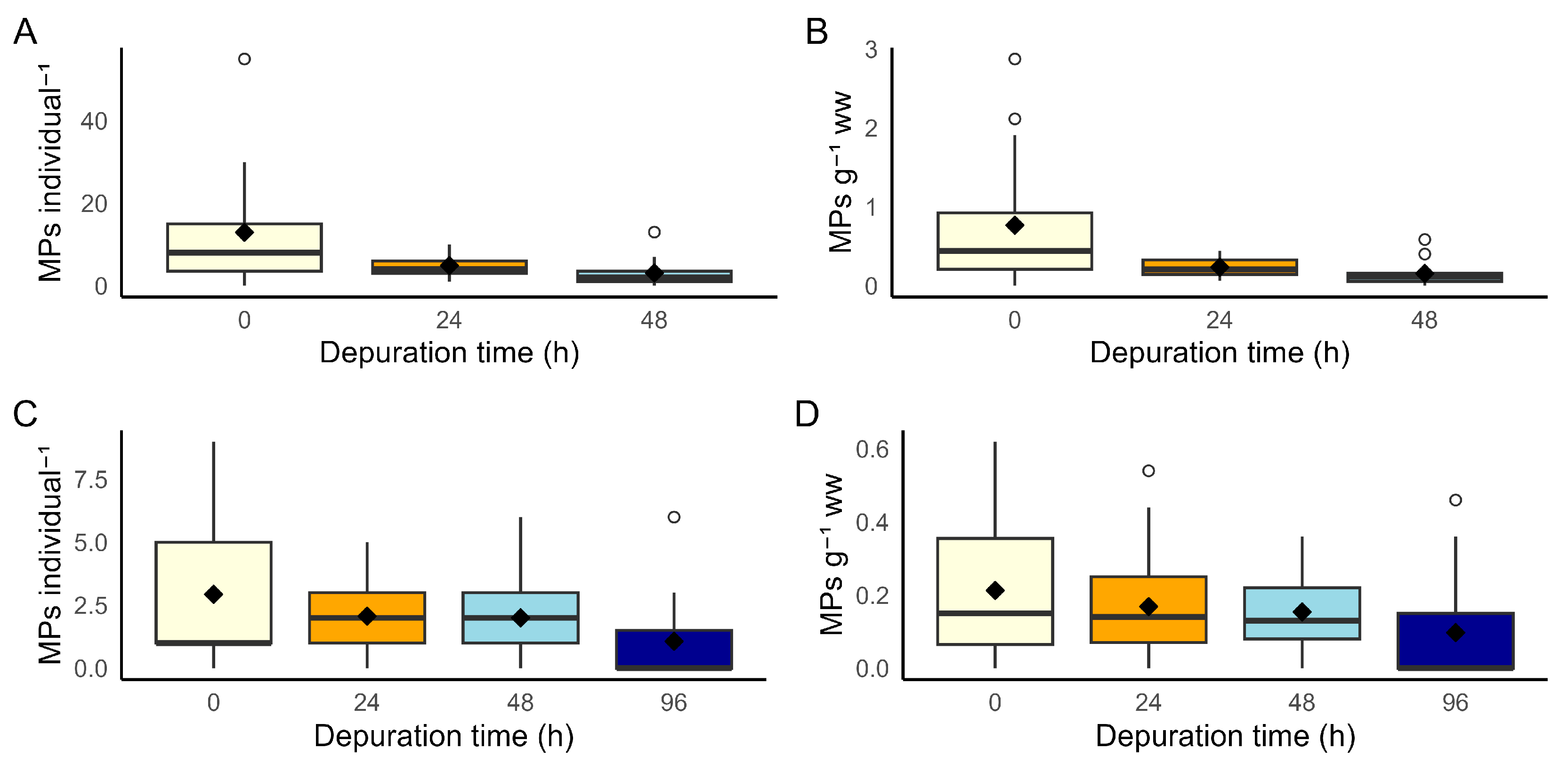
3.2. Depuration Trials
3.2.1. Laboratory Controlled Conditions
3.2.2. Commercial Conditions
4. Discussion
4.1. MP Contamination in Farmed Oysters and Surrounding Environment
4.2. Depuration Efficiency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| PET | Polyethylene terephthalate |
| PE | Polyethylene |
| PP | Polypropylene |
| HDPE | High-density polyethylene |
Appendix A
| Sampling Date | N | Shell Length (cm) | Soft Tissues Wet Weight (g) | Total MPs | Number (and Percentage of Total Number, %) of Oysters Contaminated | MPs per Individual | MPs g−1 ww | MPs ind−1 | |
|---|---|---|---|---|---|---|---|---|---|
| Field assessment | Oct 2023 (autumn) | 30 | 80 ± 7 | 21 ± 5 | 293 | 29 (97) | 0–38 | 0.5 ± 0.3 | 9.8 ± 7.5 |
| Feb 2024 (winter) | 30 | 77 ± 4 | 25 ± 3 | 65 | 25 (83) | 0–7 | 0.09 ± 0.07 | 2.2 ± 1.7 | |
| Depuration trials 1 | Nov 2023 (laboratory) | 15 | 70 ± 5 | 17 ± 2 | 194 | 13 (87) | 0–55 | 0.8 ± 0.9 | 12.9 ± 14.9 |
| Apr 2024 (commercial) | 15 | 71 ± 3 | 14 ± 2 | 44 | 12 (80) | 0–9 | 0.2 ± 0.2 | 2.9 ± 3.1 |
| Methodological Procedures | Airborne Controls | Duration of Procedure | Fibres Detected per Blank |
|---|---|---|---|
| Sample preparation | Open Petri dishes with filtered deionised water | Less than 1 h | 0–3 transparent 0–1 black |
| Microplastic extraction | Less than 30 min | 0–2 transparent | |
| Microplastic characterisation | More than 1 h | 0–5 transparent 0–2 black |
References
- Ramos, S.; Rodrigues, S.M.; Pereira, R.; Silva, D.; Almeida, C.M.R. Floatables and plastic debris in estuarine and coastal marine environments. In Treatise on Estuarine and Coastal Science, 2nd ed.; Baird, D., Elliott, M., Eds.; Elsevier: Oxford, UK, 2024; Volume 6, pp. 467–511. [Google Scholar]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Aleixo, M.; De Pablo, H.; Lopes, C.; Raimundo, J. Microplastics in wastewater: Microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. 2020, 27, 26643–26649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S.; et al. Current research trends on micro- and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef] [PubMed]
- Wayman, C.; Niemann, H. The fate of plastic in the ocean environment—A minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Kazmiruk, T.N.; Kazmiruk, V.D.; Bendell, L.I.; Magar, V. Abundance and distribution of microplastics within surface sediments of a key shellfish growing region of Canada. PLoS ONE 2018, 13, e0196005. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, L.; Chen, Q.; Kalogerakis, N.; Ji, R.; Ma, Y. Interactions between microplastics and organic pollutants: Effects on toxicity, bioaccumulation, degradation, and transport. Sci. Total Environ. 2020, 748, 142427. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Aquaculture in the EU: Mussels, Oysters and Clams Among the Top Products. European Commission. 2024. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/w/ddn-20240430-3 (accessed on 1 May 2025).
