A Rapid Method for Identifying Plant Oxidative Stress and Implications for Riparian Vegetation Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and a Selection of Native and Invasive Species
2.2. Collection of Plant Leaves and Soil
2.3. Sample Preparation Utilizing Shake Master Auto for H2O2 Quantification
2.4. Determination of H2O2 Content with Ti (SO4)2 Assay
2.5. Extraction of Photosynthetic Pigments (Chlorophyll a and Chlorophyll b)
2.6. Measurement of Soil Moisture, TN, and TP
2.7. Statistical Analyses
3. Results
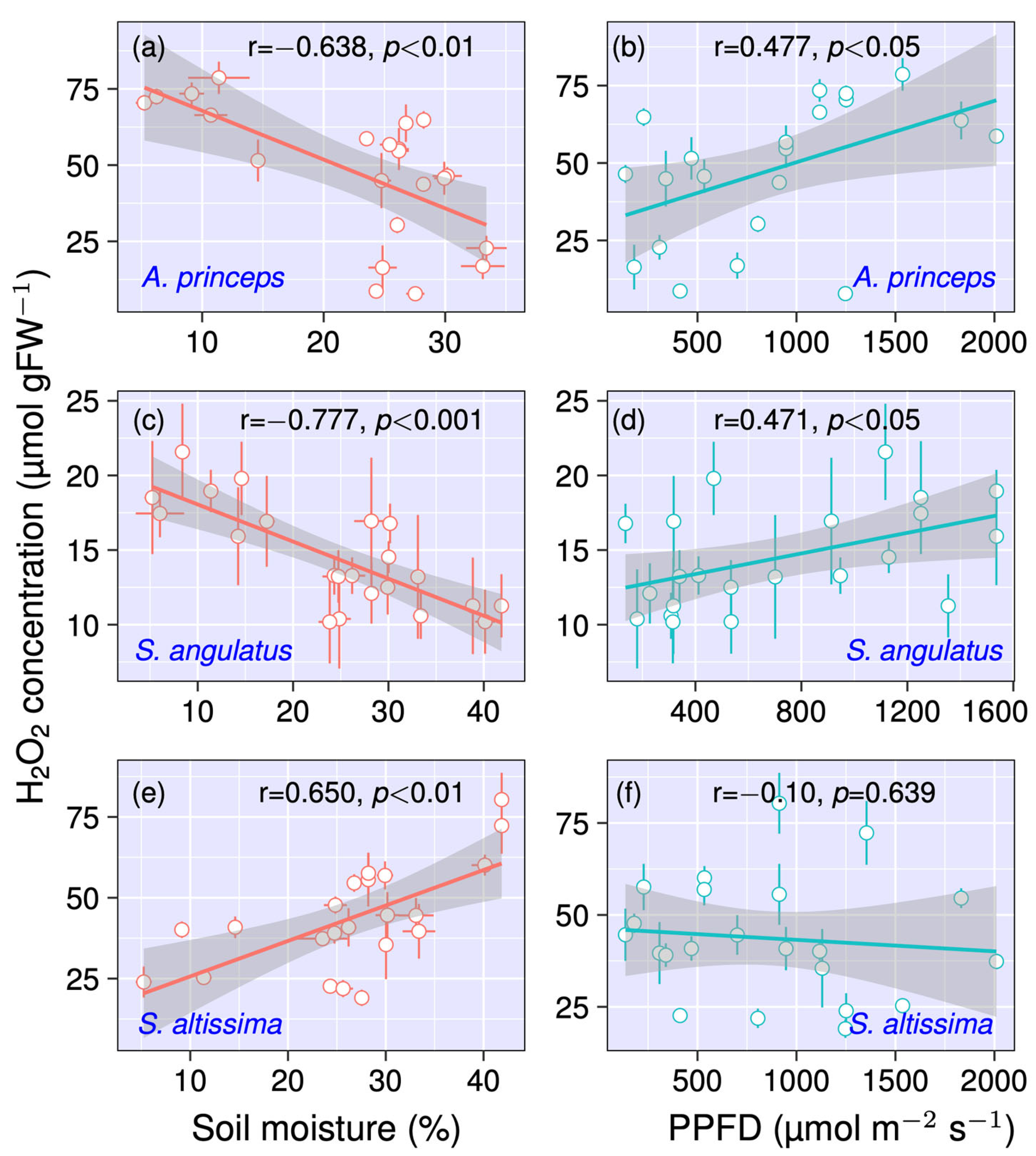
3.1. Impact of Soil Moisture and PPFD on H2O2 Concentration
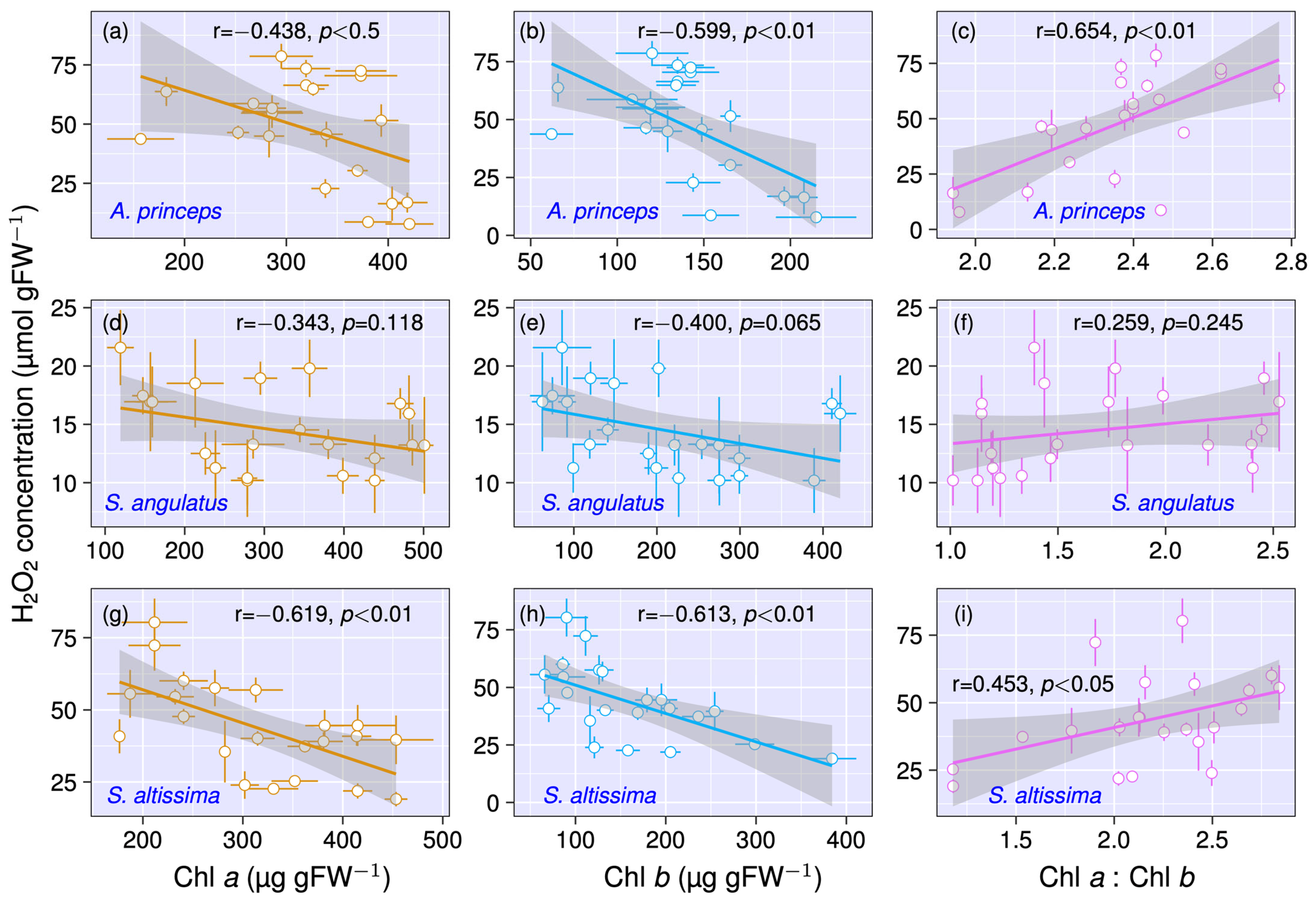
3.2. Relationship Between Chlorophyll Content and H2O2 Concentration
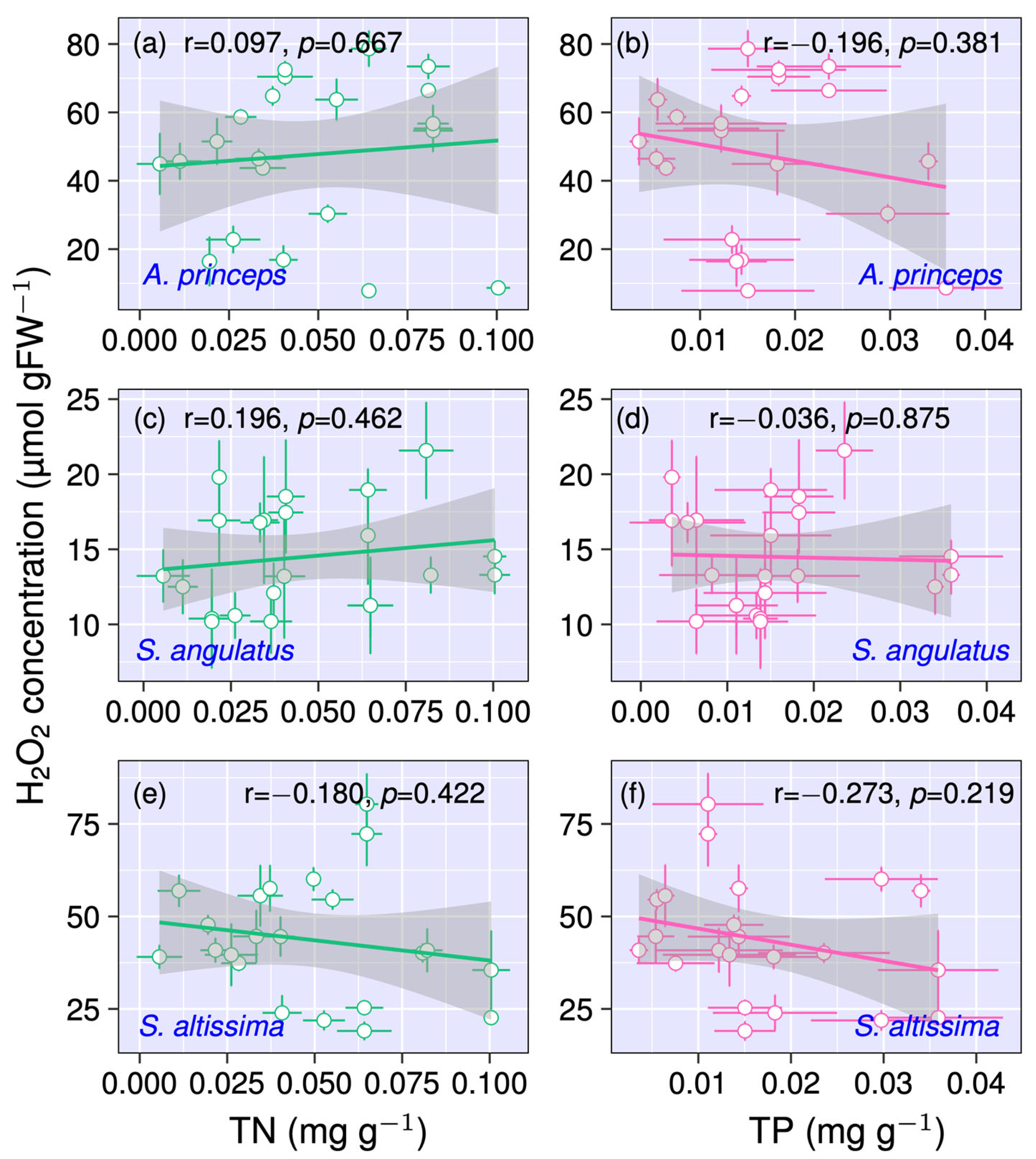
3.3. Relationship Between H2O2 Concentration and Soil Macronutrients
3.4. Connection Between Several Factors and H2O2 Concentration
4. Discussion
4.1. Evaluating the Influence of Selected Environmental Parameters
4.2. Tissue H2O2 Concentration in Vegetation Management
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, F.; Yamada, H. Effects of pasture development on the ecological functions of riparian forests in Hokkaido in northern Japan. Ecol. Eng. 2005, 24, 539–550. [Google Scholar] [CrossRef]
- Toda, Y.; Zhou, Y.; Sakai, N. Modeling of riparian vegetation dynamics and its application to sand-bed river. J. Hydro-Environ. Res. 2020, 30, 3–13. [Google Scholar] [CrossRef]
- Akamatsu, F.; Shimano, K.; Denda, M.; Ide, K.; Ishihara, M.; Toda, H. Effects of sediment removal on nitrogen uptake by riparian plants in the higher floodplain of the Chikuma River, Japan. Landsc. Ecol. Eng. 2008, 4, 91–96. [Google Scholar] [CrossRef]
- Akasaka, T.; Mori, T.; Ishiyama, N.; Takekawa, Y.; Kawamoto, T.; Inoue, M.; Mitsuhashi, H.; Kawaguchi, Y.; Ichiyanagi, H.; Onikura, N.; et al. Reconciling biodiversity conservation and flood risk reduction: The new strategy for freshwater protected areas. Divers. Distrib. 2022, 28, 1191–1201. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kohzu, A.; Ouchi, T.; Fukushima, T. Quantifying the sources and removal of nitrate in riparian and lotic environments based on land use and topographic parameters of the watershed. Ecol. Indic. 2020, 116, 106535. [Google Scholar] [CrossRef]
