Unveiling Heavy Metal Distribution in Different Agricultural Soils and Associated Health Risks Among Farming Communities of Bangladesh
Abstract
1. Introduction
2. Materials and Methods
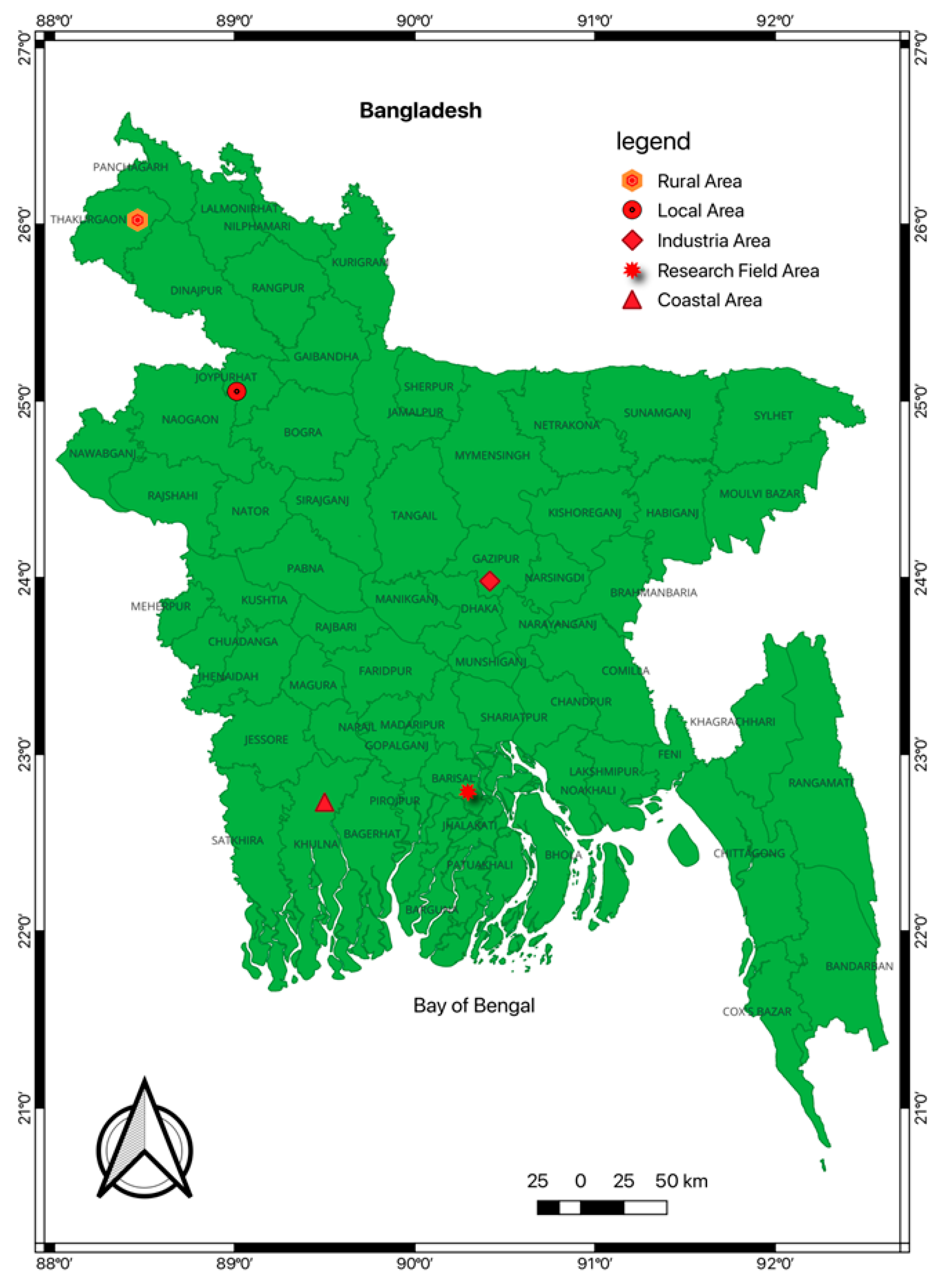
2.1. Sampling Sites
2.2. Preparation of Soil Sample
2.3. Pretreatment of the Soil Sample
2.4. Determination of Heavy Metals
2.5. Pollution Load Index (PLI)
2.6. Health Risk Evaluation
2.6.1. Estimated Daily Intake (EDI)
| Parameters | Value for Children | Value for Adults | References |
|---|---|---|---|
| Heavy metal (mg/kg) | HM | HM | This study |
| Exposure Duration (y) | 6 | 30 | [21] |
| Exposure Frequency (d/y) | 180 | 180 | |
| Average Time (Cancer) (d) | 27,740 | 27,740 | |
| Average Time (Non-cancer) (d) | 2190 | 10,950 | |
| Average Body Weight (kg) | 16.2 | 61.8 | [22] |
| Average Lifetime (y) | 76 | 76 | |
| Particle Emission Factor (m3/g) | 1.36 × 106 | 1.36 × 106 | [19] |
| Ingestion Rate (g/d) | 0.2 | 0.1 | |
| Inhalation Rate (m3/d) | 7.6 | 20 | [23] |
2.6.2. Cancer Risks Evaluation
2.7. Quality Control
2.8. Statistical Analysis
3. Results and Discussion
