1. Introduction
Unlike the period when the synthesis and use of pesticides rapidly developed, today, due to the potentially harmful effects, every compound (before its introduction into production and application) undergoes a strict registration procedure aimed at assessing the risk to human health, animal welfare, and the environmental impact [
1]. Considering that intensive crop production has largely relied particularly on the use of herbicides as the dominant plant protection products for decades, their impact on soil and water quality is a priority issue regarding environmental protection and a major cause of global concern [
2].
Soil contamination with pesticides may result from their direct application to control harmful organisms, treated seed and combined use with mineral fertilizers. Also, they can indirectly contaminate soil by runoff from treated plants, irrigation with contaminated water, drift during the treatment of surrounding crops, wind, or rain [
3,
4]. Additional sources of pollution include inappropriate disposal of packaging waste, together with unregulated dumpsites. Pesticide losses in general, depending on the application method and the type of sprayer, range from 22% to 80% [
5], so a significant part remains in the environment [
6,
7]. The behavior of pesticides in the soil is influenced by the physico-chemical properties of pesticides, applied quantity, water solubility, application method, ability of binding to the colloidal particles, soil type and fertility, climatic factors, etc. Adsorption, desorption, diffusion, and degradation processes are directly related to their persistence and leaching into the soil [
8]. A particular concern is the occurrence of phytotoxicity in subsequent crops in crop rotation, e.g., residues of certain herbicides applied in the previous crop can cause increased phytotoxicity in the following crop. Similarly, many herbicides, when applied on sandy and highly permeable soils, are quickly leached into the root zone of the cultivated plant and may exhibit phytotoxic effects. Along with the aforementioned, the pesticide application must not disturb the activities of soil microorganisms, which are essential for the cycling of nutrients in the soil. They also play a significant role in the creation and maintenance of soil fertility, influence plant growth and development, and degrade herbicides and other pollutants. Additionally, these microorganisms serve as indicators of changes in the physico-chemical soil properties, which arise under the influence of various external environmental factors [
9,
10].
Levels of pesticides in environmental samples are measured mostly in micrograms and nanograms [
11,
12,
13]. Even in small amounts, releases into the environment could result in the long-term accumulation and biomagnification of some pollutants, potentially complicating their negative effects [
13]. Pesticides have been reported to affect human health in various ways, which can lead to serious consequences such as endocrine disruption, neurological disruption, and immune and reproductive system effects [
13,
14,
15]. Consequently, the toxic effects of pesticides on non-target organisms have created great concern worldwide.
A lot of research shows that more than 60% of European soils are considered unhealthy, and around 2.8 million localities within the European Union (EU) have been identified as potentially contaminated. Furthermore, the results suggest that this situation is worsening over time [
16]. Despite the occurrence of soil contamination, which is a significant concern, the monitoring of pesticide residues in soil in the EU is not adequately regulated compared to water monitoring. There is also a lack of extensive international studies about pesticide contamination in soil, with most of the existing studies focused on individual active substances or a limited number of them [
17]. Some European countries have included reference or maximum levels of pesticides that are no longer in use in their legislation for soil, as their high persistence makes their monitoring necessary, while many currently used pesticides are not yet regulated in this way [
17,
18,
19]. The Soil Surveillance and Resilience Law could significantly change the existing conditions. In addition, the European Commission has introduced a new EU Soil Strategy for 2030, aiming to ensure that all EU soil ecosystems are in a healthy state by 2050 [
20].
Soil, in which pesticides accumulate, represents a serious ecological problem as pesticides, particularly herbicides, are transferred to other ecosystem components, including surface and groundwater. The area of water protection and contamination within the EU has been regulated since 2000 by the Water Framework Directive (WFD) [
21]. In recent years certain changes and additions have been introduced, along with proposals for new changes. One of the seventeen goals proposed by the United Nations within the 2030 Agenda for Sustainable Development includes the protection of aquatic ecosystems and the reduction in aquifer pollution [
22]. Recently, the European Commission proposed changes to this directive to address the existing shortcomings and adopted new exceptions related to its application, according to which the good status of water bodies is achieved if all parameters are qualified as good. According to the latest updates, all water bodies need to accomplish good status by 2027. The most recent results reveal that at least 35% of European surface waters do not achieve good chemical status and 51% show inadequate ecological status [
23].