- Ishikawa, N.F.; Uchida, M.; Shibata, Y.; Tayasu, I. Carbon storage reservoirs in watersheds support stream food webs via periphyton production. Ecology 2014, 95, 1264–1271. [Google Scholar] [CrossRef]
- Piras, F.; Fiore, B.; Santoro, A. Small Cultural Forests: Landscape Role and Ecosystem Services in a Japanese Cultural Landscape. Land 2022, 11, 1494. [Google Scholar] [CrossRef]
- Zheng, X.; Natuhara, Y. Landscape and local correlates with two green tree-frogs, Rhacophorus (Amphibia: Rhacophoridae) in different habitats, central Japan. Landsc. Ecol. Eng. 2020, 16, 199–206. [Google Scholar] [CrossRef]
- Mizusawa, N.; Reza, M.S.; Oikawa, C.; Kuga, S.; Iijima, M.; Kobiyama, A.; Yamada, Y.; Ikeda, Y.; Ikeda, D.; Ikeo, K.; et al. Diversity and functions of bacterial communities in water and sediment from the watershed of the Tama River flowing a highly urbanized area. Fish. Sci. 2021, 87, 697–715. [Google Scholar] [CrossRef]
- Ide, J.I.; Takeda, I.; Somura, H.; Mori, Y.; Sakuno, Y.; Yone, Y.; Takahashi, E. Impacts of hydrological changes on nutrient transport from diffuse sources in a rural river basin, western Japan. J. Geophys. Res. Biogeosci. 2019, 124, 2565–2581. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Vamsi-Krishna, L.; Schoelynck, J.; Rashid, M.H. Measurement of foliar H2O2 concentration can be an indicator of riparian vegetation management. Sci. Rep. 2022, 12, 13803. [Google Scholar] [CrossRef]
- Asaeda, T.; García de Jalón, D.; O’Hare, M.; Rahman, M. Sequential riparian vegetation alteration in Japanese river landscapes. Int. J. River Basin Manag. 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Ito, H.; Ito, S.; Matsui, T.; Marutani, T. Effect of fluvial and geomorphic disturbances on habitat segregation of tree species in a sedimentation-dominated riparian forest in warm-temperate mountainous region in southern Japan. J. For. Res. 2006, 11, 405–417. