Abstract
Shallow lakes are highly vulnerable to pollution due to their small water volume. Those that receive effluent from the drainfields of onsite wastewater treatment systems (septic tanks) may contain pharmaceuticals and personal care products (PPCPs) that escaped removal during treatment. This study examined the effects of seasonal rainfall variability on the assemblages and concentrations of fourteen PPCPs in two shallow lakes in West–Central Florida, USA: one surrounded by residents equipped with septic tanks and the other located within a nature preserve. Water samples were collected weekly during an 18-week interval from April to August 2021. Liquid chromatography–mass spectrometry analyses revealed the omnipresence of five PPCPs: theophylline, caffeine, cotinine, DEET, and testosterone, although acetaminophen, ibuprofen, and sulfamethoxazole were also common. Of all the PPCPs detected, theophylline, DEET, and acetaminophen concentrations were higher during the wet season in the septic tank-influenced lake, while caffeine, cotinine, and testosterone concentrations decreased. In the lake located in the nature preserve, theophylline, caffeine, and acetaminophen levels increased in the wet season. In contrast, cotinine, DEET, and testosterone levels decreased. Overall, more compounds were detected during the wet season, with highly hydrophobic PPCPs (fluoxetine, atorvastatin, and octocrylene) only present during this period.
1. Introduction
Pharmaceuticals and personal care products (PPCPs) use has risen with a growing and aging population [1]. New PPCPs are developed each year, adding to the already thousands of chemicals that pollute our water bodies, and their toxicity has already led to acute exposure to aquatic organisms [2,3]). PPCPs are ubiquitous, being found in the atmosphere [4], polar ice sheets [5], oceans [6], rivers and lakes [3,7], aquifers [8], and soils [9]. They bioaccumulate in flora [10] and fauna [3]. Their introduction to the environment is mainly through the release of treated water from wastewater treatment plants (WWTPs) and onsite wastewater treatment systems (OWTS), more commonly known as septic tanks. These waters are released into rivers, lakes, and drainfields. Because of the inability of these treatment systems to remove all PPCPs from the effluent, these contaminants can be incorporated into the tissue of organisms that interact with this polluted water [7].
There are 15,000 WTTPs in the USA discharging 1296 m3 of wastewater per day [11]. These facilities release treated water into water bodies through several practices. First is the intentional release into rivers and lakes, where dilution overcomes the potentially high levels of PPCPs in the effluent. Second, reclaimed water produced by tertiary-level processing of wastewater is used for municipal irrigation. Overwatering with reclaimed water, a common practice in residential areas, leads to urban runoff, which enters community stormwater ponds, local streams, and groundwater [12]. Reclaimed water for irrigation is also commonplace in the agricultural setting [13]. Finally, there is unintentional discharge when the WWTPs are overwhelmed by major storm events [14]. These facilities tend to be regional and contribute significant amounts of contaminated water to the environment. At a local level, the drainfields of OWTS are designed to slowly release human waste, including PPCPs, into the surrounding soils. OWTS are the largest contributors of wastewater entering groundwater [15]. Increased precipitation leads to effluent entering the groundwater or overland runoff [16]. The USA has over 21 million OWTS, each discharging 160 to 200 L/day per capita [17]. Assuming a minimum of two individuals per system, OWTS contribute at least 3.36 million m3 of effluent per day.
Human residences equipped with OWTS, located along lake shorelines, can discharge untreated PPCPs and contribute to water pollution. Shallow lakes play an important role in biodiversity as they provide habitats for fish, invertebrates, and waterfowl and support ecosystem services like carbon sequestration, nutrient cycling, and aquaculture [18]. However, their relatively shallow depth makes them highly sensitive to disturbances such as tropical storms, fluctuating water levels, and human activities like agriculture and urbanization, impairing their water quality and availability [19].
This study examines how seasonal changes in precipitation affect the mobilization of PPCPs from OWTS drainfields located upgradient of a residential lake. For comparison, we include a lake located in a nature preserve that has most of the same physical characteristics as the residential lake, albeit with surrounding OWTS. Specifically, we examine how the onset of the wet season in West–Central Florida will influence both the concentrations and assemblages of PPCPs in both lakes. The novelty of our study relates to the following: (1) the two lakes are small enclosed basins with no significant inputs of PPCPs from other sources besides OWTS; (2) there are few studies in karst regions with a similar sampling resolution; (3) the lakes have very similar physical characteristics, which reduces complications of different climate, geology, and soil type; and (4) there is clear climatic delineation between the dry season and the wet season.
2. Conceptual Model of Study
Our conceptual model of PPCP mobilization is illustrated in Figure 1. During the dry season, PPCPs are primarily retained in unsaturated drainfield soils through hygroscopic behavior via absorption or adsorption, thereby limiting their movement into the lake [20] (Figure 1a). With the onset of the wet season, soil saturation increases, flushing out more hydrophilic PPCPs from the drainfields/soils and helping initiate the mobilization of those with more hydrophobic properties (Figure 1b).
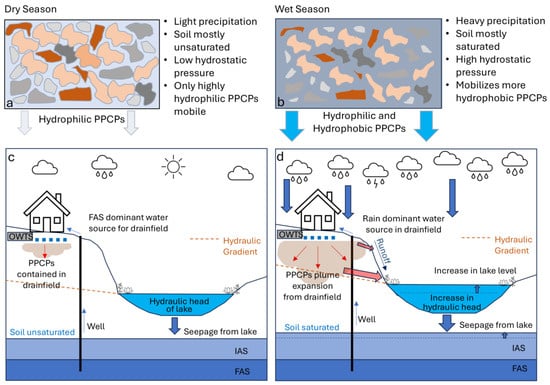
Figure 1.
Conceptual model of PPCP movement in the soil during the dry and wet seasons: (a) hygroscopic water surrounding soil particles is dark blue; (b) due to saturation, all soil pores are filled with water; mobilization of PPCPs from OWTS drainfields into a residential lake in the dry (c) and wet (d) season. The two aquifers identified are the Floridan Aquifer System (FAS) and the Intermediate Aquifer System (IAS).
Transferring this concept to our study area, specifically the residential lake, two different scenarios are evident (Figure 1a,b). For the dry season, little, if any, PPCPs reach the lake. It should be noted that the homes surrounding the lake are supplied by wells that tap the Floridan Aquifer System (FAS). The aquifer is the main source of water for the lake for this season (Figure 1c). With the onset of the wet season, heavy rainfall saturates the drainfield soils, flushing PPCPs out into the lake, overwhelming the contribution of the aquifer water. Increased groundwater flow mobilizes both stored and newly introduced PPCPs, leading to higher concentrations. While the initial precipitation may temporarily reduce PPCP levels through dilution, concentrations rise as mobilized PPCPs enter the lake. In concert, more hydrophobic PPCPs may be flushed from the drainfields and soils. Finally, although the piezometric surface of the Intermediate Aquifer System (IAS), which lies between the lake and FAS, may rise, its contribution to the lake is unlikely due to the lake’s greater hydraulic head as lake volume increases (Figure 1d). Consequently, the underlying aquifer is unlikely to contribute PPCPs or impact lake volume significantly. Previous studies [21,22,23] have noted increased PPCP concentrations in water bodies during periods of elevated rainfall due to the reduced removal efficiency of OWTS and higher mobilization rates.
3. Materials and Methods
3.1. Study Area
This study was conducted in West–Central Florida, in the Alafia River watershed (Figure 2). The residential lake (RL) is internally drained, 120 m wide, with a maximum depth of 5.95 m. It is surrounded by 18 residential homes equipped with OWTS for wastewater treatment and water wells that draw water from the Upper Floridan Aquifer (UFA). The natural lake (NL), which serves as a control site, is located 1800 m northwest of RL. It is 100 m wide and 2.95 m at its deepest point (Figure 2a). The study area’s climate, represented by the nearest town, Riverview, is classified as humid subtropical, characterized by distinct wet and dry seasons. Annual precipitation averages 764 mm in the wet season and 348 mm in the dry season. The mean annual temperature for the region is 22.2 °C.