- Ward, J.E.; Zhao, S.; Holohan, B.A.; Mladinich, K.M.; Griffin, T.W.; Wozniak, J.; Shumway, S.E. Selective ingestion and egestion of plastic particles by the blue mussel (Mytilus edulis) and eastern oyster (Crassostrea virginica): Implications for using bivalves as bioindicators of microplastic pollution. Environ. Sci. Technol. 2019, 53, 8776–8784. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, J.; Li, Z.; He, H.; Wang, Y.; Wu, H.; Xie, L.; Chen, D.; Wang, L. How does bivalve size influence microplastics accumulation? Environ. Res. 2022, 214 Pt 1, 113847. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, 4501–4530. [Google Scholar]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in fisheries and aquaculture: Status of knowledge on their occurrence and implications for aquatic organisms and food safety. FAO Fish. Aquac. Tech. Pap. 2017, 615, 126. [Google Scholar]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- EUMOFA. The EU Fish Market; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Dias, A.C.; Almeida, C.; da Costa, T.P.; Nunes, M.L.; Marques, A.; Quinteiro, P. Environmental assessment of oyster farming from a life cycle perspective. Sustain. Prod. Consum. 2025, 54, 102–114. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Sáez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Otero, X.L.; Guilhermino, L. Microplastic contamination in marine mussels from the Atlantic coast of North Portugal and human risk of microplastic intake through mussel consumption. Environ. Pollut. 2024, 352, 124133. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.J.; Vale, C.; Marques, F.; Moreirinha, C.; Costa, S.T.; Guilhermino, L.; Joaquim, S.; Matias, D.; Candeias, M.; Rudnitskaya, A. One-year variation in quantity and properties of microplastics in mussels (Mytilus galloprovincialis) and cockles (Cerastoderma edule) from Aveiro lagoon. Environ. Pollut. 2023, 333, 121949. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, L.; de los Santos, C.B.; Zardi, G.I.; Repetto, L.; Nicastro, K.R. Microplastics in commercial bivalves harvested from intertidal seagrasses and sandbanks in the Ria Formosa lagoon, Portugal. Mar. Freshw. Res. 2021, 72, 1092–1099. [Google Scholar] [CrossRef]
- Espincho, F.; Pereira, R.; Rodrigues, S.M.; Silva, D.M.; Almeida, C.M.R.; Ramos, S. Assessing microplastic contamination in zooplanktonic organisms from two river estuaries. Water 2024, 16, 992. [Google Scholar] [CrossRef]
- Pequeno, J.; Antunes, J.; Dhimmer, V.; Bessa, F.; Sobral, P. Microplastics in marine and estuarine species from the coast of Portugal. Front. Environ. Sci. 2021, 9, 579127. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental status of (micro)plastics contamination in Portugal. Ecotoxicol. Environ. Saf. 2020, 200, 110753. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Microplastics contamination along the coastal waters of NW Portugal. Case Stud. Chem. Environ. Eng. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Silva, D.; Cunha, J.; Antunes, C.; Freitas, V.; Ramos, S. Microplastic contamination in an urban estuary: Abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci. Total Environ. 2019, 659, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Vale, C.; Rudnitskaya, A.; Moreirinha, C.; Costa, S.T.; Botelho, M.J. Major characteristics of microplastics in mussels from the Portuguese coast. Environ. Res. 2021, 197, 110993. [Google Scholar] [CrossRef] [PubMed]