In parallel, Directive 2000/60/EC [
21], amended by Directives 2008/105/EC [
24] and 2013/39/EU [
25], sets limits for 45 different substances in various water bodies. Among the 45 chemical contaminants currently classified as priority substances, which include pesticides, metals, and industrial chemicals, 21 are categorized as priority hazardous substances regarding their bioaccumulation potential, persistence, and toxicity. Most of these substances are no longer in use [
25]. However, there is still an absence of extensive monitoring of pesticide residues that are currently intensively applied. This is of great importance considering the trend of exclusion from using more pesticides and the intensification of applying the remaining current ones.
The particular challenges regarding pesticide residue presence in water are canal network waterways located among arable land under intensive agricultural production. Water bodies near agricultural fields are particularly vulnerable to contamination, as pesticides from treated areas are directly transported into these aquatic systems. These water bodies accumulate significantly higher pesticide concentrations compared to more distant or larger water bodies. When rainfall occurs immediately after pesticide application on crop fields it can lead to the rapid runoff of pesticide molecules. Rainfall or irrigation water facilitates the transport of pesticides, carrying them into nearby surface waters through runoff or into groundwater through leaching.
Crop production uses vast amounts of water, which is often contaminated with substances such as pesticides originating from surrounding arable fields. Contaminated irrigation water can transfer these pollutants to other crops and the environment, affecting the quality of agricultural products and potentially entering the food chain. This study aims to improve the understanding of the environmental fate of currently used herbicides by analyzing canal water used for irrigation and the agricultural soil in the immediate vicinity. Given the existing gaps in their monitoring, special attention is paid to herbicides in use and to watercourses located on arable land. Monitoring was focused on the Danube–Tisza–Danube canal system, located in the main agricultural region of Serbia. The majority of the selected herbicides are commonly applied in crop production, reflecting the dominant agricultural practices in the study area.
2. Materials and Methods
2.1. Study Area
Danube–Tisza–Danube (DTD) is a canal system located in northern Serbia. It is a unique system built for flood control, amelioration, water supply, wastewater management, tourism, and fishing. Situated in the middle of the Pannonian plain, the canal system covers 12.700 km
2, with a total length of 929 km. Part of the system of waterways is the Bogojevo–Bečej canal (
Figure 1), which also forms part of the wider Danube–Tisa–Danube canal system. This canal is an important watercourse that connects the Tisza River and the Danube River, and other rivers in the Danube basin. It allows navigation and transport between Bečej and Bogojevo, linking to the Danube, Tisza, and smaller canals. Specific to this canal is that it is situated in the main agricultural region under intensive crop production in conventional agriculture, which implies intensive use of plant protection products.
Monitoring was conducted in late spring since this is the time of year when herbicide levels in the environment are expected to reach their peak.
2.2. Chemicals
Certified analytical standards of herbicides with a high purity grade (>98%) were obtained from the manufacturer Dr Ehrenstorfer (
Table S1). The solvents used for the extraction and chromatography were obtained from Fisher Scientific (Leicestershire, UK). Individual herbicide stock standard solutions were prepared by dissolving precisely weighed analytical standards in 10 mL of an appropriate solvent (acetonitrile or methanol), after which they were protected from light and stored at −20 °C in dark glass bottles with PTFE closures during the experiment, i.e., 2 months [
26]. Through further dilution, working standard solutions of different concentrations were prepared.