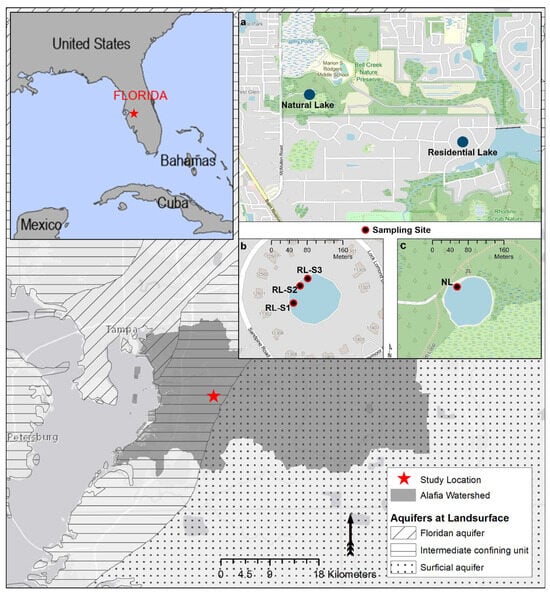
Figure 2.
Study location in Florida, Alafia River watershed, and the major aquifers found at the land surface; (a) residential (27°50′09.7″ N 82°16′46.6″ W) and natural (27°50′28.8″ N 82°17′54.4″ W) lakes; (b) sampling sites RL-S1, RL-S2, and RL-S3 at RL; (c) sampling site at NL.
The UFA represents the upper aquifer of the FAS. FAS is subdivided into three units: UFA, the Middle Confining Unit, and the Lower Floridan Aquifer. In the hydrogeological framework of Florida, the FAS, the IAS, and the Surficial Aquifer are the main aquifers. Each of these aquifer systems is exposed at the land surface in various regions of Florida. Within the study area, the FAS is geologically confined by IAS, while the Surficial Aquifer is present at the surface to the east of the study site [24] (Figure 2).
Our aim was to determine how the onset of the wet season altered the PPCPs present in the lake water. Consequently, starting sampling at the end of the dry season allows us to determine a baseline before the hydraulic regime changes in the area. As such, weekly water samples were collected from 30 April to 30 August 2021 (18 weeks). Within RL, sampling was conducted at three sites: Site 1 (RL-S1), Site 2 (RL-S2), and Site 3 (RL-S3), and at one site in NL (Figure 2b,c). The three sites from RL were selected because of their similarities: (1) distance of their OWTS from the lake, (2) hydraulic gradients, and (3) width of the shoreline vegetation. Additionally, only these three property owners allowed us access to the lake.
3.2. Water Samples
Water samples were collected in the littoral zone of RL and NL at approximately 10 am each sampling day. Sixty-nine (69) water samples were collected, including 51 from the residential and 18 from the natural lakes. Due to the accessibility issues at RL-S2, this site was not sampled on the last three dates (13, 20, 30 August). For each site, 1 L of water was collected at a depth of 35 cm below the lake surface in amber glass bottles after rinsing with the sample water twice before sample collection. The samples were placed in a cooler and transported to the Chemical Purification Analysis and Screening (CPAS) Lab at the University of South Florida (USF) in Tampa. Samples were filtered twice through vacuum-assisted Whatman 0.7 µm glass microfiber fiber (GF/F) filters, stored at 4 °C, and processed within 24 h.
The physiochemical analyses of the water samples were performed right before the water collection. YSI Pro20 Dissolved Oxygen Meter was used to measure dissolved oxygen (DO). pH and temperature were measured with Hanna Instruments H198127 pH-Temperature tester. Total dissolved solids (TDS) and electric conductivity (EC) were measured with Hanna Dissolved Solid Tester (DiST).
3.3. Precipitation Data
Precipitation data for the study were obtained from the Southwest Florida Water Management District [25] using the NexRad extraction tool. This tool provides detailed hourly rainfall data. To facilitate analysis, the hourly rainfall information was aggregated to daily totals, giving a comprehensive view of daily precipitation levels for the study area.
3.4. Chemicals, Reagents, and Materials
Fourteen compounds were selected for this study based on their documented occurrence in aquatic environments near OWTS [26,27,28,29,30,31]. These PPCPs represent a broad spectrum of substances, including antidepressants, nonsteroidal anti-inflammatory drugs, lipid regulators, anticonvulsants, antibiotics, respiratory drugs, nicotine metabolites, preservatives, stimulants, insect repellents, and sunscreen ingredients. Compounds such as octocrylene, atorvastatin, fluoxetine, testosterone, propylparaben, carbamazepine, N,N-diethyl-meta-toluamide (DEET), methylparaben, and cotinine were sourced from Neugen Labs (Tampa, FL). Other compounds, including ibuprofen, sulfamethoxazole, acetaminophen, caffeine, and theophylline, were obtained from Sigma Aldrich (St. Louis, MO, USA). Additionally, theophylline-d6 was procured from Cayman Chemical Company (Ann Arbor, MI, USA). All analytical standards and solvents used in this study were of ≥98% purity. Methanol, acetonitrile, formic acid, and ammonium fluoride were supplied by Thermo Fisher Scientific (Pittsburgh, PA, USA), and Milli-Q water from CPAS was used in the analyses.
Table 1 provides the complete list of target PPCPs, detailing their hydrophobicity, molecular size, formula, CAS number, and classification.

Table 1.
List of target compounds.
3.5. Sample Extraction and Analysis
3.5.1. Sample Extraction
Solid-phase extraction (SPE) of PPCPs from water was conducted using Oasis hydrophilic–lipophilic balance (HLB) cartridges (500 mg) from Waters Corporation (Milford, MA, USA). HLB cartridges were used due to their dual hydrophilic–lipophilic balance, which is advantageous for extracting a broad range of acidic, basic, and neutral chemicals from various matrices. Previous research has demonstrated the efficacy of HLB Oasis in extracting PPCPs from environmental water samples [32]. Elution of PPCPs was performed using methanol, which has proven effective in solid-phase extraction (SPE) in previous studies [33,34,35,36].
HLB cartridges (500 mg) were preconditioned with 6 mL of MeOH followed by 6 mL of H2O at a flow rate of 1 mL per min. A total of 1 L of sample was loaded into the cartridges at a rate of 6 mL/min and dried under vacuum. Subsequently, the analytes were eluted with 8 mL of MeOH and dried under nitrogen. Dried samples were stored in a freezer at −20 °C until the sampling campaign was completed. All samples were analyzed at the same time.
Dried extracts were reconstituted in 0.5 mL of 80:20 H2O:MeOH (v/v) and filtered through 0.45 µm polyvinylidene fluoride (PVDF) filters and transferred to vials. A total of 200 ng/mL of Theophylline-d6 (internal standard) was added to vials to monitor instrument precision. All samples were analyzed using liquid chromatography–tandem mass spectrometry (LC-MS/MS).
3.5.2. Liquid Chromatography–Mass Spectrometry
Fragment ions of each PPCP were optimized via direct infusion of each compound (0.5 mL/min) into the mass spectrometer at a flowrate of 500 µL/min. The two most abundant product ions were selected, except for octocrylene and testosterone, where only one product ion was observed.
The liquid chromatography was performed using Agilent 1260 infinity high-performance liquid chromatography (HPLC) coupled to a 6460 Triple Quadrupole Mass Spectrometer (QqQ MS) with Agilent Jet Stream Technology (AJS) with an electro spray ionization (ESI) source. The presence of contaminants was determined using a Zorbax Eclipse reversed-phase column (100 mm × 2.1 mm, 1.8 um particle size) and tandem mass spectrometry (MS/MS) with the multiple reaction monitoring (MRM) method. The data acquisition was performed in positive (ESI+) and negative (ESI−) ionization polarity modes. The injection volume was 10 μL. The mobile phase flow rate was 200 μL/min, and the column was maintained at 30 °C. In the positive mode, solvent A contained Milli-Q water with 0.1% (v/v) formic acid at 95% and acetonitrile with 0.1% (v/v) formic acid as solvent B at 5%. Solvent B increased to 100% at 12 min and held until 15 min, then returned to 5%. A post-time of 5 min was added to allow the column to re-calibrate before the next analysis, resulting in a total run-time of 21 min.
In the negative mode, solvent A contained Milli-Q water with 1 mM of ammonium fluoride at 40%, and solvent B contained 1 mM of ammonium fluoride with 50:50 (v/v) water: methanol at 60%. Solvent B increased to 100% to 2 min and held until 15 min, then returned to 0%. The injection volume was 10 μL. A post-time of 5 min was added to enable conditioning.
