Abstract
CO2-biomethanation was studied in the present manuscript by considering the direct injection of hydrogen into a conventional anaerobic digester treating sewage sludge within a simulated wastewater treatment plant (WWTP). The plant was simulated using the Python 3.12.4 software, and a Monte Carlo simulation was conducted to account for the high variability in the organic content of the wastewater and the methane potential of the sludge. Two modes of operation were studied. The first mode involves the use of an anaerobic digester to upgrade biogas, and the second mode considers using the digester as a CO2 utilization unit, transforming captured CO2. Upgrading biogas and utilizing the extra methane to generate electricity within the same plant leads to a negative economic balance (first scenario). A hydrogen injection of 1 L of H2/Lr d (volumetric H2 injection per liter of reactor per day) was required to transform the CO2 present in the biogas into methane. The benefits associated with this approach resulted in lower savings regarding heat recovery from the electrolyzer, increased electricity production, and an additional oxygen supply for the waste-activated sludge treatment system. Increasing the injection rate to values of 5 and 30 L of H2/Lr d was also studied by considering the operation of the digester under thermophilic conditions. The latter assumptions benefited from the better economy of scale associated with larger installations. They allowed for enough savings to be obtained in terms of the fuel demand for sludge drying, in addition to the previous categories analyzed in the biogas upgrading case. However, the current electricity price makes the proposal unfeasible unless a lower price is set for hydrogen generation. A standard electricity price of 7.6 c€/kWh was assumed for the analysis, but the specific operation of producing hydrogen required a price below 3.0 c€/kWh to achieve profitability.
1. Introduction
The biological treatment of municipal wastewater typically involves the conventional activated sludge process, where aerobic microorganisms break down organic material, producing a microbial biomass, which is subsequently removed through physical separation. The larger the scale of the treatment plant, the greater the amount of biological material that requires suitable disposal. Many large-scale plants commonly treat primary and waste-activated sludge through anaerobic digestion, producing biogas as an energy product and a slurry referred to as biosolids, which often serves as an organic amendment when the material complies with the country’s regulations. The land application of biosolids provides the benefit of recycling nutrients (nitrogen and phosphorus), improves soil quality, and avoids the depletion of organic carbon from soils, a feature attained at modest expenses [1,2,3,4].
The digestion of sewage sludges may be carried out under mesophilic or thermophilic conditions. In the case of digesters working under mesophilic conditions, the degradation of organics takes place at a slower pace because of the effect of temperature on kinetics. Despite the benefits of a high degradation rate in the thermophilic regime, they are insufficient to tilt the balance in favor of this process due to the lower quality of thermophilic digestate and the poor properties of the rejected supernatant [5]. In addition, no significant differences in biogas production have been reported when operating digesters under mesophilic and thermophilic conditions [6]; therefore, the increase in temperature has not always resulted in higher gas production [7,8]. For these reasons, many wastewater treatment plants (WWTPs) still operate under mesophilic conditions and typically implement other options for increasing productivity, such as co-digestion. The addition of organic waste increases the loading and aids in achieving a better balance of nutrients, which may enhance biogas formation by 13–176% [9,10,11].
Given the high energy demands of the waste-activated process, treating sewage sludge by anaerobic digestion provides the dual benefit of reducing its volatile content and generating some of the plant’s energy needs. Therefore, increasing digestion productivity is essential for improving the energy balance. García-Cascallana et al. [12] reported that if a sufficient digestion capacity was available, the biogas generated from co-digestion could even cover the full energy demand of the plant. Finding a suitable co-substrate all year round, without dealing with odor or the discomfort from waste handling operations, is often challenging. In addition, the increase in organic loading also causes an unavoidable increase in digested sludge, along with other unexpected outcomes such as solid accumulation inside the reactor, a nitrogen backload, and lower dewaterability [13].
A completely different strategy for increasing methane production may involve using hydrogen as a co-substrate. CO2 is transformed by hydrogenotrophic methanogens into methane, requiring four moles of hydrogen (H2). CO2-biomethanation has garnered the attention of the scientific community due to the ease of adaptation of anaerobic microflora and the broad application of digestion technology operating at an industrial scale. Several researchers have reported on the experimental performance and technical feasibility of the approach [14,15,16]. The strategy of increasing methane productivity by recirculating biogas has been reported by Poggio et al. [17], indicating that H2 gas transfer limitations were reduced by attaining higher circulating rates and increasing gas residence time. In situ biogas upgrading can significantly reduce energy consumption by utilizing endogenous CO2, resulting in a methane content that is compatible with the natural gas grid [18]. Martínez et al. [19] tested the conversion of H2 in anaerobic reactors treating sewage sludge at injection rates of 0.5–2.0 L of H2/Lr d. These authors reported an increase in biogas production but not in composition. After analyzing the microbial population, the reactor performance was explained by the conversion of CO2 into acetate, which was subsequently converted into methane. Nguyen et al. [20] tested hydrogen injection rates of 4.39 L of H2/d (1.0 L of H2/Lr d), achieving a H2 utilization efficiency of 92–99% with a methane composition up to 92%.
Large-scale anaerobic digesters may play a role in the hydrogen economy by utilizing the captured CO2 to produce methane, thereby serving as units for energy storage [21]. Hydrogen derived from water electrolyzers can be integrated into existing large-scale digesters without negatively impacting performance. This approach combines two benefits. The first is upgrading biogas, and the second is using excess renewable energy, which becomes available when solar and wind power account for a high share of the energy mix. Methane can be used as fuel in different sectors, such as industry and transportation. Thus, wastewater treatment plants (WWTPs) can significantly contribute to the shift towards a decarbonized economy by supplying renewable fuel that helps reduce the dependency on fossil fuels [22]. The decision to phase out coal and nuclear generation and increase the share of renewables has created a phenomenon where negative prices are more frequent in the energy spot market due to excess energy available when demand is low [23]. In addition, the Spanish shutdown in April 2025 underscored the importance of maintaining a reliable grid system with a sufficient energy storage capacity to ensure the continuity of essential services.
One way of storing this extra energy is by transforming electricity into fuel such as methane. However, alternatives like storing energy in batteries as well as potential, kinetic, or thermal energy are also possible. The conversion into chemicals seems to be the best option for long-term storage [24]. This goal can also be achieved by using catalysts in a process known as the Sabatier reaction, where noble metals (Ru, Rh, Pd, and Pt) are required to catalyze the conversion of CO2 at high pressures and temperatures between 300 and 400 °C [25,26]. The feasibility of this approach depends on the price of catalysts, the cost of producing hydrogen, and the capture of CO2 [27]. Recent work has focused on the development of new nickel-based catalysts and bimetallic catalysts [28,29] as well as the simultaneous capture and transformation of CO2 into methane by combining adsorbents and metallic catalysts. This allows for the simplification of the capture and transformation stages by having these take place in a single reactor using dual-functional materials [30,31,32]. Another interesting route for transforming CO2 is electrochemical reduction. However, the high stability of the CO2 molecule (with the C=O bond having a dissociation energy of 750 kJ/mol) translates into the need to provide a large overpotential to activate the reaction, leading to low energy efficiencies [33,34].
On the contrary, biological systems may also attain the transformation of CO2 into methane with the added advantage of milder reaction conditions. This approach offers several benefits, as previously mentioned, in addition to the advantage of utilizing existing large-scale anaerobic digesters. It leverages the experience gained from operating standard digestion units and transforms these systems into a process capable of storing energy as methane. The application of milder conditions and the use of mixed microflora make biological methanation a promising technology due to its ease of implementation. The use of existing reactors reduces the financial burden associated with installation and operating costs, as nutrient supply and specific inoculation are no longer necessary. This is one of the arguments put forward by some authors for relegating biological methanation to small-scale applications [35,36,37].