- Vital, S.A.; Cardoso, C.; Avio, C.; Pittura, L.; Regoli, F.; Bebianno, M.J. Do microplastic contaminated seafood consumption pose a potential risk to human health? Mar. Pollut. Bull. 2021, 171, 112769. [Google Scholar] [CrossRef] [PubMed]
- Brocardo, G.S.; Saldaña-Serrano, M.; Bertoldi, C.; Miranda Gomes, C.H.A.; Nogueira, D.J.; Leonel, J.; Fernandes, A.N.; Bainy, A.C.D. Microplastics in commercial bivalves and their association with farm structures: A case study in a relevant aquaculture area of Brazil. Reg. Stud. Mar. Sci. 2025, 81, 103965. [Google Scholar] [CrossRef]
- Silva, D.C.C.; Borba, A.; de Sousa, H.C.; Marques, J.C.; Gonçalves, A.M.M. Microplastic pollution in marine bivalves, surface water and sediments from an aquaculture in Portugal and possible intake by humans. Reg. Stud. Mar. Sci. 2025, 85, 104146. [Google Scholar] [CrossRef]
- Mladinich, K.; Holohan, B.A.; Shumway, S.E.; Ward, J.E. Abundance of microplastics at and near a shellfish aquaculture farm: An eastern oyster (Crassostrea virginica) transplant study. Mar. Environ. Res. 2024, 199, 106606. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Ward, J.; Shumway, S. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: A review. J. Shellfish. Res. 2018, 37, 727–746. [Google Scholar] [CrossRef]
- Talbot, R.; Chang, H. Microplastics in freshwater: A global review of factors affecting spatial and temporal variations. Environ. Pollut. 2022, 292, 118393. [Google Scholar] [CrossRef] [PubMed]
- Lerebours, A.; Bathie, M.; Kazour, M.; Amara, R.; Huet, V.; Thomas, H. Spatio-temporal contamination of microplastics in shellfish farming regions: A case study. Mar. Pollut. Bull. 2022, 181, 113842. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Lovatelli, A.; Ababouch, L.; FAO Fisheries and Aquaculture Division. Bivalve Depuration: Fundamental and Practical Aspects; FAO: Rome, Italy, 2008. [Google Scholar]
- Birnstiel, S.; Soares-Gomes, A.; da Gama, B.A.P. Depuration reduces microplastic content in wild and farmed mussels. Mar. Pollut. Bull. 2019, 140, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Covernton, G.A.; Dietterle, M.; Pearce, C.M.; Gurney-Smith, H.J.; Dower, J.F.; Dudas, S.E. Depuration of anthropogenic particles by Pacific oysters (Crassostrea gigas): Feasibility and efficacy. Mar. Pollut. Bull. 2022, 181, 113886. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.T.; Hannon, C.; Švonja, M.; Connellan, I.; Frias, J. Efficacy of microplastic depuration on two commercial oyster species from the west coast of Ireland. J. World Aquac. Soc. 2023, 54, 1217–1234. [Google Scholar] [CrossRef]
- Graham, P.; Palazzo, L.; de Lucia, G.A.; Telfer, T.C.; Baroli, M.; Carboni, S. Microplastics uptake and egestion dynamics in Pacific oysters, Magallana gigas (Thunberg, 1793), under controlled conditions. Environ. Pollut. 2019, 252, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Ertel, B.M.; Gray, A.D. Accumulation and depuration of microplastic fibers, fragments, and tire particles in the eastern oyster, Crassostrea virginica: A toxicokinetic approach. Environ. Pollut. 2022, 308, 119681. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Cabral, H.; Elliott, M. Do fish larvae have advantages over adults and other components for assessing estuarine ecological quality? Ecol. Indic. 2015, 55, 74–85. [Google Scholar] [CrossRef]