3.5.3. Quantification Analysis
The limit of detection (LOD) and limit of quantification (LOQ) were calculated based on the signal-to-noise ratios of 3 and 10, respectively. According to the International Committee on Harmonization [37],
where δ is the standard deviation of the response, and S is the slope of the calibration curve.
External calibration method using 7 points was used to quantify the concentrations of PPCPs. Details on PPCPs and chromatography information are presented in Supplementary Materials Tables S1–S4.
3.5.4. Quality Assurance and Quality Control
Instrument blanks were used to check for possible contamination. DEET was detected in blank samples at concentrations of 0.075 μg/L. The smallest concentration of DEET in the water samples was 0.5 μg/L. DEET was detected in blank water samples from several other studies [38,39], and there are known challenges with analyses for DEET, including the co-occurrence of chemically similar substances in the environment or in the solvents used for the analysis, which could result in analytical bias [39].
3.6. Statistical Analysis
Pearson correlation analysis was performed using SPSS software (version 29.0) to establish if there is a correlation between PPCP concentrations at the sampling sites in the residential lake, between PPCP concentrations in the residential and natural lake, and between PPCP concentrations and water physicochemical characteristics. The Mann–Whitney U test was used to determine if there are statistically significant differences in PPCP concentrations between the dry and wet seasons.
Three key assumptions were made in the statistical analyses. First, a PPCP was considered present if it was detected in more than 50% of the water samples (≥9 occurrences). For missing data points, the limit of detection (LOD) value was assigned. Second, only data from RL-2 and RL-3 were used to calculate the average RL values, as these sites exhibited strong correlation, allowing for a representative mean (RL-avg). Third, RL-S1 was excluded from this analysis because its concentrations significantly deviated from those of RL-2 and RL-3. Including RL-S1 would have skewed the results, creating a misleading impression of higher PPCP concentrations in the RL.
4. Results
4.1. Onset of Wet Season
The transition from the dry season to the wet season was noticeable. During the last month of the dry season, there was very little precipitation. From the end of May to mid-July 2021, regular rainfall events occurred, with most of them exceeding 10 mm, with a particularly heavy rain during the first week of July. In August, although the frequency of rain events remained similar to that of the initial six weeks of the wet season, the amounts were mostly below 10 mm (Figure 3).
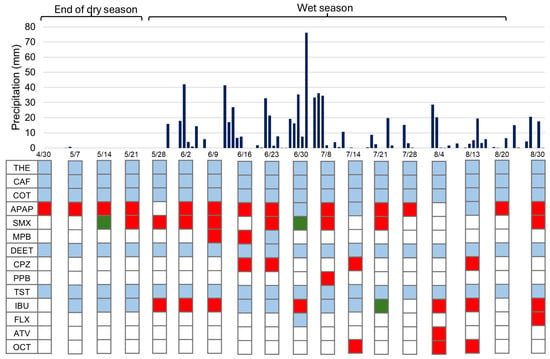
Figure 3.
Sampling campaign dates and precipitation values. Presence of PPCPs in the residential and natural lakes. The representation of PPCPs in water samples is color-coded: blue indicates presence in both lakes (in residential lake—at least one detection at RL-S1, RL-S2, or RL-S3), red represents presence only in RL (detection at RL-S1, RL-S2, or RL-S3), and green indicates presence in the NL. Theophylline (THE), caffeine (CAF), cotinine (COT), acetaminophen (APAP), sulfamethoxazole (SMX), methylparaben (MPB), N,N-Diethyl-meta-toluamide (DEET), carbamazepine (CBZ), propylparaben (PPB), testosterone (TST), ibuprofen (IBU), fluoxetine (FLX), atorvastatin (ATV), and octocrylene (OCT).
4.2. PPCPs in RL and NL
All fourteen target PPCPs were identified at least once throughout the study period (Figure 3). To investigate the impact of the OWTS on lake water quality, we compared PPCP values of the residential lake (RL-avg) with the nature preserve lake (NL). Mean concentrations of cotinine and acetaminophen were higher in the RL-avg than the NL by 5.63 μg/L and 0.26 μg/L, respectively. Conversely, theophylline, caffeine, DEET, and testosterone had higher concentrations in NL compared to RL-avg. Theophylline and caffeine had an average concentration of 24.21 μg/L and 14.82 μg/L at RL-avg and 61.79 μg/L and 95.58 μg/L at NL. The average DEET concentration at RL-avg was 8.61 μg/L, and that at NL was 23.36 μg/L. This high average at NL was mainly caused by two samples with concentrations of 182.5 μg/L (14 May) and 76.9 μg/L (20 August). Similarly, the higher average concentration of testosterone at NL was due to two sampling events, first on 30 April and second on 28 July. The testosterone average was 6.39 μg/L at NL and 1.41 μg/L at RL-avg. Maximum and minimum concentrations and the frequency of occurrences for all identified PPCPs can be found in Table S5.
4.3. Influence of Precipitation on PPCP Concentrations
When depicting the relationship, if any, between PPCP concentrations and precipitation, only those compounds that occurred throughout the entire sampling period were used (Figure 4). Also, the values of RL-avg represent the concentrations of the PPCPs in the residential lake (see Section 3). There is no consistent relationship between any of the PPCP concentrations and the onset of the wet season. Of the six PPCPs, only DEET increases in concentration throughout the wet season. For caffeine and theophylline, the onset of the wet season appears to decrease their presence in RL initially, but their concentrations gradually increase toward the latter half of this interval. Surprisingly, cotinine, testosterone, and acetaminophen concentrations vary throughout the entire sampling period.
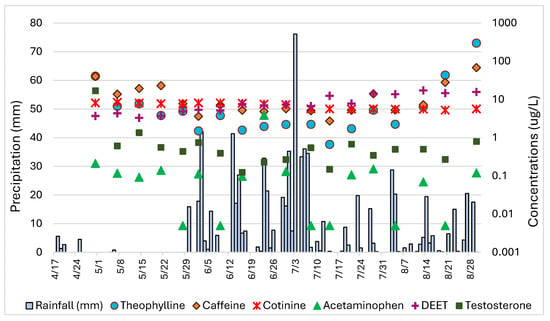
Figure 4.
PPCP concentrations for RL-avg. Rain precipitation for the study area is presented as a bar graph representing daily precipitation in mm on the left Y-axis, and PPCP concentrations are presented on the right Y-axis.
At NL, theophylline, caffeine, and acetaminophen concentrations increased during the wet season, while DEET, cotinine, and testosterone decreased. The only statistically significant difference between the seasons was found for cotinine (Mann–Whitney U = 0.5, z = −2.939, p < 0.001). Fluoxetine was only detected in the wet season. Methylparaben, carbamazepine, propylparaben, octocrylene, and atorvastatin were not detected in NL.
Seasonal variations in the detection of PPCPs across all four sampling sites show that more PPCPs were detected in the wet season when compared to the dry season (Figure 5). For example, the number of detected PPCPs increased from 7 to 13 in RL-S1, from 8 to 10 in RL-S2, and from 7 to 12 in RL-S3. The natural lake also showed an increase, from 7 PPCPs in the dry season to 10 in the wet season.
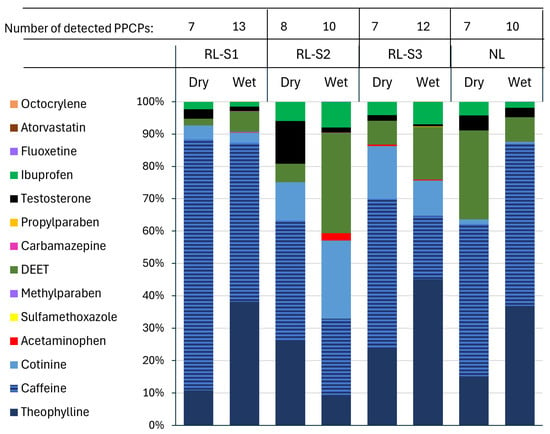
Figure 5.
Dry and wet season variation in number of detected PPCPs.
Caffeine and theophylline dominated the PPCP profiles across all four sites and seasons. Cotinine, acetaminophen, and ibuprofen were also frequently present but in lower concentrations. Compounds such as fluoxetine, testosterone, and methyl- and propylparaben appeared more frequently during the wet season.
RL showed more diverse and abundant PPCP profiles than the natural lake, particularly during the wet season. RL-S1 and RL-S3 wet season samples had the richest PPCP assemblages, including once-off detections of carbamazepine, atorvastatin, and methylparaben, which were absent in their dry season.