The idea of transforming C1 gases dates back to the 1990s. It has been previously studied not only as a method for upgrading biogas but also for transforming the H2/CO/CO2 components of syngas to produce methane [38,39,40]. The interest in this technology has experienced a resurgence due to concerns about greenhouse gas (GHG) emissions and the goals associated with achieving climate neutrality by 2050, which are at the heart of the European Green Deal [41]. Efforts to increase fermentation efficiency are associated with reducing mass transfer limitations by favoring the gas–liquid interphase, operating at lower temperatures, increasing pressure, and increasing the biomass concentration in the reactor via immobilization [16,42,43]. Other approaches include the use of biocathodes, the introduction of electrodes (electro-fermentation), and operating under thermophilic conditions using mixed cultures [44,45,46]. Nevertheless, the low development of these recent proposals and the lack of enough experience at a pilot scale make the direct injection of a gas phase into an anaerobic operating digester the most feasible option in the short term.
The present manuscript studies the feasibility of using anaerobic digesters working in WWTPs as biological units for producing extra methane thanks to CO2-biomethanation. The novelty of the present contribution is assessing the feasibility of using anaerobic digesters in WWTPs as reactor units for either upgrading biogas or as CO2-conversion systems. Different hydrogen injection rates were established, assuming the use of water electrolyzers, with the digestion unit’s main aim being the conversion of H2/CO2 mixtures into methane. The main parameters to attain profitability were assessed and the electricity price required to attain profitability was estimated.
2. Materials and Methods
The WWTP model was based on Ellacuriaga et al. [47] as well as González et al. [48] and used the assumptions of Martínez et al. [19]. Table 1 shows the list of main model assumptions, considering a 20% variation in sludge solid content and volatile solid composition. The WWTP process assumptions were based on the SuperPro designer model used in Ellacuriaga et al. [47].

Table 1.
Main model parameters used in the WWTP flow calculations.
The digester’s thermal demand was calculated using the equations described by González et al. [55]. The thermal demand considered the heat required to increase the sludge temperature from the inlet stream (15 °C in summer conditions and 5 °C in winter conditions) to the fermentation temperature (37 °C under mesophilic and 55 °C under thermophilic conditions), assuming a 95% heat transfer efficiency, 5% of heat losses in the summer, and 10% of losses in the winter period. A hydraulic retention time of 21 days was used for dimensioning the digester volume.
The digestate was subjected to dehydration using horizontal decanter centrifuges. The solid content of dehydrated sludge was 22.0 ± 4.4%. The land application of the dehydrated digestate for agronomic purposes was assumed. Sludge was transported to a nearby site located 30 km away (tortuosity factor of 1.4). A truck with a load capacity of 40 m3 and a fuel (diesel) consumption of 35 L/100 km was used to estimate the transport energy demand [56]. The lower heating value (LHV) and density of diesel were 44.8 MJ/kg and 0.84 kg/L, respectively [57,58]. The price of truck renting was 1.25 €/km with a diesel fuel price of 1.6 €/L. A combined heat and power (CHP) engine was considered with a maximum electrical efficiency of 39.7% and a maximum thermal efficiency of 52% for an electrical output of a 249–330 kW range [59]. The thermal exhaust gas temperature was assumed to be 640 °C, which allowed for the recovery of 50% of the CHP exhaust gases as heat [56,60]. The natural gas price was 45 €/MWh for the period from 1 November 2024 to 30 April 2025 [61].
Supplementary Material S1 shows a full description of the WWTP model used. A Monte Carlo simulation was performed to take into account the variability of the sludge and wastewater composition. A normal distribution was assumed for the values reported in Table 1. The Python 3.12.4 software with the ‘rng. normal’ command was used for modeling the process, running 10,000 simulations.
2.1. CO2-Biomethanation
The biological methanation of CO2 involves the following reactions, as proposed by Schwede et al. [62] and Rafrafi et al. [35]. The conversion of CO2 into acetate was demonstrated to be dependent on the dissolved CO2 levels in the reactor, with values above 2.0 ± 0.2 mmol/L favoring direct methane conversion from CO2 by keeping H2/CO2 ratios above 4.0 units [63]; therefore, in the present document, only reaction (1) was considered to estimate the methane productivity of the reactor:
CO2 + 4 H2 → CH4 + 2 H2O
CH3COOH → CH4 + CO2
Continuous stirred tank reactors (CSTR) attain better specific surface areas than other types of reactors if the gas bubbles are small and well dispersed, thus allowing better transfer efficiencies (higher KLa values) [64]. However, other reactor configurations, such as airlift reactors, perform better in terms of their mass transfer efficiency [65]. Table 2 shows a list of the different experimental work found in the literature reporting on the conversion of H2/CO2 to produce methane.

Table 2.
Main operating parameters and performance of anaerobic reactors working as CO2-methanation units.
The hydrogen injection values reported in the scientific literature range from 0.02 up to 0.56 L of H2/Lr d [66,67] when studying direct injection into the anaerobic reactor and higher values when operating with other reactor configurations, thanks to the feasibility of applying higher gas recirculation rates and thus, improving mass transfer. Laguillaumie et al. [69] applied injection rates between 0.7 and 9.4 L of H2/Lr d using a bubble column when working under thermophilic conditions and attained a gas conversion close to 100%. Illi et al. [68] reported values between 0.54 and 1.1 L of H2/Lr d using an anaerobic filter under mesophilic conditions, and Strübing et al. [71] reported an injection rate of 52.5 m3 of H2/m3 trickle bed/d operating in this case under thermophilic conditions. Haitz et al. [72] reported injection values of 4–6 L of H2/Lr d when testing a hollow fiber system. Given the complexity of operating other reactor configurations, the present work assumed a direct hydrogen injection into the digester, thereby taking advantage of the nutrients already present in the sludge, operating the reactor as a typical digestion unit, and serving H2 as a co-substrate.
Two operational modes were studied (see Figure 1), one involving mesophilic conditions for the anaerobic digester and thermophilic conditions for the other. The injection rate in the first case was assumed to be 0.2 L of H2/Lr d for the low injection case and a value of 1 L of H2/Lr d for the high injection case, based on the assumption that the maximum theoretical value was 2.27 L of H2/Lr d [75]. The second operational mode evaluated higher injection rates by establishing thermophilic conditions. In this latter mode, two injection rates were also tested: 5 L of H2/Lr d for the low injection case and 30 L of H2/Lr d for the high injection case. No increase in sludge-specific methane production (SMP) was considered for the thermophilic conditions based on reports of Gavala et al. [6] and Chen et al. [8]. The H2 injection assumptions yielded kLa values in the range of 0.23 and 0.32 h−1 when working under the mesophilic regime and for the thermophilic case the range for kLa was between 9.0 and 42.0 h−1. These values are in accordance with those reported by Liu et al. [76] when evaluating the kLa values for syngas fermentation in CSTRs.
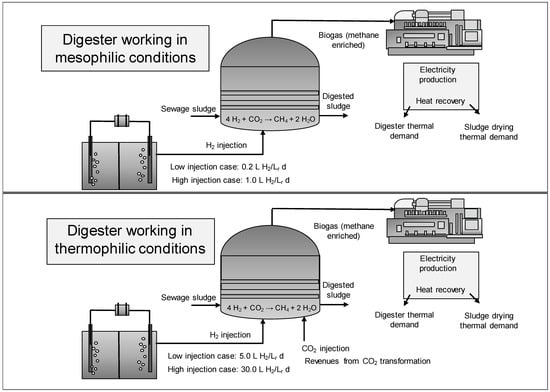
Figure 1.
Operational modes studied for direct hydrogen injection into the anaerobic digester.
2.2. Hydrogen Production from Water Electrolyzers
Alkaline electrolyzers are the most widespread technology due to their high level of maturity [77,78]. However, proton exchange membrane (PEM) electrolyzers allow for higher current densities, with the prices for these units expected to decrease significantly by 2030 [36,79]. The size of the electrolyzer is based on the hydrogen injection rates considered in the previous scenarios. The oxygen produced by the electrolyzer was assumed to be added to the air supply system of the conventional WAS unit, thus reducing the volume of fluid handled by the air compressor. The energy demand of the air compressor was 0.2 kWh/m3 of air. The electrolyzer specific energy demand was 4.3 kWh/m3 of H2 with a heat production equivalent to 20% of the power and 80% of the heat recovery capacity [80,81]. The heat recovered from the electrolyzer is used to cover the digester’s thermal demand either under mesophilic or thermophilic conditions, depending on the working mode evaluated. Under the mesophilic regime, the biological reactor works as a biogas upgrading unit. Conversely, the thermophilic regime permits higher injection rates and utilizes the biological reactor as a treatment unit capable of transforming captured CO2 (intensive CO2 conversion system). The electrolyzer water demand was estimated by assuming a conversion factor of 9 L of ultrapure water being required for producing 1 kg of H2 and 4.5 L of tap water being necessary for producing 1 L of ultrapure water.