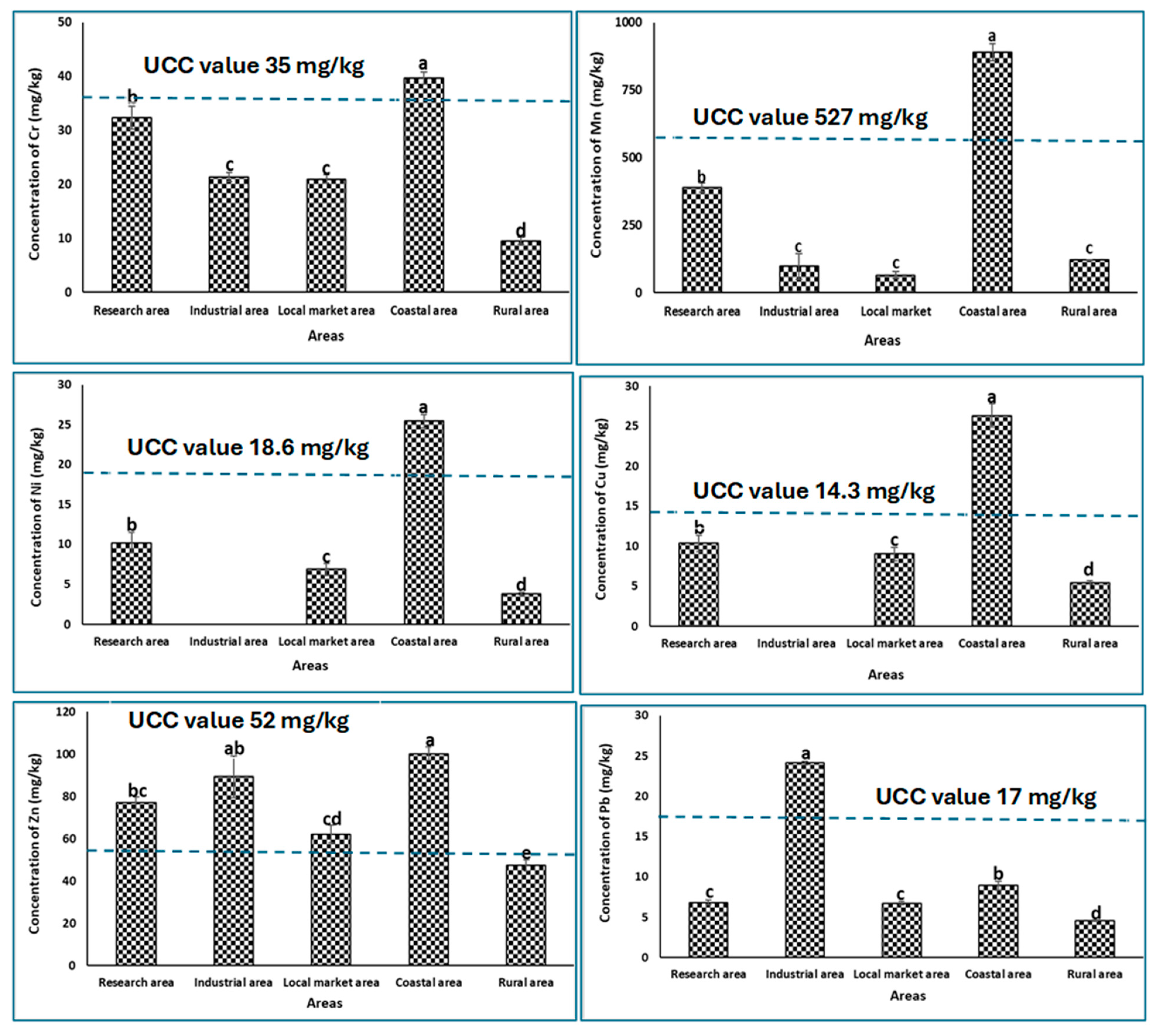
3.1. Heavy Metal Concentration in Different Agricultural Soils
| City, Country | Concentration (mg/kg) | Types of Soils | References | |||||
|---|---|---|---|---|---|---|---|---|
| Mn | Ni | Cu | Zn | Pb | Cr | |||
| North Dakota, USA | 1191 | 22.1 | 12.4 | 66.4 | 9.5 | 20.4 | Rural agricultural soil | [32] |
| Khyber-Pakhtunkhwa, Pakistan | 399 | 30.7 | 16.0 | 39.1 | 15.8 | 30.6 | City agricultural soil | [30] |
| Ahvas Metropolis, Iran | 561.8 | 109.3 | 23.8 | 56.6 | 8.3 | 67.3 | City area soil | [31] |
| Sinú River Basin, Colombia | - | 587 | 1004 | 1218 | 0.1 | - | Rural agricultural soil | [39] |
| Peloponnese, Greece | 1020 | 146.8 | 74.7 | 74.9 | 19.7 | 83.1 | Residential area | [33] |
| Dhaka, Bangladesh | 106–577 | 25–112 | 28–217 | 53–477 | 17–99 | - | Industrial soil | [40] |
| Dhaka, Bangladesh | - | 36.03–74.16 | 31.35–45.16 | 103.20–123.49 | 44.31–52.21 | 33.89–67.58 | Industrial soil | [41] |
| Bagerhat, Bangladesh | 426.36 | 26.91 | 23.75 | 48.58 | 51.11 | 27.63 | Coastal soil | [34] |
| Tangail, Bangladesh | - | 0.71–18.39 | 1.02–34.44, | - | 2.01–28.86 | 0.96–14.04 | Industrial vicinity soil | [42] |