4.4. Water Quality
Various water quality measurements were conducted concurrently with the water collection for the PPCPs (Figure 6). This was performed to determine whether changes in the water quality could explain the variability in the concentrations and assemblages of the PPCPs.
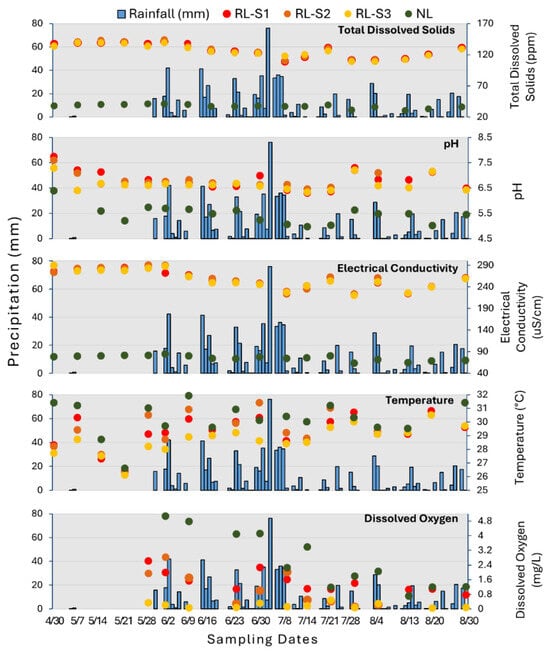
Figure 6.
Water quality parameters and precipitation for the residential lake sites and the natural lake.
RL’s TDS values at each sampling site peaked at 144 ppm, 145 ppm, and 142 ppm just before the wet season onset and then decreased slightly. In contrast, NL maintained stable TDS levels of ~100 ppm. RL exhibited pH ranging from 6.29 to 7.73, and EC varied between 221 μS/cm and 289 μS/cm. Conversely, NL exhibited pH levels ranging from 4.97 μS/cm to 6.38 μS/cm. All the water quality measurements were higher in RL than in NL. The average temperature at RL was similar, 29.3 °C, 29.4 °C, and 28.7 °C at RL-S1, RL-S2, and RL-S3, respectively. The lowest temperature occurred on 21 May. The temperature in NL was higher than in RL. With the lowest temperature of 26.6 °C and the highest of 32.2 °C. In RL, DO levels fluctuated between 0.04 mg/L and 2.82 mg/L, while in the NL, DO ranged from 0.7 mg/L to 5.25 mg/L. Generally, lower levels of DO with slightly lower temperatures were observed at RL.
Lake water physicochemical parameters, including pH, EC, and TDS, were quite homogeneous at all three sites in the lake. A positive correlation was observed between TDS and EC (Table 2). This concurrent change most probably reflects the dilution of the lake water. While there are some statistically significant correlations between the water quality parameters and certain PPCPs, there is little consistency in their relationships. For example, there are positive correlations between testosterone and pH and between DEET and temperature. Conversely, there is a negative correlation between DEET and TDS and DEET and EC. TDS and electrical EC had a positive correlation with cotinine, but neither pH nor DO was correlated with PPCPs.

Table 2.
Pearson correlation between physicochemical characteristics of the lakes and PPCP concentrations.
5. Discussion
5.1. PPCP Concentrations
The concentrations of some of our PPCPs are quite high compared to most studies. Given our unique study area, a small, enclosed lake that receives effluent from 18 OWTS, we found it impossible to find data from comparable settings. However, there are several that have similar concentrations. Ramage et al. [40] sampled the waters of a low-flow stream adjacent to several households with septic tanks. They detected cotinine and caffeine at 31 μg/L and 200 μg/L, respectively. Richards et al. [41] investigated water downstream from two water tanks, one used by two individuals and the other by five, and reported caffeine concentrations ranging from 0.48 to 5 µg/L. Similarly, Tran et al. [42] documented caffeine levels in surface waters reaching up to 14 µg/L. Daneshvar et al. [43] and Diwan et al. [44] detected 60–174 μg/L caffeine levels in Ambazari, Futala, and Gandhi Sagar lakes. Tran et al. [45] investigated concentrations in surface water samples from the sewer-fed catchment. The caffeine concentrations ranged from 14 ng/L to 144.18 μg/L, acetaminophen from below detection (<5.0 ng/L) to 4.82 μg/L, and DEET from 1.4 ng/L to 6.23 μ/L Carbamazepine concentrations ranged from below detection to 53.3 ng/L. DEET with μg/L level concentrations has been measured in waters receiving WWTP effluent. For example, Lee et al. [46] detected levels at 3.7 μg/L in stream water, while concentrations up to 15 μg/L have been found in WWTP effluent [47,48]. Regarding theophylline, only effluents from hospitals (max. 8 μg/L) [49] and PPCP manufacturing plants (max. 33.1 μg/L) [50] have similar values to our maximum concentrations [51].
5.2. PPCP Concentrations in the Dry and Wet Seasons
The results show an increase in average concentrations in the wet season for theophylline, acetaminophen, and DEET, with only the mean for DEET being statistically significant (at significance level 0.05, p < 0.001). Conversely, the average caffeine, cotinine, and testosterone concentrations decreased, with all means being statistically significant (significance level 0.05, p-values: 0.035, 0.008, and 0.018, respectively). Such results are similar to the findings of Sodré et al. [52], who found higher mean concentrations of caffeine in the dry season. As mentioned in the Introduction, we expected that the onset of the wet season would initially dilute PPCP concentrations in the lake, followed by the arrival of the PPCPs mobilized from the septic tank drainfields. This would be accompanied by decreases in both TDS and specific conductance. However, as none of the PPCPs that adhered to this supposition, except DEET, there must be other complicating factors in play.
5.2.1. Complications at the Source
An important assumption that is required to support our initial supposition is that the OWTS would be operating as expected. If the OWTS has not been properly maintained, this may change its operation regarding the removal efficiency of certain PPCPs in the tank and drainfield [53]. The PPCP concentrations in the environment also depend on how fast the compounds are removed in the OWTS. Removal and transformation of PPCPs in OWTS relies on vadose zone processes, sorption, and microbial degradation [54]. Another factor influencing the presence of PPCPs may be the age of the OWTS. Older systems may not process the effluent as well as newer ones. The pipes of the drainfield may have become clogged or crushed over time. Consequently, this diminished performance of the OWTS could complicate the delivery of PPCPs to the lake. Without a detailed examination of the OWTS (tanks and drainfields), it is impossible to tell the state of operation of these systems. However, if they were not properly maintained, then their efficiency of PPCP removal would be reduced, thereby leading to more PPCPs entering the groundwater and, subsequently, the lake.
5.2.2. Contribution of Aquifer Water
Another factor to consider is the contribution of well water to the runoff entering the lake. The study area has aquifer-fed wells as the only option for residential water. This aquifer water would enter the lake via drainfield effluent and irrigation. Aquifers can act as storage reservoirs for contaminants, including PPCPs [29]. Changes in groundwater flow patterns due to fluctuations in precipitation or pumping from wells can remobilize stored contaminants and release them into the lake [55].
To investigate the aquifers’ influence, we measured well water at site one in RL (Table 3). Only those detected at the well are included in the table. First, most of the PPCPs detected in the lake are not present in the aquifer (well) water. This suggests that most of the PPCPs originate from the OWTS. Second, besides DEET’s first sample, all PPCP levels are at least one order of magnitude greater than those from the FAS. Consequently, while there are PPCPs in the well water, the aquifer’s contribution can be considered minimal.

Table 3.
PPCP concentrations of well water at RL-S1 and lake water samples at RL-S1.
Cotinine was detected in the well water only during the first sampling. During this period of the dry season, irrigation water is sourced from the well, which taps the aquifer. If there was a short pulse of water contaminated with cotinine passing through the aquifer at the time of pumping, it would be in both the well water and the residential sampling sites. The levels in the residential sites could be higher due to the evaporation of some of the irrigation water. This may represent a single contamination event in an area upgradient from the wells. Dumping of septic tank effluent in a recharge area for the aquifer could explain a temporary high level of cotinine. Other studies have found similar episodic presence of certain PPCPs in aquifer water [56,57].