The PEM electrolyzer and auxiliary equipment costs were 1337 (2020) $/kW using the 2020 CEPCI index of 596.2, and 2023 CEPCI index of 800.8 [82,83]. The US dollar to euro conversion was 1.14$, equivalent to 1 €. The operating and maintenance costs were 5% of the initial investment. The profitability of the approach was based on the savings attained through the extra methane available and the reduction in electrical demand resulting from the use of pure oxygen in the WAS unit. The methane derived from the digester was used to produce electricity in the CHP engine. The heat recovered from the CHP engine covered the thermal demand of the digestion system and, whenever possible, the energy demand for sludge drying. The time horizon of the economic assessment was 25 years, using linear depreciation with a 10% salvage value. The depreciation period was 15 years. The net present value (NPV) and payback period were used to estimate profitability, assuming a discount rate (r) of 3.5%.
where CI stands for capital investment; CF represents cash flow, which, in the present document, is derived from the savings and revenues obtained when the reactor operates as a capture-CO2 utilization unit. In this latter case, the profitability was assessed by assuming revenues equivalent to 50 €/t CO2 and 100 €/t CO2. The present study focuses on assessing the feasibility of using existing anaerobic digesters as CO2-conversion units. For this reason, the analysis does not consider the costs associated with carbon capture or the investment costs associated with reactor installation.
3. Results and Discussion
Figure 2 shows a scheme of the WWTP where sludge digestion is also represented. The primary and secondary sludge are treated in the anaerobic digester. The amount of biogas produced was 3914 ± 1347 m3 biogas/d based on a 60% methane concentration in the biogas. Two digesters with a mean size of 3130 m3 are needed for treating the sludge flow. The model equations considered winter and summer conditions for estimating the digester’s heat demand.
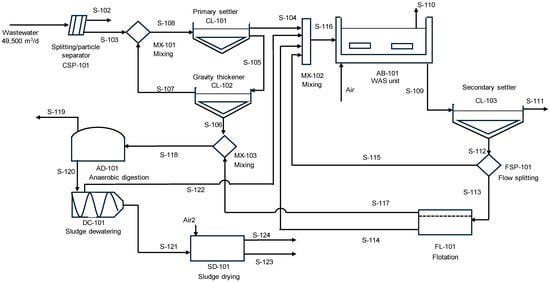
Figure 2.
Schematic representation of a WWTP with sludge digestion and thermal drying.
The main performance parameters of the WWTP are listed in Table 3. A significant amount of digested sludge is obtained (219 ± 44 m3/d). The sludge stream is subsequently dewatered, to reduce the amount of sludge requiring final disposal, thereby impacting the efficiency of sludge handling and transport costs. The energy associated with sludge drying is excessive. Transporting the dewatered sludge requires an annual energy demand that appears to be extremely high compared to the energy needed when dealing with the dried material (78,055 ± 13,971 MJ/year, equivalent to a transport cost of 10,726 ± 1919 €/year). However, the decision to dry sludge before transport involves a significantly higher amount of energy due to the excessive energy demanded for water evaporation. Thus, the transport of dewatered sludge translates into a mean annual expense of approximately 46,000 €. In contrast, the cost of drying the material reaches a cost that is almost ten times greater. Table 3 also indicates that during winter, auxiliary fuel is required if 50% of the heat generated by the CHP unit is recovered with the engine exhaust gases [56].

Table 3.
Results from the WWTP simulation derived from model equations and a Monte Carlo simulation.
Biosolid land application is an environmentally friendly option to valorize digested material, allowing the recycling of nutrients (nitrogen and phosphorus) and the retention of carbon in soils. Biosolids are rich in phosphorus content, particularly when the plant counts with an enhanced system for phosphorus removal, either a chemical or a biological one [84]. Additionally, substituting mineral phosphates for this organic amendment helps mitigate the risks associated with the presence of Cd in low-quality rock phosphate [85,86]. Nevertheless, thermal valorization emerges as a viable alternative when sludge valorization is unfeasible due to location-specific restrictions at WWTPs regarding the presence of metals or micropollutants. The energy contained in sludge can be estimated from its higher heating value (HHV), with values ranging from 12 to 14 MJ/kg [87,88,89]. In the present case, the power derived from the biosolids hardly covers the drying needs, with a value of 1047 ± 187 kW if a mean HHV of 13 MJ/kg is assumed. This simplistic estimation shows the intrinsic difficulties found when attempting sludge thermal valorization. Thermal processes such as gasification and pyrolysis may seem to be feasible technologies for obtaining valuable fuels from sludge. However, these processes may only partly compensate for the high energy demand required for drying when integrating digestion and thermal valorization, with the solid content of the feed in the integrated system having a significant impact on the global energy balance [48,90].
3.1. Addition of H2 Gas as a Co-Substrate in Anaerobic Digestion
The use of hydrogen as a co-substrate enhances the digester’s productivity while avoiding the inconvenience associated with handling external waste. However, the cost of the electrolyzer is a significant drawback. CO2-biomethanation can transform the CO2 present in biogas into methane by requiring additional hydrogen (See Figure 3). The amount of CO2 present in the biogas was 64.7 ± 22.2 m3/h; thus, upgrading the biogas into a natural gas surrogate is achieved under the assumption of the high injection case (1.0 L of H2/Lr d), but the extra amount of methane produced does not equilibrate the economic balance regarding the cost of the electrolyzer.
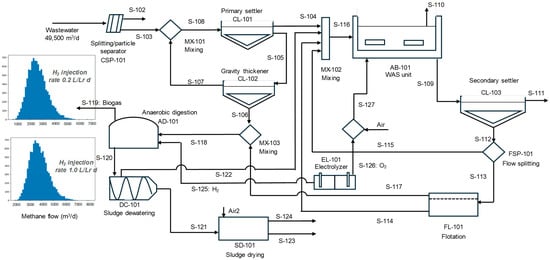
Figure 3.
Schematic representation of a WWTP with an electrolyzer producing hydrogen (stream S-125) to be used for in situ biogas upgrading and oxygen (stream S-126) used in the WAS unit. Scenario 1: Digester operating under mesophilic conditions.
Table 4 shows the main results derived from the model when assuming mesophilic conditions for the digester. If the digester’s conversion capacity for transforming CO2 into methane is considered, a yearly methane production equivalent to 5336 MWh/year would be available, translating into 240,106 € of annual savings. This extra methane is enough to cover any demand for auxiliary fuel if digestate drying is not contemplated into the scenario. However, an average extra cost of 170,200 € is still required when a thermal drying operation is included.

Table 4.
Results from the WWTP model with H2 injection. H2 injection flows into the anaerobic reactor are 0.2 and 1.0 L of H2/Lr d. Anaerobic digester works under mesophilic conditions.
The transformation of CO2 into methane offers the advantage of utilizing the existing equipment to upgrade the biogas, achieving a quality comparable to that of natural gas. However, in addition to the electrolyzer’s investment and installation costs, hydrogen production involves high electricity consumption. Currently, the lowest electricity price in Europe was reported by Finland, with a value of 0.0767 €/kWh, and the highest was that for Cyprus, with a price of 0.2578 €/kWh for the second half of the year 2024 [91]. Applying these prices to the economic balance translates into an hourly cost of producing methane between 81 and 272 € for obtaining the H2 flow required to upgrade the biogas. In contrast, the market price of methane produced for the same interval only reaches a value of 27.5 €. This discrepancy evidences the difficulties found by technologies dealing with CO2 utilization. The price of electricity to equilibrate the balance needs to drop below 0.025 €/kWh if methane market prices are kept constant.