| Kurigram, Bangladesh | - | - | - | - | 26.7 | 34.7 | Rural agricultural soil | [43] |
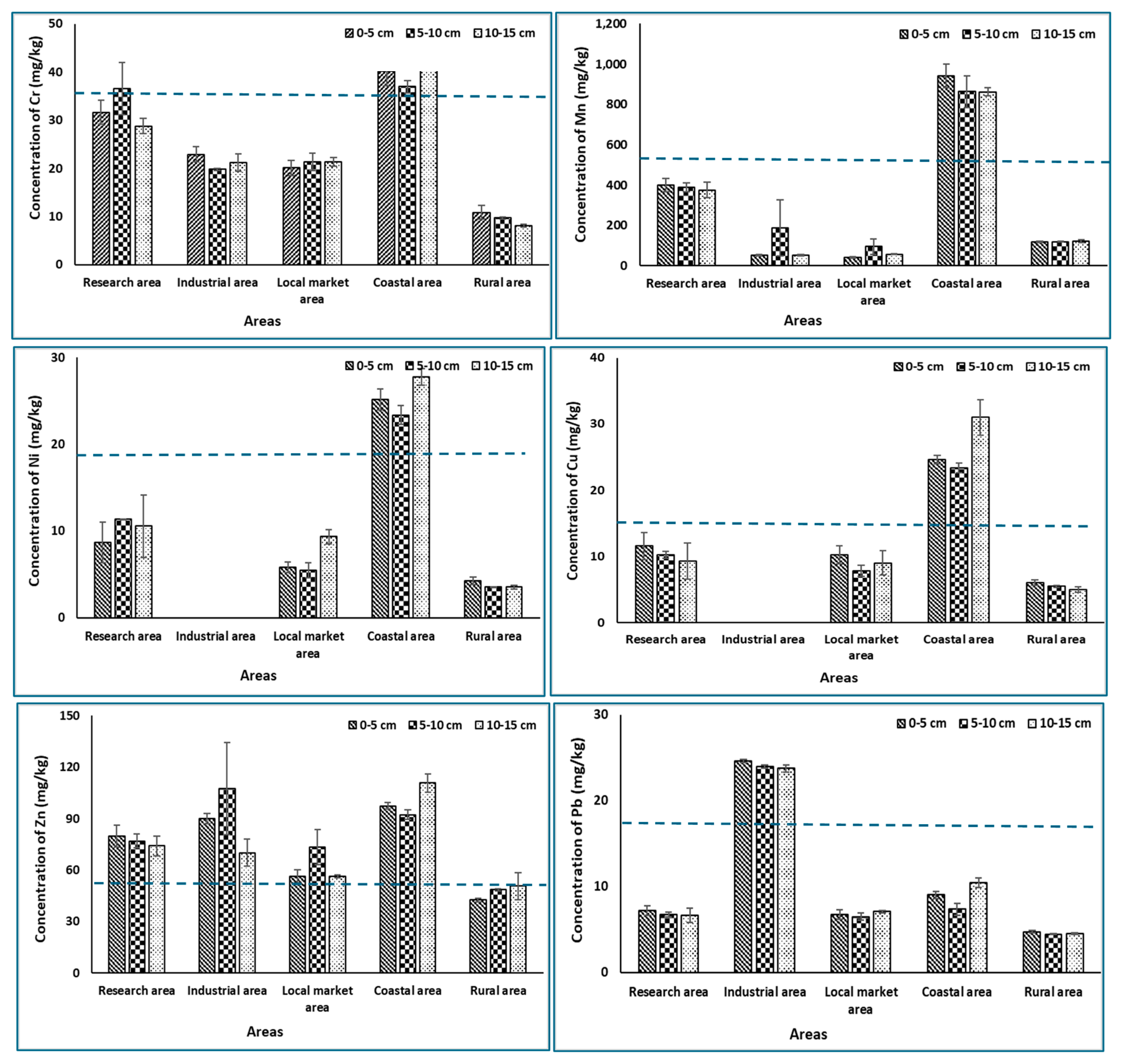
3.2. Measurement of Heavy Metal Concentration of Different Soil Depths in Different Agricultural Soils
3.3. Pearson Correlation Analysis Between Heavy Metal Concentrations
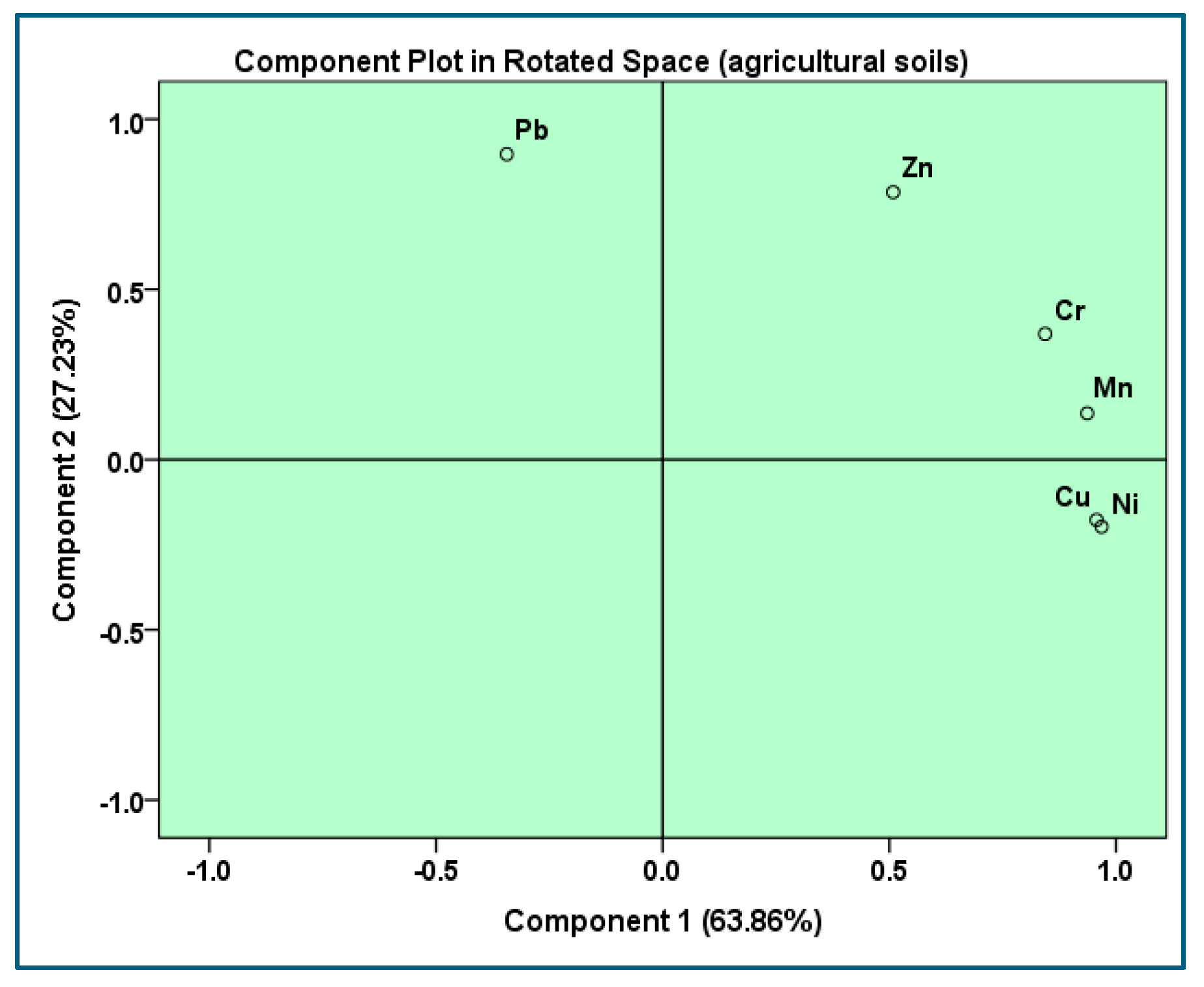
3.4. Principal Component Analysis (PCA)
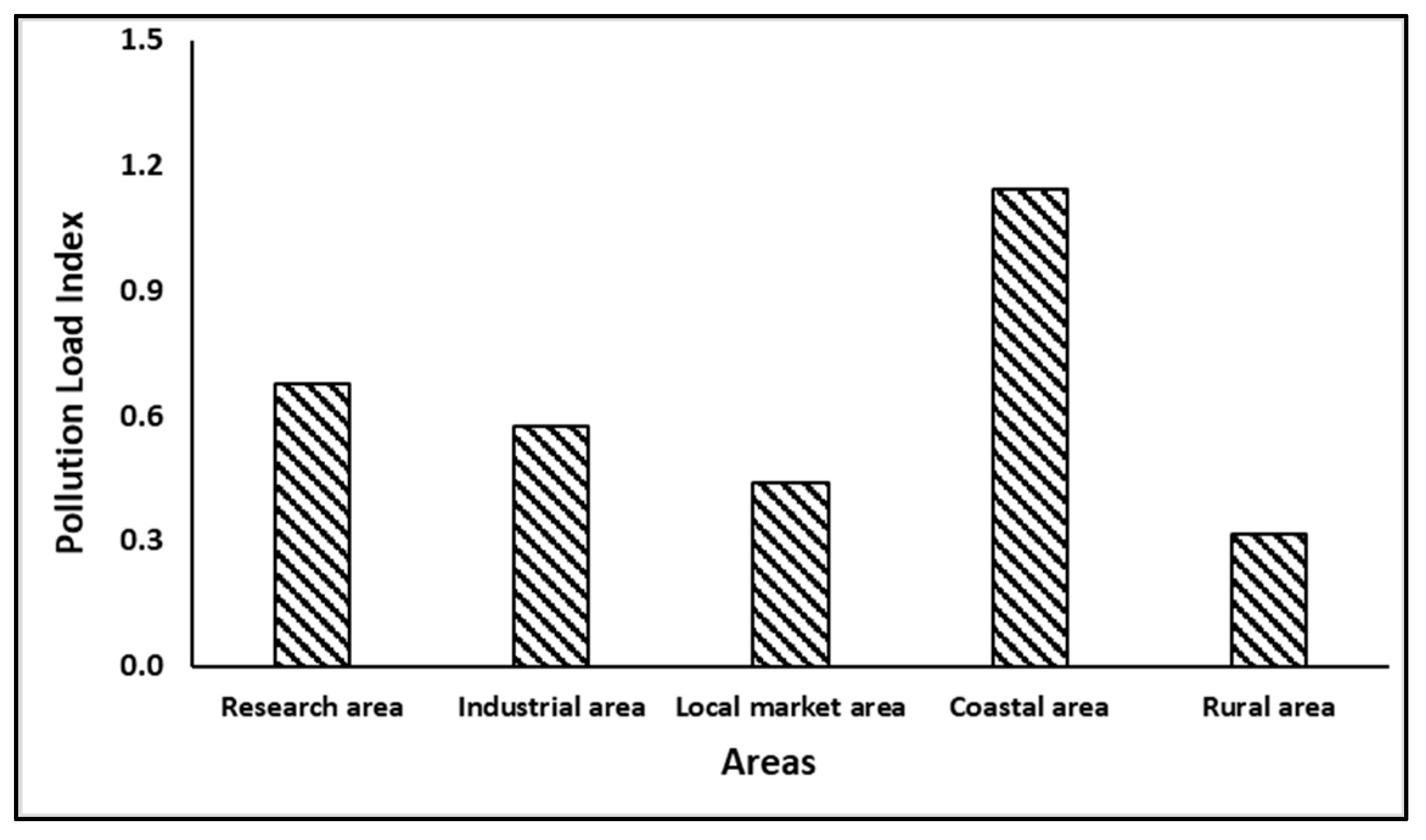
3.5. Pollution Loading Index
3.6. Average Daily Intake of Different Agricultural Soils
3.7. LADD of Heavy Metals in Different Agricultural Soils
3.8. Cumulative Cancer Risk
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The Status of Food Security and Nutrition in the World. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/1c38676f-f5f7-47cf-81b3-f4c9794eba8a/content (accessed on 21 December 2024).
- Parvez, M.S.; Czédli, H.; Hoque, M.I.; Rahman, M.M.; Anwar, A.; Uddin, A.H.M.M.; Simon, E. Accumulation of microplastics and potentially toxic elements in plant leaves along an urbanization gradient in Bangladesh. Toxics 