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Numata, S.; Hosaka, T. Mapping an invasive goldenrod of Solidago altissima in urban landscape of Japan using multi-scale remote sensing and knowledge-based classification. Ecol. Indic. 2020, 111, 105975. [Google Scholar] [CrossRef]
- Uchida, T.; Nomura, R.; Asaeda, T.; Rashid, M.H. Co-existence of Sicyos angulatus and native plant species in the floodplain of Tama River, Japan. Int. J. Biodivers. Conserv. 2012, 4, 336–347. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 2001, 418, 617–620. [Google Scholar] [CrossRef]
- Stevens, J.M.; Fehmi, J.S. Early establishment of a native grass reduces the competitive effect of a non-native grass. Restor. Ecol. 2011, 19, 399–406. [Google Scholar] [CrossRef]
- LeJeune, K.D.; McLachlan, J.S. The role of nitrogen in plant invasions: A review of the evidence. Front. Ecol. Environ. 2004, 2, 103–110. [Google Scholar]
- Asaeda, T.; Rashid, M.H.; Liping, X.; Vamsi-Krishna, L.; Barnuevo, A.; Takeuchi, C.; Rahman, M. The distribution of submerged macrophytes in response to intense solar radiation and salinity reveals hydrogen peroxide as an abiotic stress indicator. Sci. Rep. 2023, 13, 4548. [Google Scholar] [CrossRef]
- Walker, L.R.; Smith, S.D. Impacts of invasive plants on community and ecosystem properties. In Assessment and Management of Plant Invasions; Springer New York: New York, NY, USA, 1997; pp. 69–86. [Google Scholar]
- Zhang, L.; Natuhara, Y.; Zhao, J.; Cao, Y. Effects of soil properties and urbanization on the trait variations of riparian species: Evidence from central Japan. J. Biotech. Res. 2024, 16, 37–383. [Google Scholar]
- Sato, T.; Ito, S.; Mitsuda, Y.; Soen, N. Impacts of land-use history on the diversity of a riparian forest landscape in warm-temperate Kyushu, southern Japan. Landsc. Ecol. Eng. 2010, 6, 89–98. [Google Scholar] [CrossRef]
- Yamamoto, K.; Iida, S.; Tanaka, M. Soil moisture and biodiversity in riparian ecosystems of Japan. Biodivers. Conserv. 2016, 25, 1129–1144. [Google Scholar]