The electrolyzer produces oxygen in addition to hydrogen. In the present case (see Figure 3), the oxygen produced may serve as an extra supply for the air in the waste-activated sludge process. Based on the assumptions about the WAS unit, the airflow estimated was 311 m3 air/min (equivalent to 65.0 m3 O2/min). However, the oxygen flow from the electrolyzer is only 2.0 m3 O2/min, which hardly contributes to reducing the air supply by 3%. This slight decrease is also extrapolated into a small decrease in power demand.
The heat recovered from the electrolyzer helps meet the thermal demand of the digester during winter. Consequently, if sludge drying is not included, incorporating the electrolyzer reduces the need for additional fuel, resulting in significant savings. In addition, the extra methane derived from the CO2-methanation process is now available as fuel in the CHP engine. Thus, the amount of electricity is 231 kW, which translates into annual savings of 154,700 € when considering an electricity price of €0.0763/kWh (the average value reported in Spain during the second half of 2024). The previous assumptions result in a negative economic balance, even if the cost of electricity for producing hydrogen is set to zero.
Figure 4a shows the effect of reducing the installation costs by 10% and 20%, along with the proportional decrease in operating and maintenance activities. Even with this specific reduction in the equipment cost, the electricity demand was still considered zero-priced. Any price assumed for the energy demand when producing hydrogen results in a negative economic balance, given the current price of methane. Achieving a positive result is possible if the price of methane doubles (Figure 4b) or a 10% decrease in electrolyzer investment costs is assumed. However, even in the best-case scenario (a methane price of 90 €/MWh and a 20% reduction in the electrolyzer investment costs), the payback period exceeds 10 years.
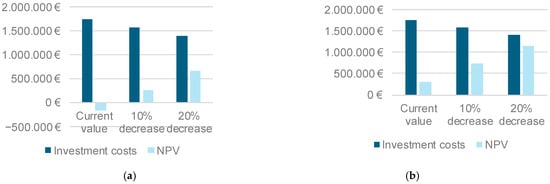
Figure 4.
Electrolyzer investment costs and NPV obtained under mesophilic digestion and a hydrogen injection rate of 1 L of H2/Lr d. (a) Methane price 45 €/MWh. (b) Methane price 90 €/MWh.
The price of the energy required for producing hydrogen may be set to zero if this energy is intended for storage or for avoiding disruptions in the electricity market whenever the production of renewable energies is excessive. Even though several researchers propose the biological reaction of transforming H2 into CH4 as a feasible option [92,93,94], the current investment price of electrolyzers and electricity prices make this approach unfeasible. Gantenbein et al. [36] reported that an electricity price of 5 c€/kWh and an electrolyzer investment cost below 1000 €/kW were needed to attain economic feasibility. Current market prices in Europe are above 7 c€/kWh and values close to 0.25 c€/kWh are offered for non-household sectors [91]. While lowering electricity prices can help decarbonize the economy by encouraging electrification in residential and industrial sectors, excessively low prices may deter investors and hinder the adoption of efficiency measures to reduce electricity consumption.
3.2. CO2-Biomethanation as a Technology for Transforming Captured CO2
The previous assumptions considered the basic approach of direct injection into the anaerobic digester, setting a 1 L of H2/Lr d as the injection rate when considering the high injection case. Using gas recirculation and other configurations can reduce mass transfer limitations, thereby allowing a higher injection value [17,73]. Pan et al. [95] reported a 72% increase in the mass transfer improvements associated with the change in reactor configuration by introducing a draft tube to allow flow circulation. Another factor to consider is the higher bottom pressure of large-scale reactors, which favors mass transfer by increasing hydrogen solubility thanks to the higher concentrations attained at the gas–liquid interface. However, this benefit may not necessarily translate into a higher mass transfer as explained by Jensen et al. [96], who reported that the effect of a reduced bubble size due to pressure (if the superficial bubble area is not increased) may offset the previous advantage. Operating under higher temperatures reduces gas solubility but also increases the reaction rates of the biological systems, thus explaining the higher injection rates used by different authors when working under thermophilic conditions [71,74]. However, when considering co-digestion with other wastes, this is not always the case, with mesophilic systems reporting higher biogas production values under certain conditions than those under the thermophilic regime [97], because the increase in the conversion rate does not necessarily lead to a higher biogas production.
The high injection rates applied under thermophilic digestion come with the identical drawback of high investment costs and excessive electricity demands. Table 5 presents the main parameters of the scenario analyzed under both low and high injection cases, considering thermophilic digestion. Oxygen derived from the water electrolysis process can be used in the WAS treatment, thus reducing energy demand by 12% and 75% under the low and high injection cases, respectively. This benefit becomes insignificant when taking into account the power demand of the electrolyzer (see Table 5). The higher thermal demand of the thermophilic digester can be supplied by the extra heat obtained from the electrolyzer (as heat recovery) even during the winter period. However, once again, this benefit is seamless based on the high electricity consumption of this equipment.

Table 5.
Results derived from the simulation when digester runs under thermophilic conditions. H2 injection flows are 5 and 30 L of H2/Lr d.
An equivalent amount of CO2 can be converted into methane, making this technology appealing because it enables the digester to function as a CO2 utilization unit rather than merely a biogas upgrading system. The annual amount of CO2 that can be transformed is 4160 t CO2 for the low injection case and 30,500 t CO2 for the high injection case. Figure 5a shows the scheme representing the introduction of a CO2 stream along with the electricity generated by the CHP engine when methane is valorized to produce heat and electricity.
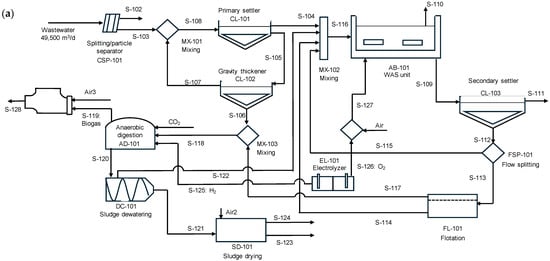
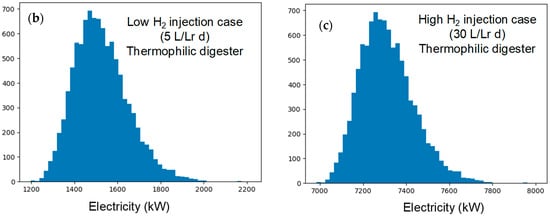
Figure 5.
(a) Schematic representation of CO2 conversion into methane and gas valorization in CHP engine. (b) Electricity production when injecting 5 L of H2/Lr d (low injection rate) and (c) Electricity production when injecting 30 L of H2/Lr d (high injection case). The anaerobic digester is assumed to work under thermophilic regimen.
The energy recovery (as electricity) of the present strategy is 22%, and the water demand of the electrolyzer accounts for approximately 100 m3/d and 640 m3/d for the low and high injection cases studied, respectively. One additional disadvantage of the hydrogen-based economy is the high water demand required for producing this valuable gas. If the water use of the isolated electrolysis step is considered, then the impact of the amount of water consumed for hydrogen production, compared with the amount of water available, may be cataloged as negligible following the criteria of Beswick et al. [98]. However, this is not the case here, where a significant amount of water is required daily to allow CO2 conversion. Despite this drawback, H2 produced from water electrolysis powered by either renewable energy or nuclear energy has the lowest impact when compared with steam reforming or when electricity is derived from an electrical grid with a high share of carbon-producing emission technologies [99].
Given the economy of scale, the balance is significantly improved (see Figure 6); however, this outcome is only achieved if no price is set for the energy demanded by the electrolyzer. The best case considered here would translate into a zero value for the NPV parameter when a price as low as 11 €/MWh is introduced into the balance sheet. The introduction of revenues linked to CO2 conversion (50 €/t CO2) does not cause a significant improvement to afford the electricity market price for hydrogen production (zero value of NPV is obtained for an electricity price of 17 €/MWh). Increasing the injection rate (high injection case) to 30 L of H2/Lr d results in a better economic balance due to the advantage from the economy of scale associated with the electrolyzer capital investment, but the fact that the price of electricity is higher than that of methane makes the whole idea of storing energy in this form unfeasible unless the price of electricity for producing hydrogen is set to zero.
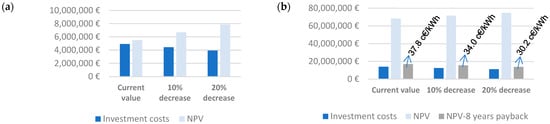
Figure 6.