2024, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Zhang, Z.; Zhao, S.; Chen, L.; Duan, C.; Zhang, X.; Fang, L. A review of microplastics in soil: Occurrence, analytical methods, combined contamination and risks. Environ. Pollut. 2022, 306, 119374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Chen, L.; Cui, Q.; Cui, Y.; Song, D.; Fang, L. Review on migration, transformation and ecological impacts of microplastics in soil. Appl. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar]
- Orellana-Mendoza, E.; Camel, V.; Yallico, L.; Quispe-Coquil, V.; Cosme, R. Effect of fertilization on the accumulation and health risk for heavy metals in native Andean potatoes in the highlands of Perú. Toxicol. Rep. 2024, 12, 594–606. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Islam, M.M.; Karim, M.R.; Zheng, X.; Li, X. Heavy metal and metalloid pollution of soil, water and foods in Bangladesh: A critical review. Int. J. Environ. Res. Public Health 2018, 15, 2825. [Google Scholar] [CrossRef]
- Rahman, S.H.; Khanam, D.; Adyel, T.M.; Islam, M.S.; Ahsan, M.A.; Akbor, M.A. Assessment of heavy metal contamination of agricultural soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: Implication of seasonal variation and indices. Appl. Sci. 2012, 2, 584–601. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Acharya, S. Heavy Metal Contamination in Food: Sources, Impact, and Remedy. In Food Safety and Quality in the Global South; Springer Nature: Singapore, 2024; pp. 233–261. [Google Scholar]
- Sharmin, S.; Wang, Q.; Islam, M.R.; Wang, W.; Enyoh, C.E. Microplastic Contamination of Non-Mulched Agricultural Soils in Bangladesh: Detection, Characterization, Source Apportionment and Probabilistic Health Risk Assessment. J. Xenobiotics 2024, 14, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, V.; Yadav, S.; Yadav, A.; Garg, S.; Poria, P.; Farooqi, F.; Dumée, L.F.; Sharma, R.S. Heavy metal pollution and risks in a highly polluted and populated Indian river–city pair using the systems approach. Environ. Sci. Pollut. Res. 2022, 29, 60212–60231. [Google Scholar] [CrossRef]
- USEPA. Methods for Collection, Storage and Manipulation of Sediment for Chemical and Toxicological Analyses: Technical Manual; US Environmental Protection Agency: Washington, DC, USA, 2001.