- Rashid, M.; Asaeda, T. Seasonal variation of carbohydrates in Pueraria montana as affected by soil characteristics in a river floodplain. In Proceedings of the 36th IAHR World Congress, The Hague, The Netherlands, 28 June–3 July 2015; pp. 1–4. [Google Scholar]
- Rashid, M.H.; Uddin, M.N.; Sarkar, A.; Parveen, M.; Asaeda, T. The growth and nutrient uptake of invasive vines on contrasting riverbank soils. River Res. Appl. 2019, 35, 749–758. [Google Scholar] [CrossRef]
- Guildford, S.J.; Hecky, R.E. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol. Oceanogr. 2000, 45, 1213–1223. [Google Scholar] [CrossRef]
- Dolman, A.M.; Mischke, U.; Wiedner, C. Lake-type-specific seasonal patterns of nutrient limitation in German lakes, with target nitrogen and phosphorus concentrations for good ecological status. Freshw. Biol. 2016, 61, 444–456. [Google Scholar] [CrossRef]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef]
- Barua, D.; Heckathorn, S.A. The interactive effects of light and temperature on heat-shock protein accumulation in Solidago altissima (Asteraceae) in the field and laboratory. Am. J. Bot. 2006, 93, 102–109. [Google Scholar] [CrossRef]
- Niinemets, Ü. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol. 2015, 205, 79–96. [Google Scholar] [CrossRef]
- Pollastrini, M.; Holland, V.; Brüggemann, W.; Bussotti, F. Chlorophyll a fluorescence analysis in forests. Ann. Di Bot. 2016, 6, 23–37. [Google Scholar]
- Guo, W.Y.; Lambertini, C.; Guo, X.; Li, X.Z.; Eller, F.; Brix, H. Phenotypic traits of the Mediterranean Phragmites australis M1 lineage: Differences between the native and introduced ranges. Biol. Invasions 2016, 18, 2551–2561. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.; Wang, P.; Hu, J.; Lu, B.; Tang, S.; Zhang, C.; Gao, P. Responses of riparian plant roots to acute combined nitrogen and phosphorus stress: Changes in root morphology and antioxidant system. Aquat. Ecol. 2023, 57, 715–732. [Google Scholar] [CrossRef]