Electrolyzer investment costs and NPV obtained under thermophilic digestion and (a) hydrogen injection rate of 5 L of H2/Lr d, assuming a zero price for electricity required for hydrogen production. (b) Hydrogen injection rate of 30 L of H2/Lr d, assuming a zero price for electricity required for hydrogen production and estimating the electricity price to obtain a payback period of 8 years.
The high injection case can result in a payback period of 8 years, provided the price of electricity increases to 37.8 c€/kWh, as long as the electrical cost of generating H2 is not factored into the equation. Figure 6b also shows the electricity price that allows a payback period of 8 years if the investment cost of the electrolyzer is reduced by 10% and 20%, respectively. This same exercise was conducted under the best-case scenario, assuming a 20% decrease in electrolyzer investment costs. The acceptable price of electricity to produce hydrogen is shown in Figure 7.
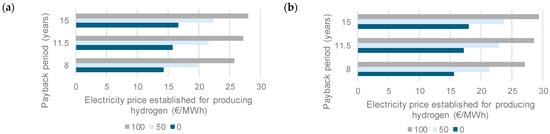
Figure 7.
Electricity price that can be afforded for producing hydrogen to obtain a payback period of 8, 11.5, or 15 years in scenario 2, high injection case. Estimations assumed no earnings for converting CO2 into methane (dark blue bars), and 50 € (light blue bars) and 100 € (gray bars) as earnings for the same transformation, (a) considering sludge dewatering and (b) considering sludge drying.
The estimated values were determined by analyzing payback periods ranging from 8 to 15 years and considering the cases where sludge was dewatered and when sludge was dried. In this assessment, it was initially assumed that no revenues were earned from transforming CO2, whereas the other scenarios considered potential earnings of €50 and €100 per ton of CO2 for the same activity. As can be seen from this figure, neither case can achieve profits at the current electricity price under the Spanish scenario (76.3 €/MWh). Figure 7b shows better results (sludge drying case) due to the savings associated with the dryer fuel demand. The greater amount of methane available for producing electricity in the plant also increases the volume of hot combustion gases, thus supplying the heat required for drying sludge.
3.3. Challenges and Opportunities for Implementing CO2-Biomethanation into WWTPs
The strategy of transforming conventional anaerobic digesters into either biogas upgrading units or CO2-conversion units to become part of an energy storage system presents numerous limitations, including the high cost of the electrolyzer and electricity prices. Additionally, other technical challenges are worth noting. Hydrogen is a small molecule with high diffusion and energetic content, resulting in a high flame propagation speed [100], which makes the handling of this fuel extremely challenging. Metal embrittlement is a significant concern when dealing with hydrogen in daily plant activities. The small size of the molecules facilitates diffusion into metal structures, causing cracks and fractures [101,102]. Safety considerations are usually associated with materials used for transporting and storing hydrogen and issues related to the daily operations of the personnel involved in handling this fuel since any leakage goes undetected by humans because it is a colorless, odorless, and tasteless flammable gas [103]; thus, specific protocols need to be implemented in conjunction with the installation of H2-sensors for detecting gas leakage and the specification of shut down protocols and isolation of areas when leakages are detected [104]. The operators of WWTPs have experience working with methane, which is a flammable gas. Nevertheless, the properties of this gas differ significantly from those of hydrogen, as methane has a higher ignition temperature, a lower flame speed, and a narrower range of flammability limits compared with hydrogen. Therefore, special training is required for operators working in zones with a higher flammability risk, which translates into higher labor and maintenance costs, thereby reducing the feasibility of the present approach even further.
Despite these challenges, the conversion of extra-renewable electricity into a chemical compound using existing biological reactors seems feasible due to the advantages of working with mixed microflora, the lack of investment required for constructing large-scale reactors, and the availability of trained personnel. Thus, the strategy leverages the experience gained from several years of reactor design and the operation of industrial reactors. Although other reactor configurations, such as gas lift, anaerobic filters, and membrane reactors, are also available, the upfront cost of building a new large-scale reactor and acquiring the electrolyzer, along with all the necessary auxiliary equipment, would annul any economic benefit.
4. Conclusions
The biological conversion of CO2 into methane offers the possibility of either upgrading biogas or transforming a conventional anaerobic digester into a CO2 utilization unit when additional captured CO2 is introduced into the system. Although the proposal may appear environmentally friendly, it entails excessive energy demands for operating the electrolyzer and involves high investments, negating any potential profitability. The present study assessed the conversion of CO2 under mesophilic and thermophilic conditions by assuming direct hydrogen injection into a digester operating in a conventional WWTP. Introducing a water electrolysis unit enables heat recovery, which can be used to cover the digester’s thermal demand. Oxygen derived from the electrolyzer can be used as a supplement to the air stream required for the WAS treatment system. However, the benefits obtained in this case are modest, resulting in a 3% reduction in airflow and covering the digester’s thermal demand during the winter period, if assuming a hydrogen injection rate of 1 L of H2/Lr d (first scenario). The increase in the hydrogen injection rate (5 and 30 L of H2/Lr d) was evaluated in the second operational mode, where the digester runs under thermophilic conditions. The economy of scale in hydrogen production favored this approach but required establishing lower electricity prices for this specific operation. The system only attains profitability if a price between 14 and 30 €/MWh is set and additional revenues are obtained from CO2-biomethanation. Setting the standard price of electricity for hydrogen production resulted in a negative NPV for any of the cases analyzed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12070245/s1, Supplementary Material S1.
Author Contributions
Conceptualization, X.G.; methodology, X.G. and R.G.; software, X.G.; formal analysis, R.G.; investigation, X.G. and R.G.; resources, X.G.; data curation, R.G.; writing—original draft preparation, X.G. and R.G.; writing—review and editing, X.G.; visualization, R.G.; supervision, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available on request to the authors of the manuscript.