- Enyoh, C.E.; Qingyue, W.; Verla, A.W.; Chowdhury, T. Index models for ecological and health risks assessment of environmental micro-and nano-sized plastics. AIMS Environ. Sci. 2022, 9, 51–65. [Google Scholar] [CrossRef]
- Zheng, N.; Liu, J.; Wang, Q.; Liang, Z. Heavy metals exposure of children from stairway and sidewalk dust in the smelting district. Northeast of China. Atmos. Environ. 2010, 44, 3239–3245. [Google Scholar] [CrossRef]
- China Statistics Bureau. China Statistical Yearbook; National Bureau of Statistics of China: Beijing, China, 2012; p. 62791819.
- Van den Berg, R. Human Exposure to Soil Contamination: A Qualitative and Quantitative Analysis towards Proposals for Human Toxicology C-Standard Values; Report No. 7252010; National Institute of Public Health and Environmental Protection: Bilthoven, The Netherlands, 1995. [Google Scholar]
- Ferreira-Baptista, L.; De Miguel, E.J. Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmos. Environ. 2005, 39, 4501–4512. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Carcinogen Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2005.
- Yang, S.; Zhao, J.; Chang, S.X.; Collins, C.; Xu, J.; Liu, X. Status assessment and probabilistic health risk modeling of metals accumulation in agriculture soils across China: A synthesis. Environ. Int. 2019, 128, 165–174. [Google Scholar] [CrossRef]
- Gomes, M.S. Determinação de Elementos Metálicos em Sedimentos Antárticos. Master Thesis, Universidade de São Paulo, São Paulo, Brazil, 1999. [Google Scholar]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia: 1. Peninsular Malaysia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Pongsakul, P.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia: 2. Thailand. Environ. Geochem. Health 2004, 26, 359–371. [Google Scholar] [CrossRef]
- Shah, S.H.A.; Sajjad, R.U.; Javed, A.; Habib, U.; Ahmad, F.; Mohamed, A. Geotechnical investigation and stabilization of soils through limestone powder at Abbottabad, Khyber-Pakhtunkhwa, Pakistan: A cost effective and sustainable approach. Front. Earth Sci. 2023, 11, 1243975. [Google Scholar] [CrossRef]
- Bahrami, S.; Moore, F.; Keshavarzi, B. Evaluation, source apportionment and health risk assessment of heavy metal and polycyclic aromatic hydrocarbons in soil and vegetable of Ahvaz metropolis. Hum. Ecol. Risk Assess. 2019, 27, 71–100. [Google Scholar] [CrossRef]
- Saleem, M.; Pierce, D.; Wang, Y.; Sens, D.A.; Somji, S.; Garrett, S.H. Heavy Metal(oid)s Contamination and Potential Ecological Risk Assessment in Agricultural Soils. J. Xenobiotics 2024, 14, 634–650. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of Heavy Metals in Agricultural Soils of Mediterranean: Insights from Argolida Basin, Peloponnese, Greece. Geoderma 2014, 221–222, 82–90. [Google Scholar] [CrossRef]
- Parvez, M.S.; Nawshin, S.; Sultana, S.; Hossain, M.S.; Rashid Khan, M.H.; Habib, M.A.; Nijhum, Z.T.; Khan, R. Evaluation of heavy metal contamination in soil samples around Rampal, Bangladesh. ACS Omega 2023, 8, 15990–15999. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chen, X.; Zheng, W.; Zhang, G.; Dong, A. Heavy Metals in the Sediments of Dongshan Bay, China: Sources, Distribution, and Controlling Factors. Water 2024, 16, 905. [Google Scholar] [CrossRef]
- Budai, P.; Clement, A. Spatial distribution patterns of four traffic-emitted heavy metals in urban road dust and the resuspension of brake-emitted particles: Findings of a field study. Transp. Res. Part D Transp. Environ. 2018, 62, 179–185. [Google Scholar] [CrossRef]