As for the comparatively high DEET levels on the first sampling day, there is no clear explanation. Sampling protocols were identical for all water collections. Additionally, the tap was run at full capacity for approximately one minute to flush “old” water from the system before sample collection began. DEET concentrations could also be a result of residential use.
Another possible source of PPCPs from the aquifer is the upward movement of water through the base of the lake from the Intermediate Aquifer System (IAS), which overlies the FAS. However, this source is unlikely, as local well logs (Figure 7) show that the IAS is over five meters below the base of the lake. Additionally, as this is an internally drained sinkhole lake [58], the excess precipitation must be removed from the watershed through seepage. Consequently, it is unlikely that the upward flow of the IAS into the RL is a major source of the PPCPs.
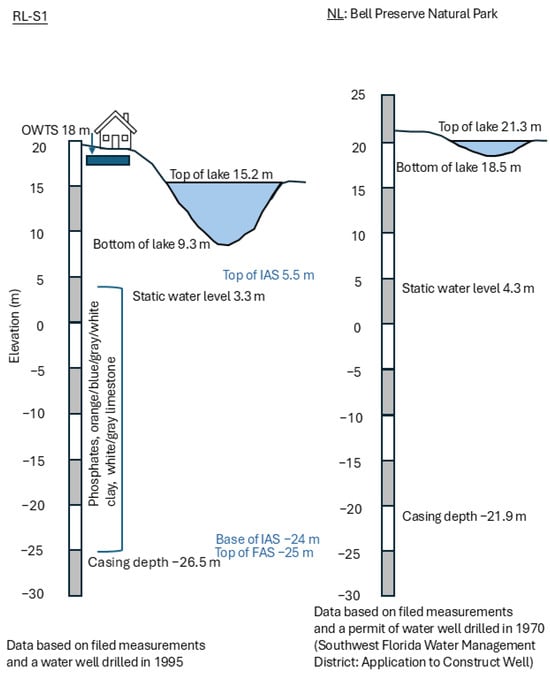
Figure 7.
Hydrogeological profiles for both lakes from well log data.
The high TDS values of the RL may provide an indication of the potential of flocculation to remove PPCPs from the water column. This process may explain the negative correlation between DEET and TDS/EC. Üstün-Odabaşı et al. [59] found a strong negative correlation between total PPCP concentrations and TDS.
The temperature and pH of the lake water impact the degradation of PPCPs. Warmer water temperatures accelerate chemical reactions, which would promote the degradation of PPCPs in both lake water and in soils [60]. Consequently, temporal changes in temperature and pH may impact the presence of PPCPs in the lake. In both RL and NL, neither temperature nor pH has consistent correlations with the concentrations of PPCPs. Such a result fits the conclusions of Du et al. [61], who investigated the role of temperature and pH, amongst others, in the attenuation of PPCPs in lake water.
5.3. PPCP Assemblages in the Dry and Wet Seasons
A second supposition of this study is that the onset of the wet season leads to an increase in more hydrophobic PPCPs in the residential lake. During the dry season, eight PPCPs were detected in the lake water: theophylline, caffeine, cotinine, acetaminophen, sulfamethoxazole, DEET, testosterone, and ibuprofen. In the wet season, along with the above PPCPs, atorvastatin, carbamazepine, methylparaben, propylparaben, fluoxetine, and octocrylene were detected. It is highly likely that the physicochemical properties of these latter PPCPs influenced their presence in the lake water [62]. A common trait of organic contaminants is that they may partition (adsorb) to soil. This accumulation can be assessed by the logarithm of octanol–water partition coefficient (log Kow), which measures the distribution of a chemical between water and an organic solvent (typically octanol). The hydrophobic PPCPs fluoxetine, atorvastatin, and octocrylene are hygroscopic in nature, which favors their absorption or adsorption onto soil particles [20]. During the wet season, these PPCPs with an affinity for soil particles (hydrophobic) are less able to sorb onto these particles due to soil saturation [63].
The PPCPs in the residential lake were classified as being hydrophilic (log Kow < 2.5), moderately hydrophobic (log Kow between 2.5 and 4.0), and highly hydrophobic (log Kow > 4). PPCPs measures that fell within the hydrophilic categories were carbamazepine, DEET, methylparaben, sulfamethoxazole, acetaminophen, cotinine, caffeine, and theophylline, in log Kow order from highest to lowest. These compounds have low sorption potential and would therefore be expected to have high occurrences in water. PPCPs found in the moderately hydrophobic classification are ibuprofen, testosterone, and propylparaben (log Kow order as described above). These PPCPs have medium sorption potential and would be expected to be present in the lake water but also remain in the OWTS drainfields and soils surrounding the lake. The final category, those of high hydrophobic class, are octocrylene, atorvastatin, and fluoxetine (the same order as above). Normally, these PPCPs remain in the soils due to their high sorption potential [64]. Consequently, as their presence in our lake water during only the wet season suggests, their sorption potential was exceeded due to the increase in both the soil saturation and hydrostatic pressure. Dai et al. [63] found that the absorption of more hydrophobic PPCPs was negligible when the soils were saturated compared to unsaturated soils. They reasoned that in unsaturated soils, the greater complexity of the flow path for the PPCPs increases the possibility of contaminant sorption as they travel along the water films surrounding the soil particles. In contrast, saturated soil allows for greater ease of travel for the PPCPs, thereby explaining their presence in the lake during the wet season. This adheres to our conceptual model (Figure 1) that the PPCPs with the highest hydrophobicity are only present when soils are saturated.
5.4. PPCPs in the Residential and the Natural Lakes
We included the NL as a control because it was not surrounded by OWTS but otherwise had similar physical properties. Unfortunately, we could only sample at one location due to the difficulty of access. While we were not surprised to find PPCPs, as they are environmentally ambiguous, the elevated concentrations of those present were unexpected. This result suggests that the NL’s source of contaminants is sourced from residential drainfields. Of the PPCPs present in the NL at certain sampling intervals, those that were at higher concentrations than the RL are also frequently detected in other environments [52,65]. Caffeine and theophylline are highly hydrophilic, explaining their presence in surface waters at high concentrations [66,67]. Caffeine in a lake located in a natural preserve is not unique since it was found in high quantities in the Arctic [68] and remote rivers of the Rocky Mountains National Park [38]. DEET, testosterone, and ibuprofen are also commonly found in aquatic environments [30,69,70]. Most studies have attributed the source of the contaminants to be WTTPs.
The pH values in NL were lower, and dissolved oxygen (DO) values were higher compared to the RL. However, as mentioned above, both lakes, for the most part, had similar trends in the PPCPs’ seasonal variability. Consequently, it can be assumed that the water quality parameters do not have a major impact on the chemical properties of the PPCP concentrations. This supposition agrees with the findings of Edwards (2014) [71], who found that the concentration of caffeine did not show any significant correlation with hydrologic parameters such as surface water temperature, pH, or dissolved oxygen, indicating its stability and slow pace of degradation. Hence, despite clear differences in the water quality parameters between the two lakes, they can be disregarded as significant influences on PPCP dissimilarities.
While the water quality parameters measured above may not explain discrepancies in the PPCP assemblages and concentrations, they may shed light on their provenance. Both pH and conductivity values for the RL are very similar to the aquifer-fed (FAS) lake in the same county, whereas NL is closer to a rain-fed lake, also in the same area (Figure 8). The RL is partially supplied by the FAS via well water, whereas there is no evidence for this source at the NL. An investigation of water levels in a groundwater well on the NL property found that the aquifer is 17.6 m below the base of the lake. Consequently, source waters are quite different for each lake, thereby suggesting that the FAS could not be the source of the PPCPs in NL. The contribution of FAS to the RL should not be overstated. The wet season RL lake volume is ~19,000 m3, and for the 123-day period of observation, ~7100 m3 of precipitation occurred over the lake. Consequently, precipitation is a significant contributor to seasonal changes in lake volume.
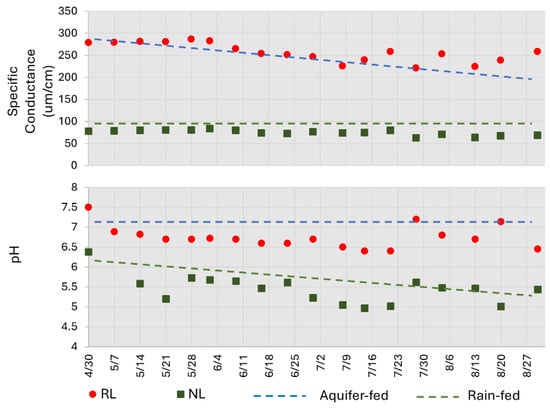
Figure 8.