Acknowledgments
We, the authors, wish to thank the WWTP of León UTE Saleal for providing advice and recommendations regarding plant modeling and performance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMP | Biochemical methane potential |
| BOD | Biological oxygen demand |
| CHP | Combined heat and power |
| COD | Chemical oxygen demand |
| HHV | Higher heating value |
| LHV | Lower heating value |
| NPV | Net present value |
| SMP | Specific methane production |
| PEM | Proton exchange membrane |
| TS | Total solid |
| VS | Volatile solid |
| WAS | Waste-activated sludge |
| WWTP | Wastewater treatment plant |
References
- O’Connor, G.A.; Elliott, H.A.; Basta, N.T.; Bastian, R.K.; Pierzynski, G.M.; Sims, R.C.; Smith, J.E. Sustainable Land Application. J. Environ. Qual. 2005, 34, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Paramashivam, D.; Dickinson, N.M.; Clough, T.J.; Horswell, J.; Robinson, B.H. Potential Environmental Benefits from Blending Biosolids with Other Organic Amendments before Application to Land. J. Environ. Qual. 2017, 46, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zoui, O.; Baroudi, M.; Drissi, S.; Abouabdillah, A.; Abd-Elkader, O.H.; Plavan, G.; Bourioug, M. Utilization of Digestate as an Organic Manure in Corn Silage Culture: An In-Depth Investigation of Its Profound Influence on Soil’s Physicochemical Properties, Crop Growth Parameters, and Agronomic Performance. Agronomy 2023, 13, 1715. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids Management and Utilizations: A Review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Gebreeyessus, G.; Jenicek, P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and Thermophilic Anaerobic Digestion of Primary and Secondary Sludge. Effect of Pre-Treatment at Elevated Temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef] [PubMed]
- Gómez, X.; Blanco, D.; Lobato, A.; Calleja, A.; Martínez-Núñez, F.; Martin-Villacorta, J. Digestion of Cattle Manure under Mesophilic and Thermophilic Conditions: Characterization of Organic Matter Applying Thermal Analysis and 1H NMR. Biodegradation 2011, 22, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, E.; Zheng, Y.; Ran, X.; Ren, Z.; Guo, J.; Dong, R. Synergistic Effect of Hydrothermal Sludge and Food Waste in the Anaerobic Co-Digestion Process: Microbial Shift and Dewaterability. Environ. Sci. Pollut. Res. 2024, 31, 18723–18736. [Google Scholar] [CrossRef] [PubMed]
- Iacovidou, E.; Ohandja, D.-G.; Voulvoulis, N. Food Waste Co-Digestion with Sewage Sludge–Realising Its Potential in the UK. J. Environ. Manag. 2012, 112, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Fernando-Foncillas, C.; Estevez, M.M.; Uellendahl, H.; Varrone, C. Co-Management of Sewage Sludge and Other Organic Wastes: A Scandinavian Case Study. Energies 2021, 14, 3411. [Google Scholar] [CrossRef]
- Chow, W.; Chong, S.; Lim, J.; Chan, Y.; Chong, M.; Tiong, T.; Chin, J.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- García-Cascallana, J.; Carrillo-Peña, D.; Morán, A.; Smith, R.; Gómez, X. Energy Balance of Turbocharged Engines Operating in a WWTP with Thermal Hydrolysis. Co-Digestion Provides the Full Plant Energy Demand. Appl. Sci. 2021, 11, 11103. [Google Scholar] [CrossRef]
- Sembera, C.; Macintosh, C.; Astals, S.; Koch, K. Benefits and Drawbacks of Food and Dairy Waste Co-Digestion at a High Organic Loading Rate: A Moosburg WWTP Case Study. Waste Manag. 2019, 95, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-type injection system as a potential H2 mass transfer technology for full-scale in situ biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Ross, A.; Camargo-Valero, M.A. Enhancing bioenergy production from food waste by in situ biomethanation: Effect of the hydrogen injection point. Food Energy Secur. 2021, 10, e288. [Google Scholar] [CrossRef]
- Hoffstadt, K.; Nikolausz, M.; Krafft, S.; Bonatelli, M.L.; Kumar, V.; Harms, H.; Kuperjans, I.B. Dioxide in a Novel Meandering Plug Flow Reactor: Start-Up Phase and Flexible Operation. Bioengineering 2024, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Poggio, D.; Sastraatmaja, A.; Walker, M.; Michailos, S.; Nimmo, W.; Pourkashanian, M. Experimental Evaluation of Continuous In-Situ Biomethanation of CO2 in Anaerobic Digesters Fed on Sewage Sludge and Food Waste and the Influence of Hydrogen Gas–Liquid Mass Transfer. Processes 2023, 11, 604. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In Situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Sotres, A.; Arenas, C.B.; Blanco, D.; Martínez, O.; Gómez, X. Improving Anaerobic Digestion of Sewage Sludge by Hydrogen Addition: Analysis of Microbial Populations and Process Performance. Energies 2019, 12, 1228. [Google Scholar] [CrossRef]
- Nguyen, P.Y.; Morgado Ferreira, A.L.; Santiago, P.-R.; Van Der Zee, F.; Lacroix, S.; Tian, J.-H.; Lens, P.N.L. Biogas Upgrading by In-Situ Biomethanation in High-Rate Anaerobic Bioreactor Treating Biofuel Condensate Wastewater. Renew. Energ. 2025, 248, 123113. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Un, C. Assessing Biogas from Wastewater Treatment Plants for Sustainable Transportation Fuel: A Detailed Analysis of Energy Potential and Emission Reductions. Gases 2025, 5, 6. [Google Scholar] [CrossRef]
- Prokhorov, O.; Dreisbach, D. The Impact of Renewables on the Incidents of Negative Prices in the Energy Spot Markets. Energy Policy 2022, 167, 113073. [Google Scholar] [CrossRef]
- Ingersoll, J.G. The Case of Renewable Methane by and with Green Hydrogen as the Storage and Transport Medium for Intermittent Wind and Solar PV Energy. Hydrogen 2024, 5, 209–229. [Google Scholar] [CrossRef]
- Sanz-Martínez, A.; Durán, P.; Mercader, V.D.; Francés, E.; Peña, J.Á.; Herguido, J. Biogas Upgrading by CO2 Methanation with Ni-, Ni–Fe-, and Ru-Based Catalysts. Catalysts 2022, 12, 1609. [Google Scholar] [CrossRef]
- Molinet-Chinaglia, C.; Shafiq, S.; Serp, P. Low Temperature Sabatier CO2 Methanation. ChemCatChem 2024, 16, e202401213. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and Motivations for the CO2 Valorization through the Sabatier Reaction Using Structured Catalysts. A Review of the Most Recent Advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Ticali, P.; Summa, P.; Mai, S.; Skorupska, K.; Behrens, M. Support effect on Ni-based mono-and bimetallic catalysts in CO2 hydrogenation. Nanoscale 2024, 16, 17378–17392. [Google Scholar] [CrossRef] [PubMed]
- Navarro de Miguel, J.C.; Bobadilla, L.F.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Exploring the Synergistic Interaction between Nickel-and Ruthenium-Based Catalysts for Carbon Dioxide Methanation Reaction. ACS Sustain. Chem. Eng. 2025, 13, 8532–8545. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Hussien, A.G.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. Integrating capture and methanation of CO2 using physical mixtures of Na-Al2O3 and mono-/ bimetallic (Ru)Ni/Pr-CeO2. Chem. Eng. J. 2024, 491, 151962. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, H.J.; Kim, J.; Yu, H.; Joo, J.B. Integration of CO2 Adsorbent with Ni-Al2O3 Catalysts for Enhanced Methane Production in Carbon Capture and Methanation: Cooperative Interaction of CO2 Spillover and Heat Exchange. Catalysts 2024, 14, 834. [Google Scholar] [CrossRef]
- Sun, H.; Sun, S.; Liu, T.; Zeng, J.; Wang, Y.; Yan, Z.; Wu, C. Integrated CO2 capture and utilization: Selection, matching, and interactions between adsorption and catalytic sites. ACS Catal. 2024, 14, 15572–15589. [Google Scholar] [CrossRef]
- Glockler, G. Carbon–oxygen bond energies and bond distances. J. Phys. Chem. 1958, 62, 1049–1054. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. 2021, 133, 20795–20816. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological methanation of H2 and CO2 with mixed cultures: Current advances, hurdles and challenges. Waste Biomass Valori. 2021, 12, 5259–5282. [Google Scholar] [CrossRef]
- Gantenbein, A.; Kroecher, O.; Biollaz, S.M.; Schildhauer, T.J. Techno-economic evaluation of biological and fluidised-bed based methanation process chains for grid-ready biomethane production. Front. Energy Res. 2022, 9, 775259. [Google Scholar] [CrossRef]
- Engstam, L.; Janke, L.; Sundberg, C.; Nordberg, Å. Optimising power-to-gas integration with wastewater treatment and biogas: A techno-economic assessment of CO2 and by-product utilisation. Appl. Energy 2025, 377, 124534. [Google Scholar] [CrossRef]
- Bredwell, M.D.; Srivastava, P.; Worden, R.M. Reactor Design Issues for Synthesis-Gas Fermentations. Biotechnol. Prog. 1999, 15, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.E.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Performance of Trickle-Bed Bioreactors for Converting Synthesis Gas to Methane. Appl. Biochem. Biotechnol. 1991, 28–29, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Elmore, B.B.; Vega, J.L.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Biological Production of Liquid and Gaseous Fuels from Synthesis Gas. Appl. Biochem. Biotechnol. 1990, 24–25, 857–873. [Google Scholar] [CrossRef]
- European Commission 2050 Long-Term Strategy: Striving to Become the World’s First Climate-Neutral Continent by 2050. Available online: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en (accessed on 14 May 2025).