- Amorim, E.; Ramos, S.; Elliott, M.; Franco, A.; Bordalo, A.A. Habitat loss and gain: Influence on habitat attractiveness for estuarine fish communities. Estuar. Coast. Shelf Sci. 2017, 197, 244–257. [Google Scholar] [CrossRef]
- European Union. Commission Implementing Regulation (EU) 2019/627 of 15 March 2019—Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625. Off. J. Eur. Union. 2019, L131, 51–100. [Google Scholar]
- Gadelha, J.R.; Rocha, A.C.; Camacho, C.; Eljarrat, E.; Peris, A.; Aminot, Y.; Readman, J.W.; Boti, V.; Nannou, C.; Kapsi, M.; et al. Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW Portuguese coast (Ria de Aveiro). Sci. Total Environ. 2019, 666, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Shumway, S.E.; Mladinich, K.; Blaschik, N.; Holohan, B.A.; Ward, J.E. A critical assessment of microplastics in molluscan shellfish with recommendations for experimental protocols, animal husbandry, publication, and future research. Rev. Fish. Sci. Aquacult. 2023, 1–133. [Google Scholar] [CrossRef]
- Silva, D.M.; Almeida, C.M.R.; Guardiola, F.; Rodrigues, S.M.; Ramos, S. Optimization of an Analytical Protocol for the Extraction of Microplastics from Seafood Samples with Different Levels of Fat. Molecules 2022, 27, 5172. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, L.; Castiglioni, M.; Rodrigues, S.M.; Freitas, V.; Bruzzoniti, M.C.; Ramos, S.; Almeida, C.M.R. Microplastic in marine environment: Reworking and optimisation of two analytical protocols for the extraction of microplastics from sediments and oysters. MethodsX 2020, 7, 101116. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Adaptation of a laboratory protocol to quantify microplastics contamination in estuarine waters. MethodsX 2019, 6, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide monitoring of microplastics in bivalves from the coastal environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Shim, W.J.; Cho, Y.; Han, G.M.; Song, Y.K.; Hong, S.H. A close relationship between microplastic contamination and coastal area use pattern. Water Res. 2020, 171, 115400. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Joint Research Centre. Guidance on the Monitoring of Marine Litter in European Seas: An Update to Improve the Harmonised Monitoring of Marine Litter under the Marine Strategy Framework Directive; Publications Office of the European Union: Luxembourg, 2021.
- Silva, D.M.; Almeida, C.M.R.; Guardiola, F.A.; Pereira, R.; Rodrigues, S.M.; Ramos, S. Uncovering Microplastics Contamination in Canned Seafood. Food Chem. 2024, 448, 139049. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis, R Package Version 0.8.26; Computer Software. 2019. Available online: https://github.com/droglenc/FSA (accessed on 15 November 2024).
- Manthopoulos, M. Presence of Microplastic in Commercial Bivalves along the Portuguese Coast, Comparing Different Aquaculture Systems. Master’s Thesis, University of Algarve, Portugal, 2022. Available online: http://hdl.handle.net/10400.1/19188 (accessed on 25 June 2025).