- Yin, M.; Wang, S.; Wang, Y.; Wei, R.; Liang, Y.; Zuo, L.; Huo, M.; Huang, Z.; Lang, J.; Zhao, X.; et al. Impact of Abiotic Stress on Rice and the Role of DNA Methylation in Stress Response Mechanisms. Plants 2024, 13, 2700. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K.; Senbayram, M. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1981, 12, 345–354. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of Hydrogen Peroxide in the Regulation of Senescence in Pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Abeynayaka, H.D.L. Hydrogen peroxide can be a plausible biomarker in cyanobacterial bloom treatment. Sci. Rep. 2022, 12, 12. [Google Scholar] [CrossRef]
- Asaeda, T.; Rahman, M.; Liping, X.; Schoelynck, J. Hydrogen peroxide variation patterns as abiotic stress responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef]
- Rahman, M.; Asaeda, T.; Abeynayaka, H.D.L.; Fukahori, K. An Assessment of the Effects of Light Intensities and Temperature Changes on Cyanobacteria’s Oxidative Stress via the Use of Hydrogen Peroxide as an Indicator. Water 2023, 15, 2429. [Google Scholar] [CrossRef]
- Satterfield, C.N.; Bonnell, A.H. Interferences in titanium sulfate method for hydrogen peroxide. Anal. Chem. 1955, 27, 1174–1175. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Rahman, M.; Asaeda, T.; Fukahori, K.; Imamura, F.; Nohara, A.; Matsubayashi, M. Hydrogen Peroxide Measurement Can Be Used to Monitor Plant Oxidative Stress Rapidly Using Modified Ferrous Oxidation Xylenol Orange and Titanium Sulfate Assay Correlation. Int. J. Plant Biol. 2023, 14, 546–557. [Google Scholar] [CrossRef]
- Rashid, M.H.; Asaeda, T.; Uddin, M.N. Litter-mediated allelopathic effects of kudzu (Pueraria montana) on Bidens pilosa and Lolium perenne and its persistence in soil. Weed Biol. Manag. 2010, 10, 48–56. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Gardner, W.H. Water content. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; Volume 5, pp. 493–544. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C., Braun-Howland, E.B., Baxter, T.E., Eds.; APHA Press: Baltimore, MD, USA, 2023; Available online: https://www.standardmethods.org/24theditioncitation (accessed on 9 July 2025).