- Merlin, O.H.; Salvador, G.L.; Vitturi, L.M.; Pistolato, M.; Rampazzo, G. Geochemical characteristics of western Ross Sea (Antarctica) sediments. Mar. Geol. 1991, 99, 209–229. [Google Scholar] [CrossRef]
- Santos, I.R.; Silva-Filho, E.V.; Schaefer, C.E.; Albuquerque-Filho, M.R.; Campos, L.S. Heavy metal contamination in coastal sediments and soils near the Brazilian Antarctic Station, King George Island. Mar. Pollut. Bull. 2005, 50, 185–194. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Heavy metal contamination of soil and vegetation in the vicinity of industries in Bangladesh. Water Air Soil Pollut. 1999, 115, 347–361. [Google Scholar] [CrossRef]
- Ahmad, J.U.; Goni, M.A. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ. Monit. Assess. 2010, 166, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.R.; Proshad, R.; Islam, M.S.; Sayeed, A.; Uddin, M.; Gao, J.; Zhang, D. Heavy metals toxicity of surface soils near industrial vicinity: A study on soil contamination in Bangladesh. Arch. Agric. Environ. Sci. 2019, 4, 356–368. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ziku, A.L.M.E.; Choudhury, T.R.; Ahmed, J.U.; Mottaleb, M.A. Heavy metal contaminations in vegetables, soils and river water: A comprehensive study of Chilmari, Kurigram, Bangladesh. Int. J. Environ. Ecol. Fam. Urban Stud. 2015, 5, 29–42. [Google Scholar]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Islam, M.R.; Wang, Q.; Guo, Y.; Wang, W.; Sharmin, S.; Enyoh, C.E. Physico-chemical characterization of food wastes for potential soil application. Processes 2023, 11, 250. [Google Scholar] [CrossRef]
- Diganta, M.T.M.; Sharmi, T.T.; Saifullah, A.S.M.; Uddin, M.J.; Sajib, A.M. Appraisal of heavy metal contamination in road dust and human health risk in a Municipality of Bangladesh. Environ. Eng. Manag. J. 2020, 19, 2165–2177. [Google Scholar]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Rana, M.S.; Wang, Q.; Wang, W.; Enyoh, C.E.; Islam, M.R.; Isobe, Y.; Kabir, M.H. Sources, distribution, and health implications of heavy metals in street dust across industrial, capital city, and peri-urban areas of Bangladesh. Atmosphere 2024, 15, 1088. [Google Scholar] [CrossRef]
| Cr | Mn | Ni | Cu | Zn | Pb | |
|---|---|---|---|---|---|---|
| Research area | ||||||
| Cr | 1 | |||||
| Mn | 0.309 | 1 | ||||
| Ni | 0.417 | 0.769 * | 1 | |||
| Cu | 0.477 | 0.768 * | 0.794 * | 1 | ||
| Zn | 0.399 | 0.839 ** | 0.672 * | 0.839 ** | 1 | |
| Pb | 0.306 | 0.949 ** | 0.795 * | 0.885 ** | 0.883 ** | 1 |
| Industrial area | ||||||
| Cr | 1 | |||||
| Mn | −0.138 | 1 | ||||
| Ni | 0.650 | 0.137 | 1 | |||
| Cu | 0.559 | −0.276 | 0.095 | 1 | ||
| Zn | −0.052 | 0.024 | −0.201 | 0.106 | 1 | |
| Pb | 0.462 | −0.212 | 0.404 | 0.614 | 0.459 | 1 |
| Local market area | ||||||
| Cr | 1 | |||||
| Mn | 0.678 * | 1 | ||||
| Ni | 0.216 | −0.109 | 1 | |||
| Cu | 0.029 | −0.013 | 0.317 | 1 | ||
| Zn | 0.635 | 0.944 ** | −0.186 | −0.074 | 1 | |
| Pb | 0.638 | 0.246 | 0.545 | −0.034 | 0.317 | 1 |
| Coastal area | ||||||
| Cr | 1 | |||||
| Mn | −0.071 | 1 | ||||
| Ni | 0.387 | 0.422 | 1 | |||
| Cu | 0.495 | 0.070 | 0.849 ** | 1 | ||
| Zn | 0.555 | 0.131 | 0.787 * | 0.721 * | 1 | |
| Pb | 0.583 | 0.412 | 0.959 ** | 0.847 ** | 0.848 ** | 1 |
| Rural area | ||||||
| Cr | 1 | |||||
| Mn | 0.177 | 1 | ||||
| Ni | 0.368 | 0.095 | 1 | |||
| Cu | 0.216 | −0.547 | 0.271 | 1 | ||
| Zn | −0.275 | 0.747 * | −0.254 | −0.252 | 1 | |
| Pb | −0.074 | −0.079 | 0.411 | 0.694 * | 0.169 | 1 |
| Areas | Cr | Mn | Ni | Cu | Zn | Pb |
|---|---|---|---|---|---|---|
| Ingestion | ||||||
| Research area | 2.58 × 10−11 | 3.10 × 10−10 | 8.13 × 10−12 | 8.26 × 10−12 | 6.14 × 10−11 | 5.47 × 10−12 |