Comparison of natural/residential lakes with aquifer-augmented and rainfed lakes from NW Hillsborough County.
The most likely explanation for the PPCPs in NL is the residential area south of the preserve. The small residential area is approximately 200 m from the lake. These homes use OWTS and are at a higher elevation than the lake itself. This differential creates a shallow hydraulic gradient sloping toward the lake. Consequently, the same process occurs as in RL. The high permeability of the Chandler soil unit, which covers the entire study area [72], would help promote the somewhat free movement of groundwater advection. If OWTS are indeed the primary source of PPCPs for NL, then the concentration of these contaminants should mirror one another. As shown in the results, the trends for most PPCPs are very similar for both lakes. Of course, it is unlikely that both residential homes use the same PPCPs, which would explain the differences between the lakes.
The higher average DEET concentration in NL may provide additional evidence on how the specific location of the two study sites may influence the concentrations. The Bell Creek Preserve has stagnant marshes south and east of the lake. This environment could be a source of a greater abundance of mosquitoes, which would call for a greater application of DEET by park visitors compared to RL, which lacks a similar marsh.
5.5. Implications for Management of Shallow Lakes
Given the results of this study, we can suggest some potential solutions to decreasing the concentrations of PPCPs in these shallow lakes. The first pertains to improving the effectiveness of PPCP removal in the residential OWTS, although these measures may be cost-prohibitive at such a small scale. These technologies include advanced oxidation processes, membrane filtration, and activated carbon adsorption [73]. The simplest option, which is currently being implemented in some areas, is to connect residential areas to the municipal sewer system. However, for neighborhoods that are more isolated, one possible option could be directing these homes to decentralized, small-scale treatment plants that utilize some of the above technologies. It is beyond the scope of this paper to calculate the costs of the alternative approaches.
From a policy/regulation perspective, there are a number of approaches that can be considered, not necessarily being adopted exclusively from one another. Monitoring lake water quality regarding PPCPs is essential to determine the presence of elevated levels and potential sources of such contaminants [74]. Increasing public awareness is a cost-effective approach, which involves both informing the residents of proper disposal of PPCPs and how their actions impact their local environment [75,76]. Next, developing site-specific risk assessments helps identify PPCPs of concern and whether they pose any ecological risks to lake organisms [77]. Finally, a more holistic approach is adopting the practice of Integrated Watershed Management [73], which incorporates various aspects such as land use, sewage infrastructure, and catchment hydrology.
6. Conclusions
This pilot study investigated how precipitation, with the onset of the rainy season, influences the concentrations and assemblages of 14 PPCPs in two lakes, the first surrounded by OWTS and the second located in a nature preserve. Our conceptual model of the influence of the wet season on PPCP concentrations in the RL was not supported by the statistical analyses. Sampling soil water may allow for a more definitive explanation as to why. This sampling would shed light on the spatial and temporal transition of the contaminants between the OWTS and the lake.
In addition to the first hypothesis, we posited that more hydrophobic PPCPs would appear in the lake due to greater soil saturation as precipitation increased. We found that the assemblage of PPCPs did change, as those with more hydrophobic properties were detected only in the wet season. The saturation of the soil overcomes the propensity of these PPCPs to adhere to soil particles, thereby allowing the release into the lake.
The contribution of variable water sources influencing the PPCP concentrations in RL was not initially considered important. However, as the residential homes are supplied by well water from the FAS, which is included for irrigation in the dry season, aquifer water is an important component of the study. The RL does share similarities with other aquifer-fed lakes. However, well water collected from a residential well showed that PPCP concentrations were very small compared to the lake water. Consequently, the OWTS surrounding the lake are the main source of PPCPs in the RL. The OWTS are also the main contributor of PPCPs in the NL, despite being located away from a residential area.
There were several limitations in the study. Only two lakes were analyzed, and including more in future research would allow for more definitive conclusions. Second, the entire dry season was not sampled. However, our interest was the onset of the wet season, not the full hydraulic year. Finally, more sites could have been sampled at both lakes. While three sites seemed reasonable for the RL as well as sampling at the same depth and time of day, it appears that the influence of the total depth of the water column may have been more significant than we first envisioned. Consequently, several more sites at both lakes should be included for the next phase of this study. However, this was the first study on shallow lakes in a karst setting and provides new foci for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12070219/s1.
Author Contributions
Conceptualization, P.v.B. and E.B.-J.; methodology, E.B.-J., L.C., and P.v.B.; software, E.B.-J. and L.C.; validation, E.B.-J., P.v.B., and L.C.; formal Analysis, E.B.-J. and L.C.; investigation, P.v.B. and E.B.-J.; resources, P.v.B., L.C., and E.B.-J.; data curation, E.B.-J.; writing—original draft preparation, E.B.-J. and P.v.B.; writing—review and editing, E.B.-J. and P.v.B.; visualization, E.B.-J. and P.v.B.; supervision, P.v.B.; project administration, P.v.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank the homeowners for granting access to the residential lake and Rae and Jenna for helping with sample collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, J.; O’Brien, J.; Du, P.; Li, X.; Ort, C.; Mueller, J.F.; Thai, P.K. Measuring Selected PPCPs in Wastewater to Estimate the Population in Different Cities in China. Sci. Total Environ. 2016, 568, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Wang, Y.; Hamid, N.; Deng, S.; Li, W. Prioritizing Selected PPCPs on the Basis of Environmental and Toxicogenetic Concerns: A Toxicity Estimation to Confirmation Approach. J. Hazard. Mater. 2019, 380, 120828. [Google Scholar] [CrossRef]
- Brausch, J.M.; Connors, K.; Brooks, B.W.; Rand, G.M. Human Pharmaceuticals in the Aquatic Environment: A Review of Recent Toxicological Studies and Considerations for Toxicity Testing. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: Boston, MA, USA, 2012; pp. 1–99. [Google Scholar]
- Duong, H.T.; Kadokami, K.; Nguyen, D.T.; Trinh, H.T.; Doan, N.H.; Mizukawa, H.; Takahashi, S. Occurrence, Potential Sources, and Risk Assessment of Pharmaceuticals and Personal Care Products in Atmospheric Particulate Matter in Hanoi, Vietnam. Environ. Sci. Pollut. Res. 2023, 30, 34814–34826. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, P.; Wu, Z.; Zhang, S.; Wei, L.; Mi, L.; Kuester, A.; Gandrass, J.; Ebinghaus, R.; Yang, R.; et al. Legacy and Emerging Organic Contaminants in the Polar Regions. Sci. Total Environ. 2022, 835, 155376. [Google Scholar] [CrossRef]
- Arpin-Pont, L.; Martínez-Bueno, M.J.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the Marine Environment: A Review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the Environment: Plant Uptake, Translocation, Bioaccumulation, and Human Health Risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Silori, R.; Shrivastava, V.; Singh, A.; Sharma, P.; Aouad, M.; Mahlknecht, J.; Kumar, M. Global Groundwater Vulnerability for Pharmaceutical and Personal Care Products (PPCPs): The Scenario of Second Decade of 21st Century. J. Environ. Manag. 2022, 320, 115703. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Chang, A.C. Degradation and Adsorption of Selected Pharmaceuticals and Personal Care Products (PPCPs) in Agricultural Soils. Chemosphere 2009, 77, 1299–1305. [Google Scholar] [CrossRef]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative Uptake and Translocation of Pharmaceutical and Personal Care Products (PPCPs) by Common Vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef]
- Center for Sustainable Systems. U.S. Wastewater Treatment Factsheet; Pub. No. CSS04-14; Center for Sustainable Systems: Ann Arbor, MI, USA, 2024. [Google Scholar]
- Karnjanapiboonwong, A.; Suski, J.G.; Shah, A.A.; Cai, Q.; Morse, A.N.; Anderson, T.A. Occurrence of PPCPs at a Wastewater Treatment Plant and in Soil and Groundwater at a Land Application Site. Water. Air. Soil Pollut. 2011, 216, 257–273. [Google Scholar] [CrossRef]
- Boxall, A.B.A. Fate and Transport of Veterinary Medicines in the Soil Environment. In Fate of Pharmaceuticals in the Environment and in Water Treatment Systems; Aga, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 123–137. [Google Scholar]
- Aga, D.S.; Samara, F.; Dronjak, L.; Kanan, S.; Mortula, M.; Vahapoglu, L. Rising Water, Rising Risks: The Hidden Dangers of Emerging Contaminants in Climate-Intensified Storms. ACS ES&T Water 2024, 4, 2785–2788. [Google Scholar]
- Perkins, R. Septic Tanks, Lot Size and Pollution of Water Table Aquifers. J. Environ. Health 1984, 46, 298–304. [Google Scholar]
- Gao, Q.; Blum, K.M.; Gago-Ferrero, P.; Wiberg, K.; Ahrens, L.; Andersson, P.L. Impact of On-Site Wastewater Infiltration Systems on Organic Contaminants in Groundwater and Recipient Waters. Sci. Total Environ. 2019, 651, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Design Manual: Onsite Wastewater Treatment and Disposal Systems; U.S. Environmental Protection Agency: Washington, DC, USA, 1980.