- Baechler, B.R.; Granek, E.F.; Hunter, M.V.; Conn, K.E. Microplastic concentrations in two Oregon bivalve species: Spatial, temporal, and species variability. Limnol. Oceanogr. Lett. 2020, 5, 54–65. [Google Scholar] [CrossRef]
- Ding, J.; Sun, C.; He, C.; Li, J.; Ju, P.; Li, F. Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 2021, 782, 146830. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, J.; Teng, J.; Ren, J.; Zheng, P.; Zhu, X.; Liu, Y.; Sun, X.; Yuan, S.; Wang, Q. Seasonal change of microplastics uptake in the Pacific oysters Crassostrea gigas cultured in the Yellow Sea and Bohai Sea, China. Mar. Pollut. Bull. 2022, 185, 114341. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Poirier, L.; Pham, Q.T.; Lagarde, F.; Zalouk-Vergnoux, A. Factors influencing the microplastic contamination of bivalves from the French Atlantic coast: Location, season and/or mode of life? Mar. Pollut. Bull. 2018, 129, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Saldaña-Serrano, M.; Bastolla, C.L.V.; Mattos, J.J.; Lima, D.; Freire, T.B.; Nogueira, D.J.; De-la-Torre, G.E.; Righetti, B.P.H.; Zacchi, F.L.; Gomes, C.H.A.M.; et al. Microplastics and linear alkylbenzene levels in oysters Crassostrea gigas driven by sewage contamination at an important aquaculture area of Brazil. Chemosphere 2022, 307, 136039. [Google Scholar] [CrossRef] [PubMed]
- Fernández-González, V.; Andrade, J.M.; Ferreiro, B.; López-Mahía, P.; Muniategui-Lorenzo, S. Monitorization of polyamide microplastics weathering using attenuated total reflectance and microreflectance infrared spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120162. [Google Scholar] [CrossRef] [PubMed]
- Ruangpanupan, N.; Ussawarujikulchai, A.; Prapagdee, B.; Chavanich, S. Seasonal variation in the abundance of microplastics in three commercial bivalves from Bandon Bay, Gulf of Thailand. Mar. Pollut. Bull. 2023, 197, 115600. [Google Scholar] [CrossRef] [PubMed]
- Dridi, S.; Romdhane, M.S.; Elcafsi, M.H. Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas, in relation to the gametogenic cycle and environmental conditions of the Bizert lagoon, Tunisia. Aquaculture 2007, 263, 238–248. [Google Scholar] [CrossRef]
- Mao, Y.; Zhou, Y.; Yang, H.; Wang, R. Seasonal variation in metabolism of cultured Pacific oyster, Crassostrea gigas, in Sanggou Bay, China. Aquaculture 2006, 253, 322–333. [Google Scholar] [CrossRef]
- Barr, J.M.; Munroe, D.; Rose, J.M.; Calvo, L.; Cheng, K.M.; Bayer, S.; Kreeger, D. Seasonal Feeding Behavior of Aquaculture Eastern Oysters (Crassostrea virginica) in the Mid-Atlantic. Estuaries Coasts 2024, 47, 789–804. [Google Scholar] [CrossRef]
- Rezania, S.; Park, J.; Md Din, M.F.; Mat Taib, S.; Talaiekhozani, A.; Kumar Yadav, K.; Kamyab, H. Microplastics pollution in different aquatic environments and biota: A review of recent studies. Mar. Pollut. Bull. 2018, 133, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Truchet, D.M.; López, A.D.F.; Ardusso, M.G.; Rimondino, G.N.; Buzzi, N.S.; Malanca, F.E.; Spetter, C.V.; Severini, M.D.F. Microplastics in Bivalves, Water and Sediments from a Touristic Sandy Beach of Argentina. Mar. Pollut. Bull. 2021, 173, 113023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, J.; Razanajatovo, R.M.; Jiang, H.; Zou, H.; Zhu, W. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 648, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Dhanker, R.; Bhawna; Tomar, A.; Raza, S.; Sharma, A. Fishing gears and nets as a source of microplastic. In Microplastic Pollution; Shahnawaz, M., Adetunji, C.O., Dar, M.A., Zhu, D., Eds.; Springer: Singapore, 2024; pp. 127–140. [Google Scholar] [CrossRef]