- R Core Team. R: A Language And Environment For Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 July 2025).
- Drexler, M.; Ainsworth, C.H. Generalized additive models used to predict species abundance in the Gulf of Mexico: An ecosystem modeling tool. PLoS ONE 2013, 8, e64458. [Google Scholar] [CrossRef]
- Rahman, M.; Asaeda, T.; Fukahori, K.; Rashid, M.H.; Matsuo, A. Diurnal and Thermal Variations in an Oxidative Stress Biomarker of Pseudanabaena foetida: A Model for Environmental Adaptation in Aquatic Ecosystems. Stresses 2025, 5, 20. [Google Scholar] [CrossRef]
- Roach, T.; Miller, R.; Aigner, S.; Kranner, I. Diurnal changes in the xanthophyll cycle pigments of freshwater algae correlate with the environmental hydrogen peroxide concentration rather than non-photochemical quenching. Ann. Bot. 2015, 116, 519–527. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Morris, J.T.; Shaffer, G.P.; Nyman, J.A. Brinson review: Perspectives on the influence of nutrients on the sustainability of coastal wetlands. Wetlands 2013, 33, 975–988. [Google Scholar] [CrossRef]
- Egli, P.; Schmid, B. Seasonal dynamics of biomass and nitrogen in canopies of Solidago altissima and effects of a yearly mowing treatment. Acta Oecologica 2000, 21, 63–77. [Google Scholar] [CrossRef]
- Fengxi, Z.; Shihui, Y.; Meihui, L.; Xinyu, L.; Zhang, X.; Yong, C.; Hong, Z. Adaptive strategies of structures that enhance invasion in Sicyos angulatus. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 1323–1330. [Google Scholar]
- Ashworth, S.M.; Foster, B.L.; Kindscher, K. Relative contributions of adjacent species set and environmental conditions to plant community richness and composition in a group of created wetlands. Wetlands 2006, 26, 193–204. [Google Scholar] [CrossRef]
- Chapin, F.S., III. Effects of plant traits on ecosystem and regional processes: A conceptual framework for predicting the consequences of global change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Luo, Y.; Elser, J.; Wang, Y.P.; Loladze, I.; Zhang, Q.; Dennis, S. Down-regulation of tissue N: P ratios in terrestrial plants by elevated CO2. Ecology 2015, 96, 3354–3362. [Google Scholar] [CrossRef]
- Bratt, A.R.; Finlay, J.C.; Welter, J.R.; Vculek, B.A.; Van Allen, R.E. Co-limitation by N and P characterizes phytoplankton communities across nutrient availability and land use. Ecosystems 2020, 23, 1121–1137. [Google Scholar] [CrossRef]