2.3. Soil Sampling and Extraction
Soil samples were collected during May and June 2024, at 260 localities, from two depths (0–30 cm and 30–60 cm), along a canal (in total 520 soil samples). From each sampling site approximately 500 g of soil sample was taken by the agrochemical probe. All samples, collected in a cloth bag, were transported to the laboratory. Afterwards, soil samples were air-dried, ground, and sieved to determine the granulometric composition, textural class, and basic chemical properties.
pH values for each sample were determined in water (H
2O) and potassium chloride (KCl) according to the SRPS EN ISO 10390:2022 method [
27]. The content of calcium carbonate (CaCO
3) was determined according to the SRPS ISO 10693:2005 [
28], organic matter content was estimated by the volumetric dichromate method (HRN EN ISO 14235) [
29] and defined according to Tyurin, while the base saturation was determined according to Kappen based on the method.
Herbicide extraction from soil samples was performed using the QuEChERS-based method [
30]. Previously prepared soil samples (dried and ground) (5 g) were transferred to a polypropylene cuvette (50 mL), then 20 mL of acetonitrile containing 1% HCOOH was added alongside 10 mL of ultrapure water (Fisher Scientific, Leicestershire, UK). Afterwards, the CHROMABOND
® QuEChERS (Sigma-Aldrich, Bellefonte, PA, USA) buffer salt mixture (4 g MgSO
4, 1 g NaCl, 0.5 g C
6H
6Na
2O
7·1.5 H
2O, 1 g Na
3C
6H
5O
7·2 H
2O) along with Ceramic Homogenizers were added, and then the samples were hand shaken for 1 min and vortexed for 3 min. After that, the samples were left in an ultrasonic bath for 10 min, followed by centrifugation (Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) for 5 min at 4000 rpm. An aliquot of the supernatant was transferred into a 15 mL cuvette and left in the refrigerator at +4 °C, for 2 h. Afterwards, the CHROMABOND QuEChERS Clean-up salt mixture (900 mg MgSO