- Wright, L.S.; Napper, I.E.; Thompson, R.C. Potential microplastic release from beached fishing gear in Great Britain’s region of highest fishing litter density. Mar. Pollut. Bull. 2021, 173, 113115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Liu, Y.; Yu, K.; Zhang, H.; Yu, H.; Jiang, J. Neglected microplastics pollution in the nearshore surface waters derived from coastal fishery activities in Weihai, China. Sci. Total Environ. 2021, 768, 144484. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; AftabUddin, S.; Akhter, F.; Nusrat, N.; Rahaman, A.; Sikder, M.N.A.; Monwar, M.M.; Chowdhury, M.S.N.; Jiang, S.; Shi, H.; et al. Surface water, sediment, and biota: The first multi-compartment analysis of microplastics in the Karnafully river, Bangladesh. Mar. Pollut. Bull. 2022, 180, 113820. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Strezov, V.; Weldekidan, H.; Kumar, R.; Kan, T.; Sarkodie, S.A.; He, J.; Dastjerdi, B.; Wilson, S.P. Interrelationship of microplastic pollution in sediments and oysters in a seaport environment of the eastern coast of Australia. Sci. Total Environ. 2019, 695, 133924. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, L.; Ruan, H.D.; Chen, J.; Lu, J.; Lee, C.-H.; Jiang, S.Y. Quantitative and qualitative determination of microplastics in oyster, seawater and sediment from the coastal areas in Zhuhai, China. Mar. Pollut. Bull. 2021, 164, 112000. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Yao, Q.; Zhang, J.; Wang, D. Effects of seasonal variation and resuspension on microplastics in river sediments. Environ. Pollut. 2021, 286, 117403. [Google Scholar] [CrossRef] [PubMed]
- Wootton, N.; Sarakinis, K.; Varea, R.; Reis-Santos, P.; Gillanders, B.M. Microplastic in oysters: A review of global trends and comparison to southern Australia. Chemosphere 2022, 307, 136065. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Q.; Huang, Y.; Jiang, Y.; Li, J.; Michal, J.J.; Jiang, Z.; Xu, Y.; Lan, W. Long-term trends of microplastics in seawater and farmed oysters in the Maowei Sea, China. Environ. Pollut. 2021, 273, 116450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Q.; Li, Y.; Tan, S.; Kang, Z.; Yu, X.; Lan, W.; Cai, L.; Wang, J.; Shi, H. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China. Sci. Total Environ. 2019, 658, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Newell, R.I.E. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish. Res. 2004, 23, 51–61. [Google Scholar]
- Ding, J.; Sun, C.; Li, J.; Shi, H.; Xu, X.; Ju, P.; Jiang, F.; Li, F. Microplastics in global bivalve mollusks: A call for protocol standardization. J. Hazard. Mater. 2022, 438, 129490. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.V.; Nobre, C.R.; Moreno, B.B.; Semensatto, D.; Sanz-Lazaro, C.; Moreira, L.B.; Castro, Í.B. Oysters and mussels as equivalent sentinels of microplastics and natural particles in coastal environments. Sci. Total Environ. 2023, 874, 162468. [Google Scholar] [CrossRef] [PubMed]
- Ó Briain, O.; Marques Mendes, A.R.; McCarron, S.; Healy, M.G.; Morrison, L. The role of wet wipes and sanitary towels as a source of white microplastic fibres in the marine environment. Water Res. 2020, 182, 116021. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester textiles as a source of microplastics from households: A mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Šaravanja, A.; Pušić, T.; Dekanić, T. Microplastics in wastewater by washing polyester fabrics. Materials 2022, 15, 2285. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Hussien, N.A.; Mohammadein, A.; Tantawy, E.M.; Khattab, Y.; Al Malki, J.S. Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA. Open Life Sci. 2021, 16, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Gooch, J.W. Polyvinyl stearate. In Encyclopedic Dictionary of Polymers; Gooch, J.W., Ed.; Springer: New York, NY, USA, 2011; p. 578. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Riccardi, N.; Magni, S.; Modesto, V.; Fossati, M.; Binelli, A. First comparative assessment of contamination by plastics and non-synthetic particles in three bivalve species from an Italian sub-alpine lake. Environ. Pollut. 2023, 330, 121752. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhang, L.; Li, R.; Wang, Y.; Guo, J.; Yu, K.; Wang, S. Underestimated microplastic pollution derived from fishery activities and “hidden” in deep sediment. Environ. Sci. Technol. 2020, 54, 2210–2217. [Google Scholar] [CrossRef] [PubMed]