- Gurnell, A. Plants as river system engineers. Earth Surf. Process. Landf. 2014, 39, 4–25. [Google Scholar] [CrossRef]
- Liébault, F.; Piégay, H. Causes of 20th century channel narrowing in mountain and piedmont rivers of southeastern France. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group 2002, 27, 425–444. [Google Scholar] [CrossRef]
- Lach, J.; Wyżga, B. Channel incision and flow increase of the upper Wisłoka River, southern Poland, subsequent to the reafforestation of its catchment. Earth Surf. Process. Landf. J. Br. Geomorphol. Res. Group 2002, 27, 445–462. [Google Scholar] [CrossRef]
- Annala, M.J.; Lehosmaa, K.; Ahonen, S.H.K.; Karttunen, K.; Markkola, A.M.; Puumala, I.; Mykrä, H. Effect of riparian soil moisture on bacterial, fungal and plant communities and microbial decomposition rates in boreal stream-side forests. For. Ecol. Manag. 2022, 519, 120344. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar]

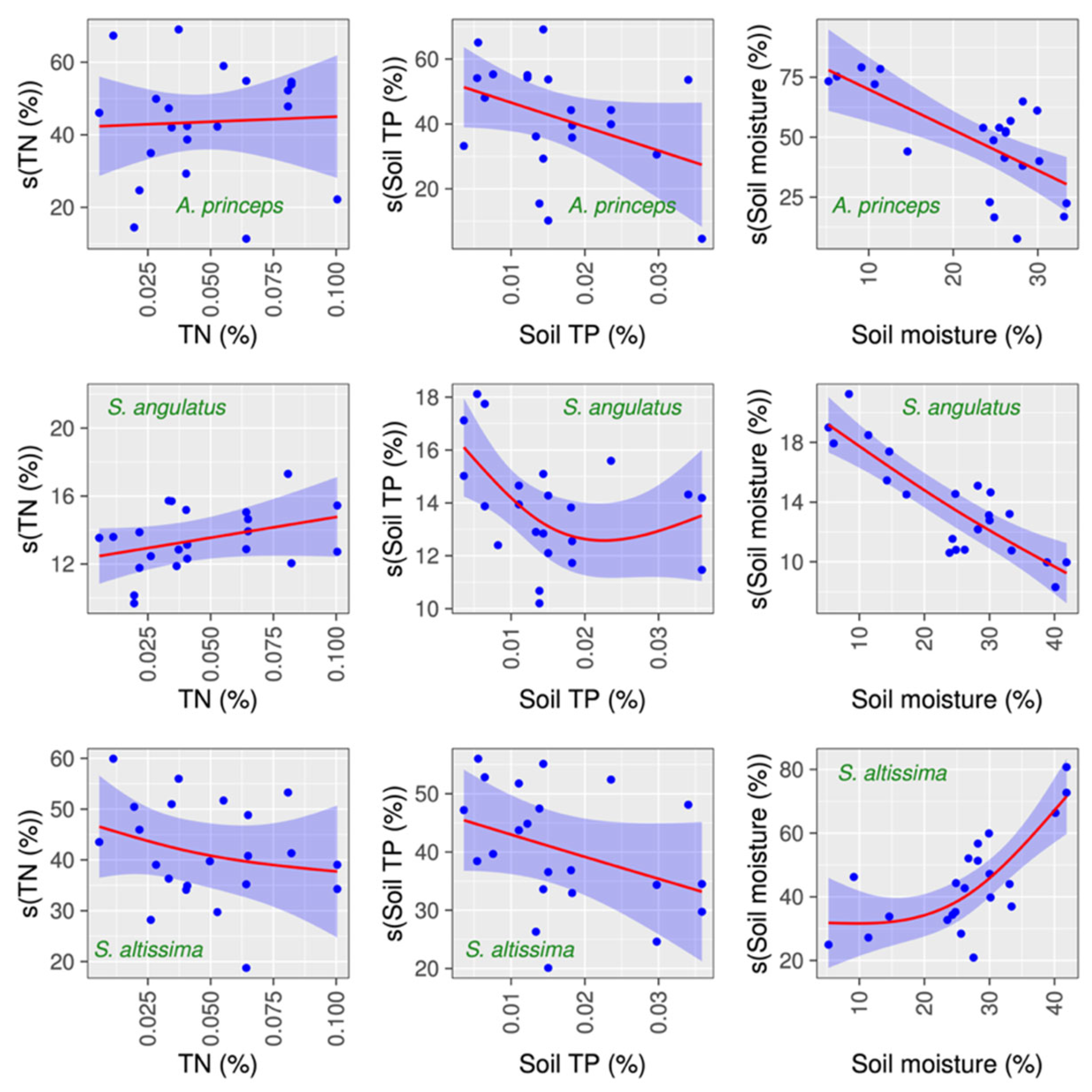
| Indicators of Smoothness (Approximately) | ||||
|---|---|---|---|---|
| Parameters | EDF | Ref. EDF | F | p-Value |
| s (soil moisture) | 1 | 1 | 15.245 | <0.0001 |
| s(TN) | 1 | 1 | 0.038 | 0.847 |
| s(TP) | 1 | 1 | 2.962 | 0.102 |
| Indicators of Smoothness (Approximately) | ||||
|---|---|---|---|---|
| Parameters | EDF | Ref. EDF | F | p-Value |
| s (soil moisture) | 1.286 | 1.487 | 31.908 | <0.001 |
| s(TN) | 1 | 1 | 2.504 | 0.1699 |
| s(TP) | 1.847 | 1.974 | 3.754 | 0.0578 |
| Indicators of Smoothness (APPROXIMATELY) | ||||
|---|---|---|---|---|
| Parameters | EDF | Ref. EDF | F | p-Value |
| s (soil moisture) | 1.878 | 1.982 | 12.477 | <0.001 |
| s(TN) | 1.178 | 1.322 | 0.974 | 0.455 |
| s(TP) | 1.008 | 1.017 | 2.17 | 0.522 |
| Candidate Models | df | Loglik | AIC | ΔAIC | Weight |
|---|---|---|---|---|---|
| A. princeps | |||||
| H2O2~ s(SM) | 3 | −92.74 | 192.82 | 0 | 0.51 |
| H2O2~ s(PPFD) + s(SM) | 4 | −91.61 | 193.58 | 0.76 | 0.35 |
| H2O2~ s(SM) + s(TN) + s(TP) * | 5 | −91.05 | 195.84 | 3.02 | 0.11 |
| H2O2~ s(PPFD) + s(SM) + s(TN) + s(TP) | 6 | −90.39 | 198.37 | 5.55 | 0.03 |
| H2O2~ s(PPFD) + s(TN) + s(TP) | 5 | −95.45 | 204.65 | 11.83 | 0 |
| S. angulatus | |||||
| H2O2~ s(SM) | 3 | −47.68 | 102.68 | 0 | 0.42 |