4, 150 mg Diamino (PSA), 150 mg C18), was added into the cuvettes, which were then hand shaken for 1 min and centrifuged for 5 min at 4000 rpm. The extract was evaporated using a nitrogen stream, dissolved in 1 mL of acetonitrile, filtered through a 0.45 µm membrane filter (Macherey-Nagel GmbH & Co. KG, Duren, Germany), and analyzed.
2.4. Water Sampling and Extraction
Specifically, one hundred water samples were collected using an extendable sampling pole at different points along the canal from Bogojevo to Bečej, during May and June 2024. The samples were collected into glass bottles (volume 1 L) from each sampling site, with the addition of 1 mL of formic acid. Afterwards, the bottles were sealed and transferred to the laboratory. The samples were stored in a refrigerator at +4 °C for approximately 24 to 48 h, until extraction.
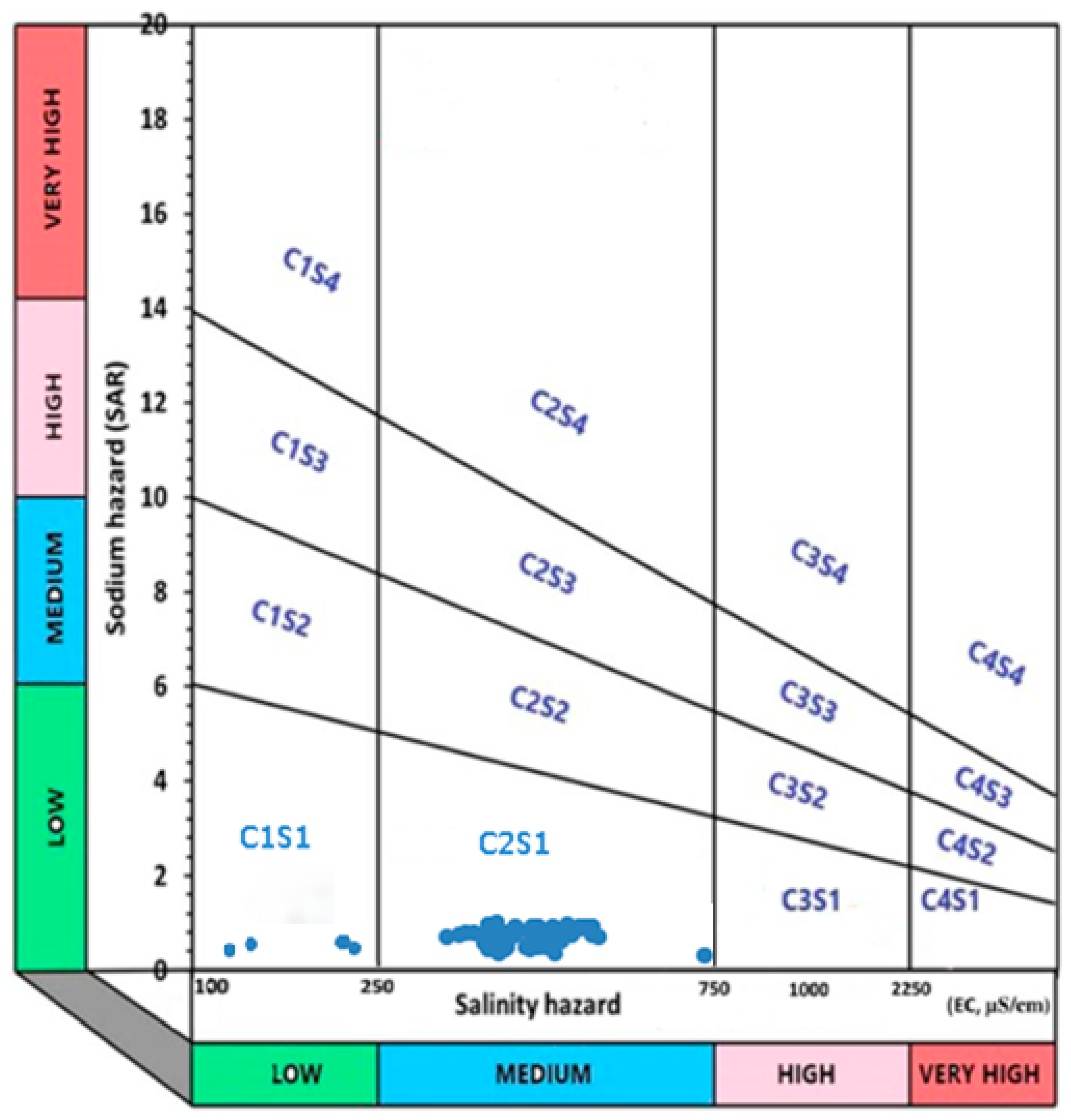
Physico-chemical water quality parameters including pH value, electrical conductivity, Ca, Mg, Na presence, and the Na adsorption ratio were analyzed.
Herbicide extraction from water was performed by solid-phase disk extraction using EmporeTM C-18 disks of a diameter of 47 mm (Cat no. 2215 CDC Analytical). Before extraction, the disk was conditioned with 5 mL of methanol and 5 mL of water. Before the disk was dried, the water sample (1000 mL) was passed through the disk at a flow rate of approximately 10 mL/min, using an ENVI 6.1 disk holder manifold (Supelco Cat No 57173 and 57174) that maintained a constant vacuum. Afterwards, the disk was dried under a vacuum for 30 min and the analytes were eluted using a mobile phase (methanol containing 0.05% HCOOH) (2 × 5 mL each). Then the extracts were evaporated in a stream of nitrogen to dryness, and after that reconstituted in the mobile phase (1 mL) and analyzed for the content of 41 herbicides.