- Meerhoff, M.; Beklioğlu, M. Shallow Lakes and Ponds. In Limnology; Academic Press: Cambridge, MA, USA, 2024; pp. 859–892. [Google Scholar]
- Beklioğlu, M.; Meerhoff, M.; Davidson, T.A.; Ger, K.A.; Havens, K.; Moss, B. Preface: Shallow Lakes in a Fast Changing World: The 8th International Shallow Lakes Conference. Hydrobiologia 2016, 778, 9–11. [Google Scholar] [CrossRef]
- Ng, A.; Weerakoon, D.; Lim, E.; Padhye, L.P. Fate of Environmental Pollutants. Water Environ. Res. 2019, 91, 1294–1325. [Google Scholar] [CrossRef]
- Ma, R.; Wang, B.; Yin, L.; Zhang, Y.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Characterization of Pharmaceutically Active Compounds in Beijing, China: Occurrence Pattern, Spatiotemporal Distribution and Its Environmental Implication. J. Hazard. Mater. 2017, 323, 147–155. [Google Scholar] [CrossRef]
- Corada-Fernández, C.; Candela, L.; Torres-Fuentes, N.; Pintado-Herrera, M.G.; Paniw, M.; González-Mazo, E. Effects of Extreme Rainfall Events on the Distribution of Selected Emerging Contaminants in Surface and Groundwater: The Guadalete River Basin (SW, Spain). Sci. Total Environ. 2017, 605–606, 770–783. [Google Scholar] [CrossRef]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Wu, D.; Yu, G.; Barcelo, D. Rainfall Influences Occurrence of Pharmaceutical and Personal Care Products in Landfill Leachates: Evidence from Seasonal Variations and Extreme Rainfall Episodes. Environ. Sci. Technol. 2021, 55, 4822–4830. [Google Scholar] [CrossRef]
- Upchurch, S.; Scott, T.M.; Alfieri, M.C. Hydrogeology of Florida. In The Karst Systems of Florida: Understanding Karst in Geologically Young Terrain; LaMoreaux, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 93–145. [Google Scholar]
- SWFWMD NexRad RADAR Rainfall Estimates. Available online: https://edis.ifas.ufl.edu/publication/AE517 (accessed on 2 February 2022).
- Yang, Y.Y.; Toor, G.S.; Wilson, P.C.; Williams, C.F. Micropollutants in Groundwater from Septic Systems: Transformations, Transport Mechanisms, and Human Health Risk Assessment. Water Res. 2017, 123, 258–267. [Google Scholar] [CrossRef]
- Schaider, L.A.; Ackerman, J.M.; Rudel, R.A. Septic Systems as Sources of Organic Wastewater Compounds in Domestic Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ. 2016, 547, 470–481. [Google Scholar] [CrossRef]
- Conn, K.E.; Siegrist, R.L.; Barber, L.B.; Meyer, M.T. Fate of Trace Organic Compounds during Vadose Zone Soil Treatment in an Onsite Wastewater System. Environ. Toxicol. Chem. 2010, 29, 285–293. [Google Scholar] [CrossRef]
- Katz, B.G.; Griffin, D.W.; McMahon, P.B.; Harden, H.S.; Wade, E.; Hicks, R.W.; Chanton, J.P. Fate of Effluent-Borne Contaminants beneath Septic Tank Drainfields Overlying a Karst Aquifer. J. Environ. Qual. 2010, 39, 1181. [Google Scholar] [CrossRef]
- Del Rosario, K.L.; Mitra, S.; Humphrey, C.P.; O’Driscoll, M.A. Detection of Pharmaceuticals and Other Personal Care Products in Groundwater beneath and Adjacent to Onsite Wastewater Treatment Systems in a Coastal Plain Shallow Aquifer. Sci. Total Environ. 2014, 487, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.R.; Weick, R.J.; Johnson, J.M.; Cahill, J.D.; Smith, S.G.; Rich, B.J. Organic Wastewater Compounds, Pharmaceuticals, and Coliphage in Ground Water Receiving Discharge from Onsite Wastewater Treatment Systems near La Pine, Oregon: Occurrence and Implications for Transport; Usgs Sir 2005-5055; U.S. Geological Survey: Reston, VA, USA, 2005; 98p.
- Gilart, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. Determination of Pharmaceuticals in Wastewaters Using Solid-Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. J. Sep. Sci. 2012, 35, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of 90 Emerging Contaminants in Liquid and Solid Environmental Matrices by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Althakafy, J.T.; Kulsing, C.; Grace, M.R.; Marriott, P.J. Liquid Chromatography—Quadrupole Orbitrap Mass Spectrometry Method for Selected Pharmaceuticals in Water Samples. J. Chromatogr. A 2017, 1515, 164–171. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The Fate of Pharmaceuticals and Personal Care Products (PPCPs), Endocrine Disrupting Contaminants (EDCs), Metabolites and Illicit Drugs in a WWTW and Environmental Waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of Pharmaceutical Residues, Personal Care Products, Lifestyle Chemicals, Illicit Drugs and Metabolites in Wastewater and Receiving Surface Waters of Krakow Agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Validation of Analytical Procedures; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2024.
- Battaglin, W.A.; Bradley, P.M.; Iwanowicz, L.; Journey, C.A.; Walsh, H.L.; Blazer, V.S. Pharmaceuticals, Hormones, Pesticides, and Other Bioactive Contaminants in Water, Sediment, and Tissue from Rocky Mountain National Park, 2012–2013. Sci. Total Environ. 2018, 643, 651–673. [Google Scholar] [CrossRef]
- Anumol, T.; Merel, S.; Clarke, B.O.; Snyder, S.A. Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry for Rapid Analysis of Trace Organic Contaminants in Water. Chem. Cent. J. 2013, 7, 104. [Google Scholar] [CrossRef]
- Ramage, S.; Camacho-Muñoz, D.; Petrie, B. Enantioselective LC-MS/MS for Anthropogenic Markers of Septic Tank Discharge. Chemosphere 2019, 219, 191–201. [Google Scholar] [CrossRef]
- Richards, S.; Paterson, E.; Withers, P.J.A.; Stutter, M. Septic Tank Discharges as Multi-Pollutant Hotspots in Catchments. Sci. Total Environ. 2016, 542, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hu, J.; Ong, S.L. Simultaneous Determination of PPCPs, EDCs, and Artificial Sweeteners in Environmental Water Samples Using a Single-Step SPE Coupled with HPLC-MS/MS and Isotope Dilution. Talanta 2013, 113, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, A.; Aboulfadl, K.; Viglino, L.; Broséus, R.; Sauvé, S.; Madoux-humery, A.; Weyhenmeyer, G.A.; Prévost, M. Chemosphere Evaluating Pharmaceuticals and Caffeine as Indicators of Fecal Contamination in Drinking Water Sources of the Greater Montreal Region. Chemosphere 2012, 88, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Lundborg, C.; Tamhankar, A.J. Seasonal and Temporal Variation in Release of Antibiotics in Hospital Wastewater: Estimation Using Continuous and Grab Sampling. PLoS ONE 2013, 8, e68715. [Google Scholar] [CrossRef]
- Tran, N.H.; Li, J.; Hu, J.; Ong, S.L. Occurrence and Suitability of Pharmaceuticals and Personal Care Products as Molecular Markers for Raw Wastewater Contamination in Surface Water and Groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4727–4740. [Google Scholar] [CrossRef]
- Lee, C.J.; Mau, D.P.; Rasmussen, T.J. Effects of Nonpoint and Selected Point Contaminant Sources on Stream-Water Quality and Relation to Land Use in Johnson County, Northeastern Kansas, October 2002 Through June 2004; U.S. Geological Survey: Reston, VA, USA, 2005.