- Gavala, H.N.; Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V. Gas Biological Conversions: The Potential of Syngas and Carbon Dioxide as Production Platforms. Waste Biomass Valorization 2021, 12, 5303–5328. [Google Scholar] [CrossRef]
- Tauber, J.; Möstl, D.; Vierheilig, J.; Saracevic, E.; Svardal, K.; Krampe, J. Biological Methanation in an Anaerobic Biofilm Reactor—Trace Element and Mineral Requirements for Stable Operation. Processes 2023, 11, 1013. [Google Scholar] [CrossRef]
- Dykstra, C.M.; Pavlostathis, S.G. Zero-Valent Iron Enhances Biocathodic Carbon Dioxide Reduction to Methane. Environ. Sci. Technol. 2017, 51, 12956–12964. [Google Scholar] [CrossRef] [PubMed]
- Diender, M.; Uhl, P.S.; Bitter, J.H.; Stams, A.J.M.; Sousa, D.Z. High Rate Biomethanation of Carbon Monoxide-Rich Gases via a Thermophilic Synthetic Coculture. ACS Sustain. Chem. Eng. 2018, 6, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, B.S.; Chandrasekaran, S.; Deutzmann, J.S.; Kracke, F.; Cornell, C.; Worthington, M.A.; Freyman, M.C.; Jue, M.L.; Spormann, A.M.; Pang, S.H.; et al. Additively Manufactured High Surface Area 3D Cathodes for Efficient and Productive Electro-Bio-Methanation. ACS Electrochem. 2025, 1, 523–534. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; González, R.; Gómez, X. Feasibility of Coupling Hydrogen and Methane Production in WWTP: Simulation of Sludge and Food Wastes Co-Digestion. Energy Nexus 2024, 14, 100285. [Google Scholar] [CrossRef]
- González, R.; González-Rojo, S.; Gómez, X. Integrating Gasification into Conventional Wastewater Treatment Plants: Plant Performance Simulation. Eng 2025, 6, 100. [Google Scholar] [CrossRef]
- Longo, S.; Mauricio-Iglesias, M.; Soares, A.; Campo, P.; Fatone, F.; Eusebi, A.L.; Akkersdijk, E.; Stefani, L.; Hospido, A. ENERWATER–A Standard Method for Assessing and Improving the Energy Efficiency of Wastewater Treatment Plants. Appl. Energy 2019, 242, 897–910. [Google Scholar] [CrossRef]
- Pons, M.N.; Spanjers, H.; Baetens, D.; Nowak, O.; Gillot, S.; Nouwen, J.; Schuttinga, N. Wastewater Characteristics in Europe-A Survey. European Water Management Online 2004, 4. Available online: https://www.researchgate.net/profile/Henri_Spanjers/publication/237790819_Wastewater_Characteristics_in_Europe_-_A_Survey/links/54aaa4460cf2bce6aa1d5229.pdf (accessed on 14 May 2025).
- Oliveira, S.C.; Von Sperling, M. Reliability Analysis of Wastewater Treatment Plants. Water Res. 2008, 42, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Fierro, J.; Sánchez, M.E.; Gómez, X. Anaerobic Co-Digestion of FOG and Sewage Sludge: Study of the Process by Fourier Transform Infrared Spectroscopy. Int. Biodeterior. Biodegrad. 2012, 75, 1–6. [Google Scholar] [CrossRef]
- Thorin, E.; Olsson, J.; Schwede, S.; Nehrenheim, E. Co-Digestion of Sewage Sludge and Microalgae–Biogas Production Investigations. Appl. Energy 2018, 227, 64–72. [Google Scholar] [CrossRef]
- Song, Y.-J.; Oh, K.-S.; Lee, B.; Pak, D.-W.; Cha, J.-H.; Park, J.-G. Characteristics of Biogas Production from Organic Wastes Mixed at Optimal Ratios in an Anaerobic Co-Digestion Reactor. Energies 2021, 14, 6812. [Google Scholar] [CrossRef]
- González, R.; Blanco, D.; Cascallana, J.G.; Carrillo-Peña, D.; Gómez, X. Anaerobic Co-Digestion of Sheep Manure and Waste from a Potato Processing Factory: Techno-Economic Analysis. Fermentation 2021, 7, 235. [Google Scholar] [CrossRef]
- González, R.; García-Cascallana, J.; Gómez, X. Energetic Valorization of Biogas. A Comparison between Centralized and Decentralized Approach. Renew. Energ. 2023, 215, 119013. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the Density and the Viscosities of Biodiesel–Diesel Fuel Blends. Renew. Energ. 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Yilmaz, N. Comparative Analysis of Biodiesel–Ethanol–Diesel and Biodiesel–Methanol–Diesel Blends in a Diesel Engine. Energy 2012, 40, 210–213. [Google Scholar] [CrossRef]
- Jenbacher Jenbacher de Tipo 2: J208. Available online: https://www.jenbacher.com/es/motores-de-gas/tipo-2 (accessed on 14 May 2025).
- Simons, G.; Barsun, S. Chapter 23: Combined Heat and Power Evaluation Protocol. In The Uniform Methods Project: Methods for Determining Energy-Efficiency Savings for Specific Measures; National Renewable Energy Laboratory: Golden, CO, USA, 2017; ISBN NREL/SR-7A40-68579. Available online: https://docs.nrel.gov/docs/fy18osti/70472.pdf (accessed on 14 May 2025).
- Trading Economics. EU Natural Gas TTF. Available online: https://tradingeconomics.com/commodity/eu-natural-gas (accessed on 14 May 2025).
- Schwede, S.; Bruchmann, F.; Thorin, E.; Gerber, M. Biological Syngas Methanation via Immobilized Methanogenic Archaea on Biochar. Energy Procedia 2017, 105, 823–829. [Google Scholar] [CrossRef]
- Paillet, F.; Crestey, E.; Gaval, G.; Haddad, M.; Lebars, F.; Nicolitch, O.; Camacho, P. Utilization of Dissolved CO2 to Control Methane and Acetate Production in Methanation Reactor. Bioresour. Technol. 2025, 416, 131722. [Google Scholar] [CrossRef] [PubMed]
- Thema, M.; Weidlich, T.; Hörl, M.; Bellack, A.; Mörs, F.; Hackl, F.; Kohlmayer, M.; Gleich, J.; Stabenau, C.; Trabold, T.; et al. Biological CO2-Methanation: An Approach to Standardization. Energies 2019, 12, 1670. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Syngas Fermentation to Biofuel: Evaluation of Carbon Monoxide Mass Transfer Coefficient (KLa) in Different Reactor Configurations. Biotechnol. Prog. 2010, 26, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hao, W.; Li, Y.; Zhou, H. Biological Methanation of H2 and CO2 in a Continuous Stirred Tank Reactor. J. Clean. Prod. 2022, 370, 133518. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Dong, R.; Xiong, W.; Li, Y.; Zhu, Y. Integration of In-Situ and Ex-Situ Power-to-Gas (PtG) Strategy for Simultaneous Bio-Natural Gas Production and CO2 Emission Reduction. Chemosphere 2023, 344, 140370. [Google Scholar] [CrossRef] [PubMed]
- Illi, L.; Lecker, B.; Lemmer, A.; Müller, J.; Oechsner, H. Biological Methanation of Injected Hydrogen in a Two-Stage Anaerobic Digestion Process. Bioresour. Technol. 2021, 333, 125126. [Google Scholar] [CrossRef] [PubMed]
- Laguillaumie, L.; Rafrafi, Y.; Moya-Leclair, E.; Delagnes, D.; Dubos, S.; Spérandio, M.; Paul, E.; Dumas, C. Stability of Ex Situ Biological Methanation of H2/CO2 with a Mixed Microbial Culture in a Pilot Scale Bubble Column Reactor. Bioresour. Technol. 2022, 354, 127180. [Google Scholar] [CrossRef] [PubMed]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High performance biological methanation in a thermophilic anaerobic trickle bed reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Anaerobic Thermophilic Trickle Bed Reactor as a Promising Technology for Flexible and Demand-Oriented H2/CO2 Biomethanation. Appl. Energy 2018, 232, 543–554. [Google Scholar] [CrossRef]
- Haitz, F.; Jochum, O.; Lasota, A.; Friedrich, A.; Bieri, M.; Stalder, M.; Schaub, M.; Hochberg, U.; Zell, C. Continuous Biological Ex Situ Methanation of CO2 and H2 in a Novel Inverse Membrane Reactor (IMR). Processes 2024, 12, 2305. [Google Scholar] [CrossRef]
- Akimoto, S.; Tsubota, J.; Angelidaki, I.; Hidaka, T.; Fujiwara, T. Pilot-Scale in-Situ Biomethanation of Sewage Sludge: Effects of Gas Recirculation Method. Bioresour. Technol. 2024, 413, 131524. [Google Scholar] [CrossRef] [PubMed]
- Pokorna, D.; Varga, Z.; Andreides, D.; Zabranska, J. Adaptation of Anaerobic Culture to Bioconversion of Carbon Dioxide with Hydrogen to Biomethane. Renew. Energ. 2019, 142, 167–172. [Google Scholar] [CrossRef]
- Bensmann, A.; Hanke-Rauschenbach, R.; Heyer, R.; Kohrs, F.; Benndorf, D.; Reichl, U.; Sundmacher, K. Biological Methanation of Hydrogen within Biogas Plants: A Model-Based Feasibility Study. Appl. Energy 2014, 134, 413–425. [Google Scholar] [CrossRef]
- Liu, K.; Phillips, J.R.; Sun, X.; Mohammad, S.; Huhnke, R.L.; Atiyeh, H.K. Investigation and Modeling of Gas-Liquid Mass Transfer in a Sparged and Non-Sparged Continuous Stirred Tank Reactor with Potential Application in Syngas Fermentation. Fermentation 2019, 5, 75. [Google Scholar] [CrossRef]
- Gambou, F.; Guilbert, D.; Zasadzinski, M.; Rafaralahy, H. A Comprehensive Survey of Alkaline Electrolyzer Modeling: Electrical Domain and Specific Electrolyte Conductivity. Energies 2022, 15, 3452. [Google Scholar] [CrossRef]
- Santos, A.L.; Cebola, M.-J.; Santos, D.M.F. Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers. Energies 2021, 14, 3193. [Google Scholar] [CrossRef]
- Krishnan, S.; Koning, V.; Theodorus de Groot, M.; De Groot, A.; Mendoza, P.G.; Junginger, M.; Kramer, G.J. Present and future cost of alkaline and PEM electrolyser stacks. Int. J. Hydrogen Energy 2023, 48, 32313–32330. [Google Scholar] [CrossRef]
- Hydrogen EnergyTM Hy.GEN-E®: Electrolysis. Available online: https://www.hygreenenergy.com/electrolyzers/pem-electrolyzers/ (accessed on 14 May 2025).