2.5. Method Validation
The method was validated by examining the linearity of the detector response, determining the extraction yield, reproducibility of measurement, and limit of detection. The linearity of the detector response for 41 herbicides ranged from 0.9906 to 0.9998. To test the extraction yield of herbicide residues, an appropriate amount of salt was added to 1 L of deionized water to ensure the same ionic strength and conductivity of approximately 388 µS/cm (average value of the tested samples). The water was then spiked in triplicate with a solution of a standard mixture of herbicides of individual concentrations of about 0.1 µg/L, using TPP (triphenylphosphate) as an internal standard. The spiked water samples were conditioned by adding 5 mL of methanol to modify the polarity and extracted in the same way as real water samples from irrigation canals. A control laboratory soil sample, spiked by adding 1 mL of a mixed herbicide standard with individual concentrations of approximately 5 µg/mL to 500 g of soil, was used to determine the soil extraction yield. The spiked soil was then homogenized in a rotary shaker for 24 h, after which the extractions were repeated three times in the same manner as with real samples. The achieved yield was up to 96% for water samples and up to 103% for spiked soil samples [
31]. The obtained pesticide residue concentrations were not corrected for the achieved yields of individual pesticides. The limit of detection (LOD) was from 0.1 µg/kg to 1 µg/kg for soil and from 0.0007 µg/L to 0.0139 µg/L for water samples. Average achieved reproducibility (RSD%) was 23.2%.
As control laboratory samples, spiked samples of deionized water and soil were prepared and analyzed in the same manner as described for determining the extraction yield. After each spiked control laboratory sample, pure solvent was passed through the chromatograph as a sample to confirm that no analyte carryover had occurred.
2.6. Herbicide Residues Analysis
The content of herbicide residues in soil and water samples was analyzed using GC-MS/MS and LC-MS/MS due to the different natures of herbicides.
2.6.1. The GC-MS/MS Conditions
Herbicide residue analysis was performed by a Thermo Trace 1300 TSQ 9000 (Thermo Scientific, Austin, TX, USA), operated through X-Calibur software ver. 3.0. Chromatographic separation was achieved using an HP-5-MS column (30 m length × 0.25 mm internal diameter, 0.25 μm film thickness). High-purity helium (99.999 vol%, Linde) served as the carrier gas at a constant flow rate of 1 mL/min. The oven-temperature program started at 60 °C with a 1 min hold, followed by an increase of 10 °C/min up to 180 °C (held for 2 min), and then a ramp of 5 °C/min up to a final temperature of 280 °C, which was maintained for 5 min. The injector was set to 250 °C in the splitless mode, with an injection volume was 1 μL. The ion source temperature was maintained at 230 °C and the interface at 280 °C. Mass spectrometric detection was performed using electron ionization at 70 eV. In selected ion monitoring (SIM) mode, specific target ions were monitored within designated time windows based on their corresponding retention times.
2.6.2. The LC-MS/MS Conditions
The herbicides were quantified using an LC-MS/MS VANQUISH CORE/TSQ Quantis Plus on a Thermo Scientific™ Hypersil GOLD™ aQ C18 Polar Endcapped HPLC Column, 100 mm × 2.1 mm × 1.9 μm (Cat No 25302-102130).
The mobile phase was composed of methanol with 0.1% acetonitrile (solvent A) according to the following program: 0–1 min: 90% B, 10% A; 1–5 min: linear gradient to 50% B, 50% A; 5–10 min: linear gradient to 10% B, 90% A; 10–12 min: hold at 10% B, 90% A; 12–15 min: re-equilibration to initial conditions. The total run analysis was 15 min. Column temperature was set at 35 °C and the flow rate was 0.4 mL/min.