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A National Reconnaissance for Pharmaceuticals and Other Organic Wastewater Contaminants in the United States—II) Untreated Drinking Water Sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef]
- Phillips, P.J.; Schubert, C.; Argue, D.; Fisher, I.; Furlong, E.T.; Foreman, W.; Gray, J.; Chalmers, A. Concentrations of Hormones, Pharmaceuticals and Other Micropollutants in Groundwater Affected by Septic Systems in New England and New York. Sci. Total Environ. 2015, 512–513, 43–54. [Google Scholar] [CrossRef]
- Azuma, T.; Arima, N.; Tsukada, A.; Hirami, S.; Matsuoka, R.; Moriwake, R.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; et al. Detection of Pharmaceuticals and Phytochemicals Together with Their Metabolites in Hospital Effluents in Japan, and Their Contribution to Sewage Treatment Plant Influents. Sci. Total Environ. 2016, 548–549, 189–197. [Google Scholar] [CrossRef]
- Kleywegt, S.; Payne, M.; Ng, F.; Fletcher, T. Environmental Loadings of Active Pharmaceutical Ingredients from Manufacturing Facilities in Canada. Sci. Total Environ. 2019, 646, 257–264. [Google Scholar] [CrossRef]
- Wilschnack, M.; Cartmell, E.; Yates, K.; Petrie, B. Septic Tanks as a Pathway for Emerging Contaminants to the Aquatic Environment–Need for Alternative Rural Wastewater Treatment? Environ. Pollut. 2024, 362, 124988. [Google Scholar] [CrossRef] [PubMed]
- Sodré, F.F.; Santana, J.S.; Sampaio, T.R.; Brandão, C.C.S. Seasonal and Spatial Distribution of Caffeine, Atrazine, Atenolol and Deet in Surface and Drinking Waters from the Brazilian Federal District. J. Braz. Chem. Soc. 2018, 29, 1854–1865. [Google Scholar] [CrossRef]
- Schaider, L.A.; Rodgers, K.M.; Rudel, R.A. Review of Organic Wastewater Compound Concentrations and Removal in Onsite Wastewater Treatment Systems. Environ. Sci. Technol. 2017, 51, 7304–7317. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Toor, G.S.; Wilson, P.C.; Williams, C.F. Septic Systems as Hot-Spots of Pollutants in the Environment: Fate and Mass Balance of Micropollutants in Septic Drainfields. Sci. Total Environ. 2016, 566–567, 1535–1544. [Google Scholar] [CrossRef]
- McEachran, A.D.; Shea, D.; Nichols, E.G. Pharmaceuticals in a Temperate Forest-Water Reuse System. Sci. Total Environ. 2017, 581–582, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hrkal, Z.; Pastuszek, F. Behaviour of PPCP Substances in a Fluvial Aquifer after Infiltration of Treated Wastewater. Appl. Sci. 2023, 13, 9348. [Google Scholar] [CrossRef]
- Dodgen, L.K.; Kelly, W.R.; Panno, S.V.; Taylor, S.J.; Armstrong, D.L.; Wiles, K.N.; Zhang, Y.; Zheng, W. Characterizing Pharmaceutical, Personal Care Product, and Hormone Contamination in a Karst Aquifer of Southwestern Illinois, USA, Using Water Quality and Stream Flow Parameters. Sci. Total Environ. 2017, 578, 281–289. [Google Scholar] [CrossRef]
- Haber, J.D.; Mayfield, G.; Loper, J.E. Sinkhole Formation at Lake Grady, Florida. In Karst Studies in West Central Florida: USF Seminar in Karst Environments; The University of South Florida and the Southwest Florida Water Management District: Tampa, FL, USA, 2003; pp. 53–64. [Google Scholar]
- Üstün-Odabaşı, S.; Maryam, B.; Özdemir, N.; Büyükgüngör, H. Occurrence and Seasonal Variations of Pharmaceuticals and Personal Care Products in Drinking Water and Wastewater Treatment Plants in Samsun, Turkey. Environ. Earth Sci. 2020, 79, 311. [Google Scholar] [CrossRef]
- Bertin, S.; Yates, K.; Petrie, B. Enantiospecific Behaviour of Chiral Drugs in Soil. Environ. Pollut. 2020, 262, 114364. [Google Scholar] [CrossRef]
- Du, R.; Duan, L.; Zhang, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Analysis on the Attenuation Characteristics of PPCPs in Surface Water and Their Influencing Factors Based on a Compilation of Literature Data. Water Res. 2023, 242, 120203. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303. [Google Scholar] [CrossRef]
- Dai, Y.; Zhuang, J.; Chen, X. Synergistic Effects of Unsaturated Flow and Soil Organic Matter on Retention and Transport of PPCPs in Soils. Environ. Res. 2020, 191, 110135. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Xu, N.; Pan, B.; Ni, J. Pharmaceuticals and Personal Care Products (PPCPs) in Water, Sediment and Freshwater Mollusks of the Dongting Lake Downstream the Three Gorges Dam. Chemosphere 2022, 301, 134721. [Google Scholar] [CrossRef]
- Katsikaros, A.G.; Chrysikopoulos, C.V. Occurrence and Distribution of Pharmaceuticals and Personal Care Products (PPCPs) Detected in Lakes around the World—A Review. Environ. Adv. 2021, 6, 100131. [Google Scholar] [CrossRef]
- Gonzalez-Rey, M.; Tapie, N.; Le Menach, K.; Dévier, M.H.; Budzinski, H.; Bebianno, M.J. Occurrence of Pharmaceutical Compounds and Pesticides in Aquatic Systems. Mar. Pollut. Bull. 2015, 96, 384–400. [Google Scholar] [CrossRef]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barceló, D. Pharmaceuticals, Pesticides, Personal Care Products and Microplastics Contamination Assessment of Al-Hassa Irrigation Network (Saudi Arabia) and Its Shallow Lakes. Sci. Total Environ. 2020, 701, 135021. [Google Scholar] [CrossRef] [PubMed]
- Kallenborn, R.; Brorström-Lundén, E.; Reiersen, L.O.; Wilson, S. Pharmaceuticals and Personal Care Products (PPCPs) in Arctic Environments: Indicator Contaminants for Assessing Local and Remote Anthropogenic Sources in a Pristine Ecosystem in Change. Environ. Sci. Pollut. Res. 2018, 25, 33001–33013. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; Gan, J.; Anderson, M.A. Degradation and Sorption of Commonly Detected PPCPs in Wetland Sediments under Aerobic and Anaerobic Conditions. J. Soils Sediments 2012, 12, 1164–1173. [Google Scholar] [CrossRef]
- Ng, B.; Quinete, N.; Maldonado, S.; Lugo, K.; Purrinos, J.; Briceño, H.; Gardinali, P. Understanding the Occurrence and Distribution of Emerging Pollutants and Endocrine Disruptors in Sensitive Coastal South Florida Ecosystems. Sci. Total Environ. 2021, 757, 143720. [Google Scholar] [CrossRef]
- Edwards, Q.A.; Kulikov, S.M.; Garner-O’Neale, L.D. Caffeine in Surface and Wastewaters in Barbados, West Indies. Springerplus 2015, 4, 57. [Google Scholar] [CrossRef]
- USDA. Soil Survey of Hillsborough County, Florida; USDA: Washington, DC, USA, 1989.
- Wang, J.; Wang, S. Removal of Pharmaceuticals and Personal Care Products ( PPCPs ) from Wastewater: A Review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.Y.C.; Peake, B.M.; Braund, R. Disposal Practices for Unused Medications around the World. Environ. Int. 2011, 37, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. Pharmaceuticals and Personal Care Products (PPCPs): What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Bagnis, S.; Fitzsimons, M.F.; Snape, J.; Tappin, A.; Comber, S. Processes of Distribution of Pharmaceuticals in Surface Freshwaters: Implications for Risk Assessment. Environ. Chem. Lett. 2018, 16, 1193–1216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).