- van der Roest, E.; Bol, R.; Fens, T.; van Wijk, A. Utilisation of Waste Heat from PEM Electrolysers–Unlocking Local Optimisation. Int. J. Hydrogen Energy 2023, 48, 27872–27891. [Google Scholar] [CrossRef]
- Badgett, A.; Brauch, J.; Thatte, A.; Rubin, R.; Skangos, C.; Wang, X.; Ahluwalia, R.; Pivovar, B.; Ruth, M. Updated Manufactured Cost Analysis for Proton Exchange Membrane Water Electrolyzers; (No. NREL/TP-6A20-87625); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2024. [CrossRef]
- The University of Manchester Chemical Engineering Plant Cost Index. Available online: https://www.training.itservices.manchester.ac.uk/public/gced/CEPCI.html?reactors/CEPCI/index.html (accessed on 14 May 2025).
- Vineyard, D.; Karthikeyan, K.G.; Barak, P. BioWin Modeling of CalPrex Phosphorus Re-covery from Wastewater Predicts Substantial Nuisance Struvite Reduction. Environments 2024, 11, 48. [Google Scholar] [CrossRef]
- Garske, B.; Ekardt, F. Economic Policy Instruments for Sustainable Phosphorus Management: Taking into Account Climate and Biodiversity Targets. Environ. Sci. Eur. 2021, 33, 56. [Google Scholar] [CrossRef]
- Walsh, M.; Schenk, G.; Schmidt, S. Realising the Circular Phosphorus Economy Delivers for Sustainable Development Goals. NPJ Sustain. Agric. 2023, 1, 2. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Frandsen, F.J.; Henriksen, U.B. Low Temperature Circulating Fluidized Bed Gasification and Co-Gasification of Municipal Sewage Sludge. Part 1: Process Performance and Gas Product Characterization. Waste Manag. 2017, 66, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.L.F.; da Silva, J.C.G.; Languer, M.P.; Batistella, L.; Di Domenico, M.; da Silva Filho, V.F.; Moreira, R.d.F.P.M.; José, H.J. Assessing the Bioenergy Potential of High-Ash Anaerobic Sewage Sludge Using Pyrolysis Kinetics and Thermodynamics to Design a Sustainable Integrated Biorefinery. Biomass Convers. Biorefin. 2022, 12, 693–704. [Google Scholar] [CrossRef]
- Januševičius, T.; Mažeikienė, A.; Danila, V.; Paliulis, D. The Characteristics of Sewage Sludge Pellet Biochar Prepared Using Two Different Pyrolysis Methods. Biomass Convers. Biorefin. 2024, 14, 891–900. [Google Scholar] [CrossRef]
- Mediboyina, M.K.; Murphy, F. Environmental Assessment of a Waste-to-Energy Cascading System Integrating Forestry Residue Pyrolysis and Poultry Litter Anaerobic Digestion. Energies 2024, 17, 1511. [Google Scholar] [CrossRef]
- Eurostat Electricity Price Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics (accessed on 14 May 2025).
- Bellini, R.; Bassani, I.; Vizzarro, A.; Azim, A.; Vasile, N.; Pirri, C.; Verga, F.; Menin, B. Biological Aspects, Advancements and Techno-Economical Evaluation of Biological Methanation for the Recycling and Valorization of CO2. Energies 2022, 15, 4064. [Google Scholar] [CrossRef]
- Khesali Aghtaei, H.; Püttker, S.; Maus, I.; Heyer, R.; Huang, L.; Sczyrba, A.; Reichl, U.; Benndorf, D. Adaptation of a Microbial Community to Demand-Oriented Biological Methanation. Biotechnol. Biofuels Bioprod. 2022, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Colantoni, S.; Cruz Viggi, C.; Aulenta, F. Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators. Catalysts 2023, 13, 859. [Google Scholar] [CrossRef]
- Pan, Z.; Hui, Y.; Hu, X.; Yu, J.; Zhang, H.; Feng, X.; Guo, K. A Novel Electrolytic Gas Lift Reactor for Efficient Microbial Electrosynthesis of Acetate from Carbon Dioxide. Bioresour. Technol. 2024, 393, 130124. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Ottosen, L.D.M.; Kofoed, M.V.W. H2 Gas-Liquid Mass Transfer: A Key Element in Biological Power-to-Gas Methanation. Renew. Sust. Energ. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Rattanapan, C.; Sinchai, L.; Tachapattaworakul Suksaroj, T.; Kantachote, D.; Ounsaneha, W. Biogas Production by Co-Digestion of Canteen Food Waste and Domestic Wastewater under Organic Loading Rate and Temperature Optimization. Environments 2019, 6, 16. [Google Scholar] [CrossRef]
- Beswick, R.R.; Oliveira, A.M.; Yan, Y. Does the green hydrogen economy have a water problem. ACS Energy Lett. 2021, 6, 3167–3169. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Longo, S.; Cellura, M.; Miccichè, G.; Ferraro, M. Critical Review of Life Cycle Assessment of Hydrogen Produc-tion Pathways. Environments 2024, 11, 108. [Google Scholar] [CrossRef]
- Salazar, V.; Kaiser, S. Influence of the flow field on flame propagation in a hydrogen-fueled internal combustion engine. SAE Int. J. Engines 2011, 4, 2376–2394. [Google Scholar] [CrossRef]
- Beachem, C.D. A new model for hydrogen-assisted cracking (hydrogen “embrittlement”). Met. Mater. Trans. B 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Kim, M.; Kim, H.-J.; Suh, J.; Kang, N. Role of Hydrogen and Temperature in Hydrogen Embrittlement of Equimolar CoCrFeMnNi High-entropy Alloy. Met. Mater. Int. 2021, 27, 166–174. [Google Scholar] [CrossRef]
- Cavaliere, P. Safety issues and regulations. In Water Electrolysis for Hydrogen Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 729–791. [Google Scholar] [CrossRef]
- Hydrogen Production Plant Design & Operational Safety Considerations. Available online: https://www.rishabheng.com/blog/hydrogen-production-plant-design-and-safety-considerations/ (accessed on 2 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).