4. Discussion
Global pesticide use in agriculture reached 3.7 million tons of active substances in 2022, marking a 4% increase from 2021, a 13% rise over the past decade, and was twice the amount used in 1990. Compared to the 1990s, the last decade saw a 121% increase in herbicide use, 54% in fungicides and bactericides, and 48% in insecticides. During this time, the composition of pesticides used also shifted—herbicides increased from 40% to 50% of total use, while the shares of insecticides and fungicides/bactericides declined to 22% each from 26% and 25%, respectively [
38]. Bearing in mind all of the above, there is a growing concern about the presence of herbicides in the environment, particularly in waters and soils that are directly exposed to anthropogenic influences through agricultural production. The issue of herbicide-related pollution needs more attention in certain agricultural regions, putting a heavy strain on the environment. Watercourses near arable areas are particularly exposed due to the intensive application of plant protection products, mainly herbicides. Also, irrigation canals are especially exposed to contamination. One good example is the Vojvodina region (Serbia) through which the Danube–Tisza–Danube canal network passes. In this region, agriculture plays a vital role in the economy, and to maintain the production levels, large amounts of herbicides per hectare are required. Therefore, constant monitoring of the impact of these activities on the environment is necessary, and to identify potential risks for aquatic ecosystems and soils, extensive studies are crucial to check the presence of pollutants, with a special emphasis on currently used herbicides [
19].
Pesticides often find their way into surface water through various natural processes, including surface and subsurface runoff and leaching through soil layers [
39]. They can also be drifted by wind and soil erosion during their application [
40,
41] on agricultural areas. Highly soluble herbicides in water are more likely to be transported during rain or irrigation [
42]. It is shown that the amount and type of pesticide residues found in surface waters also depend on several factors, such as application technique, soil management, crops, and climate conditions [
43].
At the same time, this water is used for irrigation and is an irreplaceable resource in the production of a wide range of crops. Therefore, in addition to the occurrence of phytotoxicity, there is a danger of food contamination and a possibility of harmful effects on the aquatic biocenosis. For this reason, it is important to control the quality of the environment in terms of herbicide residues. In line with the goals of sustainable agriculture and environmental protection, herbicide residues in surface watercourses have become a major focus of research in recent years [
2,
42,
43,
44,
45,