- Covernton, G.A.; Collicutt, B.; Gurney-Smith, H.J.; Pearce, C.M.; Dower, J.F.; Ross, P.S.; Dudas, S.E. Microplastics in bivalves and their habitat in relation to shellfish aquaculture proximity in coastal British Columbia, Canada. Aquac. Environ. Interact. 2019, 11, 357–374. [Google Scholar] [CrossRef]
- Kolandhasamy, P.; Su, L.; Li, J.; Qu, X.; Jabeen, K.; Shi, H. Adherence of microplastics to soft tissue of mussels: A novel way to uptake microplastics beyond ingestion. Sci. Total Environ. 2018, 610–611, 635–640. [Google Scholar] [CrossRef] [PubMed]

| Sampling Date | Samples | Number and Colour of Fibres | Polymer Type | |
|---|---|---|---|---|
| Field assessment | October 2023 (autumn) | Oysters | 3 transparent, 2 blue, 1 grey and 1 black | Adipate |
| 4 transparent, 3 grey and 1 black | Cellulose | |||
| 2 black and 2 transparent | PET | |||
| Water 1 | - | - | ||
| Sediment | 1 transparent | Adipate | ||
| 7 transparent, 2 grey, 5 blue and 1 red | Cellulose | |||
| February 2024 (winter) | Oysters | 2 transparent, 1 blue and 1 green | Adipate | |
| 1 black and 1 blue | Cellulose | |||
| 1 transparent | PP | |||
| 1 black | Poly(vinyl stearate) | |||
| 1 black and 1 grey | Rayon | |||
| Water | 1 blue | Adipate | ||
| 4 blue and 3 transparent | Cellulose | |||
| 2 black and 1 grey | PET | |||
| Sediment | 10 transparent, 1 grey and 1 blue | Cellulose | ||
| 1 transparent and 1 blue | PET | |||
| Depuration trials | November 2023 (laboratory) | Oysters | 1 green, 5 blue, 1 black, 1 transparent, 1 grey and 1 green | Adipate |
| 4 grey, 3 transparent, 3 black, 3 blue, 1 purple, 1 green and 1 red | Cellulose | |||
| 1 transparent, 1 grey and 1 blue | Cellophane | |||
| 1 transparent and 1 black | Polyacrylate | |||
| 3 transparent and 1 black | PET | |||
| 3 transparent, 1 yellow and 1 black | PE | |||
| 1 transparent | Rayon | |||
| April 2024 (commercial) | Oysters | 6 transparent, 5 blue, 1 black and 1 grey | Cellulose | |
| 1 grey and 1 black | PET | |||
| 1 transparent and 1 blue | PP | |||
| 1 transparent | Rayon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, C.; Silva, D.M.; Espincho, F.; Rodrigues, S.M.; Pereira, R.; Almeida, C.M.R.; Ramos, S.; Freitas, V. Assessing Microplastic Contamination and Depuration Effectiveness in Farmed Pacific Oysters (Crassostrea gigas). Environments 2025, 12, 254. https://doi.org/10.3390/environments12080254
Moura C, Silva DM, Espincho F, Rodrigues SM, Pereira R, Almeida CMR, Ramos S, Freitas V. Assessing Microplastic Contamination and Depuration Effectiveness in Farmed Pacific Oysters (Crassostrea gigas). Environments. 2025; 12(8):254. https://doi.org/10.3390/environments12080254
Chicago/Turabian StyleMoura, Cláudia, Diogo M. Silva, Francisca Espincho, Sabrina M. Rodrigues, Rúben Pereira, C. Marisa R. Almeida, Sandra Ramos, and Vânia Freitas. 2025. "Assessing Microplastic Contamination and Depuration Effectiveness in Farmed Pacific Oysters (Crassostrea gigas)" Environments 12, no. 8: 254. https://doi.org/10.3390/environments12080254
APA StyleMoura, C., Silva, D. M., Espincho, F., Rodrigues, S. M., Pereira, R., Almeida, C. M. R., Ramos, S., & Freitas, V. (2025). Assessing Microplastic Contamination and Depuration Effectiveness in Farmed Pacific Oysters (Crassostrea gigas). Environments, 12(8), 254. https://doi.org/10.3390/environments12080254