| H2O2~ s(SM) + s(TN) + s(TP) * | 6.13 | −42.74 | 103.64 | 0.96 | 0.26 |
| H2O2~ s(PPFD) + s(SM) | 4 | −46.94 | 104.24 | 1.55 | 0.19 |
| H2O2~ s(PPFD) + s(SM) + s(TN) + s(TP) | 6.85 | −41.88 | 105.07 | 2.39 | 0.13 |
| H2O2~ s(PPFD) + s(TN) + s(TP) | 5.43 | −54.16 | 123.67 | 20.98 | 0 |
| S. altissima | |||||
| H2O2~ s(SM) | 3.79 | −83.52 | 176.74 | 0 | 0.63 |
| H2O2~ s(SM) + s(TN) + s(TP) * | 6.06 | −80.49 | 178.85 | 2.1 | 0.22 |
| H2O2~ s(PPFD) + s(SM) | 4.78 | −83.51 | 179.99 | 3.25 | 0.12 |
| H2O2~ s(PPFD) + s(SM) + s(TN) + s(TP) | 6.91 | −80.68 | 182.93 | 6.19 | 0.03 |
| H2O2~ s(PPFD) + s(TN) + s(TP) | 5 | −90.82 | 195.39 | 18.65 | 0 |
| Variables | A. princeps | S. angulatus | S. altissima | |||
|---|---|---|---|---|---|---|
| Tolerance | VIF | Tolerance | VIF | Tolerance | VIF | |
| s(SM).1 | 0.645 | 1.551 | 0.588 | 1.701 | 0.837 | 1.195 |
| s(SM).2 | 0.779 | 1.283 | 0.777 | 1.287 | 0.859 | 1.164 |
| s(TN).1 | 0.665 | 1.503 | 0.368 | 2.718 | 0.681 | 1.469 |
| s(TN).2 | 0.562 | 1.778 | 0.400 | 2.499 | 0.623 | 1.606 |
| s(TP).1 | 0.748 | 1.337 | 0.879 | 1.138 | 0.903 | 1.108 |
| s(TP).2 | 0.710 | 1.408 | 0.350 | 2.853 | 0.585 | 1.710 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Asaeda, T.; Fukahori, K.; Rashid, M.H.; Kawashima, H.; Akimoto, J.; Anta, R.T. A Rapid Method for Identifying Plant Oxidative Stress and Implications for Riparian Vegetation Management. Environments 2025, 12, 247. https://doi.org/10.3390/environments12070247
Rahman M, Asaeda T, Fukahori K, Rashid MH, Kawashima H, Akimoto J, Anta RT. A Rapid Method for Identifying Plant Oxidative Stress and Implications for Riparian Vegetation Management. Environments. 2025; 12(7):247. https://doi.org/10.3390/environments12070247
Chicago/Turabian StyleRahman, Mizanur, Takashi Asaeda, Kiyotaka Fukahori, Md Harun Rashid, Hideo Kawashima, Junichi Akimoto, and Refah Tabassoom Anta. 2025. "A Rapid Method for Identifying Plant Oxidative Stress and Implications for Riparian Vegetation Management" Environments 12, no. 7: 247. https://doi.org/10.3390/environments12070247
APA StyleRahman, M., Asaeda, T., Fukahori, K., Rashid, M. H., Kawashima, H., Akimoto, J., & Anta, R. T. (2025). A Rapid Method for Identifying Plant Oxidative Stress and Implications for Riparian Vegetation Management. Environments, 12(7), 247. https://doi.org/10.3390/environments12070247