46]. Pesticides drifting from fields intonearby protected areas represents a serious risk to the stability of these sensitive ecosystems. The growing use of pesticides in soybean and sugarcane cultivation has contributed to the degradation of soil and water quality, putting the rich biodiversity at risk [
47].
Beyond their effects on human health, pesticides threaten aquatic environments by polluting ecosystems and potentially entering the food chain. They also have a strong impact on flora and fauna by altering microbial diversity, reducing fertility, and disrupting essential ecosystem processes [
48].
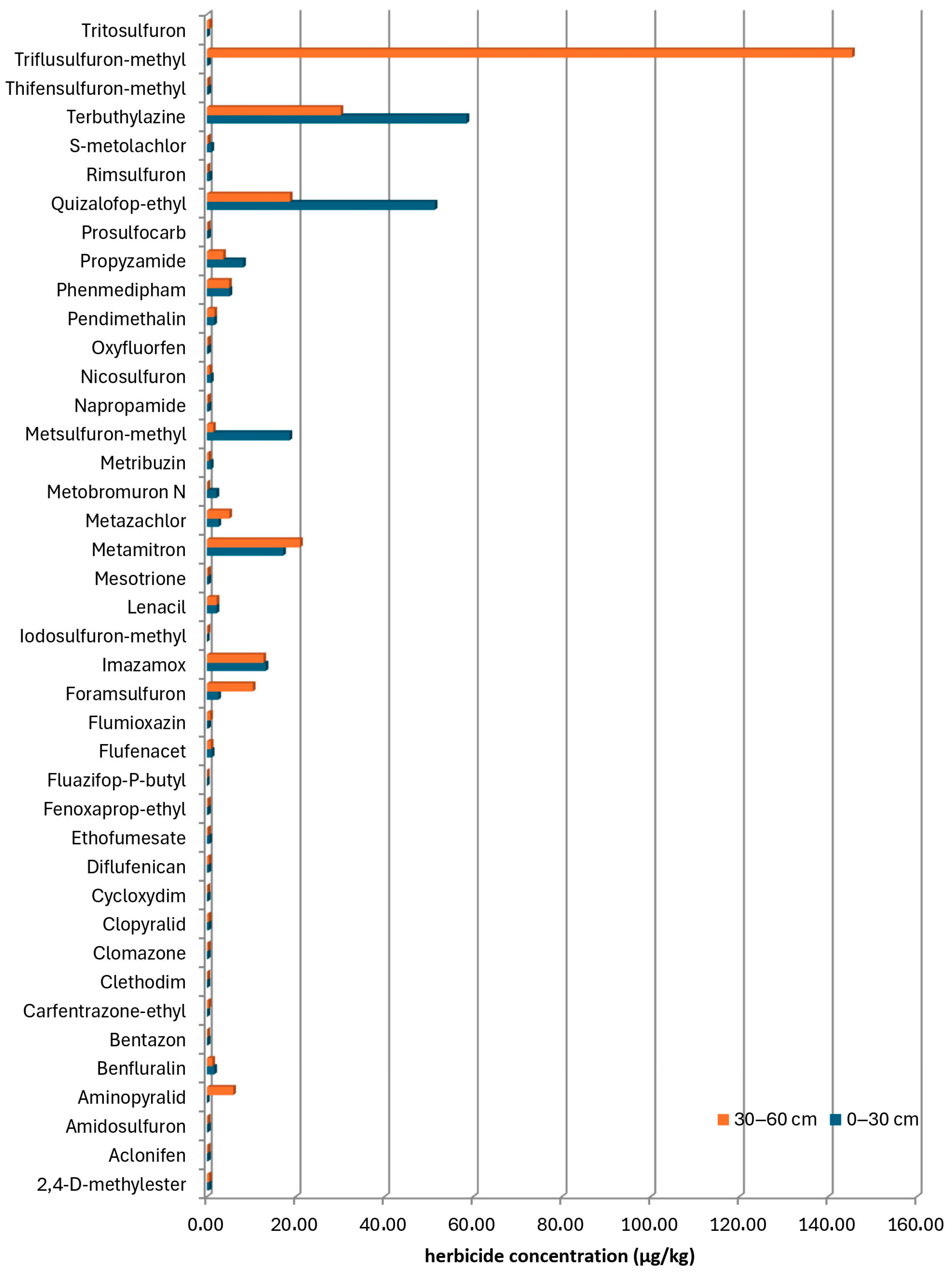
Out of the 41 active substances analyzed in the canal water of the DTD network, 28 compounds were identified, with s-metolachlor and terbuthylazine having the highest frequency of occurrence. These herbicides were applied after sowing and before the emergence of maize, which is the dominant crop in this area. Due to the atmospheric precipitation, they were easily washed off from the treated surfaces and subsequently reached watercourses. However, the total herbicide residues in canal water samples were below the maximum allowed concentrations prescribed in Directive 2013/39/EC [
25] for surface water, as well as below the limit values for pesticides in drinking water according to 98/83/EC [
32].
In contrast, a study examining pesticide contamination in river waters within an agricultural zone identified quizalofop-ethyl, trifluralin, and pendimethalin. Additionally, the insecticide tebufenpyrad was detected at a concentration of 0.330 mg/L [
2]. A separate investigation involving 287 surface water samples, including rainwater, streams, ponds, springs, and rivers, collected from 20 agricultural sites in a sub-basin area revealed the frequent presence of herbicides such as atrazine, clomazone, haloxyfop-methyl, and glyphosate. Conversely, no detectable levels were found for clethodim, chlorimuron-ethyl, diuron, fluazifop-p-butyl, imazamox, mesotrione, metsulfuron, nicosulfuron, or pendimethalin. During the rainy season, nearly all samples (about 99%) tested positive for residues of at least one of the monitored herbicides [
49]. Since water and soil are deeply interconnected in agricultural landscapes, especially in lowland regions such as Vojvodina, herbicide residues detected in surface water are often mirrored by their presence in the surrounding soil. Therefore, it is equally important to research how these compounds behave in both parts of the environment. Soil and sediments serve as primary reservoirs for herbicides as these substances tend to accumulate there, influencing their subsequent distribution to other environmental compartments. Studies that examine these issues are carried out worldwide [
19,
50,
51,
52,
53,
54,
55]. Herbicides’ persistence in the environment largely depends on how well they bind to the soil. This process is influenced not only by the weather, especially rainfall, but also by the soil’s characteristics, such as its organic matter and clay content, mineral composition, pH level, and temperature [
56,
57].
In this study, the dominant herbicides present in the surface soil layer were clopyralid, 2,4-D-methyl ester, terbuthylazine, fenoxaprop-ethyl, and aclonifen, which are intensively applied in the production of sugar beet, sunflower, soybean, wheat, and oilseed rape to control a wide range of weeds. A potential scenario for the high concentrations of terbuthylazine and quizalofop-ethyl residues in the soil on individual sites could be its application shortly before sampling. As a pre-emergence herbicide, terbuthylazine tends to remain concentrated near the soil surface, resulting in high residue levels in the upper soil layer. On the other side, in soil depths of 30–60 cm, with the frequency of occurrence identical to that in the surface layer, the same herbicides were present. However, the presence of herbicides from the sulfonylurea group (triflusulfuron-methyl) in the concentration of 145.470 µg/kg is a consequence of its application on the field under the sugar beet, slightly before sampling [
58]. Considering that the soils are alkaline and only a small amount of sulfonylurea herbicides can be adsorbed and remain in soil samples [
8], the persistence of these herbicides is expected to be short. Herbicide residues monitoring in agricultural soil in the Republic of Serbia, in 2023, showed higher concentrations of s-metolachlor and clopyralid in the soil samples [
19].
The analyzed soil is characterized by an alkaline reaction and a high CaCO3 content, classifying it as weakly to strongly calcareous. The average organic matter content indicates moderate to good humus supply, which is considered typically adequate for calcareous soils. However, extremely low values at certain localities point to humus-poor soils, which are unsuitable for agricultural production. The very high base saturation reflects the soil’s strong alkaline nature. Soil with these characteristics generally shows a reduced mobility of pesticides, which can explain the low level of herbicides in canal water compared to land in the immediate vicinity.
5. Conclusions
The increased use of herbicides in agriculture has led to growing concerns about their environmental impact. The issue of herbicide-related pollution needs more attention in certain agricultural regions where the watercourses are in intensive production areas. Special attention should be paid to currently used herbicides, since there are gaps in their monitoring. This research represents the determination of currently used herbicides in irrigation canal water and soil near those watercourses within the main agricultural region of Serbia, as a model for an area under intensive crop production in conventional agriculture.
Analysis of the canal water showed that although the water samples contained at least one active substance, the amounts of herbicide residues were below the maximum allowable concentrations of environmental quality standards, even for drinking water.
The soil’s mechanical and chemical properties were also analyzed. The average pH values of the analyzed soil samples indicated an alkaline reaction, while according to the average content of CaCO3 the soils were classified as weakly to strongly carbonated but moderately to well supplied with organic matter. The most abundant herbicides in the soil samples were clopyralid, 2,4-D-methyl ester, terbuthylazine, fenoxaprop-ethyl, and aclonifen. Terbuthylazine and quizalofop-ethyl were detected in the highest amounts, suggesting their possible recent application shortly before sampling. Moreover, the concentrations of herbicide residues in the soil at two depths were highly similar, without a significant difference. These results emphasize the importance of introducing regulations to control soil pollution, including the setting of MRL values.
Due to their indispensable role, herbicides are still an important element in agricultural production. However, in order to maintain the appropriate quality of the complete ecosystem, it is necessary to carry out long-term monitoring programs of currently used herbicides in soils and waters, especially in regions with intensive agricultural activities.