| Industrial area | 1.70 × 10−11 | 7.72 × 10−11 | 4.16 × 10−12 | 2.22 × 10−12 | 7.12 × 10−11 | 1.92 × 10−11 |
| Local market area | 1.67 × 10−11 | 5.11 × 10−11 | 5.48 × 10−12 | 7.19 × 10−12 | 4.96 × 10−11 | 5.39 × 10−12 |
| Coastal area | 3.16 × 10−11 | 7.10 × 10−10 | 2.03 × 10−11 | 2.10 × 10−11 | 8.00 × 10−11 | 7.13 × 10−12 |
| Rural area | 7.62 × 10−12 | 9.59 × 10−11 | 3.01 × 10−12 | 4.36 × 10−12 | 3.77 × 10−11 | 3.64 × 10−12 |
| Inhalation | ||||||
| Research area | 3.80 × 10−9 | 4.56 × 10−8 | 1.20 × 10−9 | 1.21 × 10−9 | 9.03 × 10−9 | 8.04 × 10−10 |
| Industrial area | 2.50 × 10−9 | 1.14 × 10−8 | 6.11 × 10−10 | 3.26 × 10−10 | 1.05 × 10−8 | 2.83 × 10−9 |
| Local market area | 2.46 × 10−9 | 7.51 × 10−9 | 8.06 × 10−10 | 1.06 × 10−9 | 7.29 × 10−9 | 7.93 × 10−10 |
| Coastal area | 4.65 × 10−9 | 1.04 × 10−7 | 2.98 × 10−9 | 3.09 × 10−9 | 1.18 × 10−8 | 1.05 × 10−9 |
| Rural area | 1.12 × 10−9 | 1.41 × 10−8 | 4.43 × 10−10 | 6.41 × 10−10 | 5.55 × 10−9 | 5.36 × 10−10 |
| Dermal | ||||||
| Research area | 1.03 × 10−9 | 1.24 × 10−8 | 3.24 × 10−10 | 3.30 × 10−10 | 2.45 × 10−9 | 2.18 × 10−10 |
| Industrial area | 6.79 × 10−10 | 3.08 × 10−9 | 1.66 × 10−10 | 8.84 × 10−11 | 2.84 × 10−9 | 7.68 × 10−10 |
| Local market area | 6.66 × 10−10 | 2.04 × 10−9 | 2.19 × 10−10 | 2.87 × 10−10 | 1.98 × 10−9 | 2.15 × 10−10 |
| Coastal area | 1.26 × 10−9 | 2.83 × 10−8 | 8.10 × 10−10 | 8.38 × 10−10 | 3.19 × 10−9 | 2.84 × 10−10 |
| Rural area | 3.04 × 10−10 | 3.82 × 10−9 | 1.20 × 10−10 | 1.74 × 10−10 | 1.51 × 10−9 | 1.45 × 10−10 |
| Areas | Cr | Mn | Ni | Cu | Zn | Pb |
|---|---|---|---|---|---|---|
| Ingestion | ||||||
| Research area | 6.52 × 10−11 | 7.82 × 10−10 | 2.05 × 10−11 | 2.09 × 10−11 | 1.55 × 10−10 | 1.38 × 10−11 |
| Industrial area | 4.3 × 10−11 | 1.95 × 10−10 | 1.1 × 10−11 | 5.6 × 10−12 | 1.8 × 10−10 | 4.86 × 10−11 |
| Local market area | 4.22 × 10−11 | 1.29 × 10−10 | 1.38 × 10−11 | 1.82 × 10−11 | 1.25 × 10−10 | 1.36 × 10−11 |
| Coastal area | 7.99 × 10−11 | 1.79 × 10−9 | 5.13 × 10−11 | 5.3 × 10−11 | 2.02 × 10−10 | 1.8 × 10−11 |
| Rural area | 1.92 × 10−11 | 2.42 × 10−10 | 7.6 × 10−12 | 1.1 × 10−11 | 9.53 × 10−11 | 9.2 × 10−12 |
| Inhalation | ||||||
| Research area | 4.9 × 10−9 | 5.88 × 10−8 | 1.54 × 10−9 | 1.57 × 10−9 | 1.17 × 10−8 | 1.04 × 10−9 |
| Industrial area | 3.23 × 10−9 | 1.46 × 10−8 | 7.9 × 10−10 | 4.2 × 10−10 | 1.35 × 10−8 | 3.65 × 10−9 |
| Local market area | 3.17 × 10−9 | 9.69 × 10−9 | 1.04 × 10−9 | 1.36 × 10−9 | 9.4 × 10−9 | 1.02 × 10−9 |
| Coastal area | 6 × 10−9 | 1.35 × 10−7 | 3.85 × 10−9 | 3.98 × 10−9 | 1.52 × 10−8 | 1.35 × 10−9 |
| Rural area | 1.45 × 10−9 | 1.82 × 10−8 | 5.71 × 10−10 | 8.27 × 10−10 | 7.16 × 10−9 | 6.91 × 10−10 |
| Dermal | ||||||
| Research area | 1.82 × 10−9 | 2.18 × 10−8 | 5.72 × 10−10 | 5.81 × 10−10 | 4.32 × 10−9 | 3.85 × 10−10 |
| Industrial area | 1.2 × 10−9 | 5.43 × 10−9 | 2.9 × 10−10 | 1.6 × 10−10 | 5.01 × 10−9 | 1.35 × 10−9 |
| Local market area | 1.17 × 10−9 | 3.59 × 10−9 | 3.86 × 10−10 | 5.06 × 10−10 | 3.49 × 10−9 | 3.79 × 10−10 |
| Coastal area | 2.23 × 10−9 | 4.99 × 10−8 | 1.43 × 10−9 | 1.48 × 10−9 | 5.62 × 10−9 | 5.01 × 10−10 |
| Rural area | 5.36 × 10−10 | 6.74 × 10−9 | 2.12 × 10−10 | 3.07 × 10−10 | 2.66 × 10−9 | 2.56 × 10−10 |
| Area | Cr | Zn | Pb | Ni | Cumulative CR | |
|---|---|---|---|---|---|---|
| Ingestion | Inhalation | Ingestion | Ingestion | Inhalation | ||
| Research area | 5.54 × 10−13 | 2.06 × 10−6 | 1.32 × 10−12 | 8.42 × 10−11 | 1.29 × 10−9 | 2.06 × 10−6 |
| Industrial area | 3.66 × 10−13 | 1.36 × 10−6 | 1.53 × 10−12 | 2.96 × 10−10 | 6.64 × 10−10 | 1.36 × 10−6 |
| Local market area | 3.59 × 10−13 | 1.33 × 10−6 | 1.06 × 10−12 | 8.30 × 10−11 | 8.74 × 10−10 | 1.33 × 10−6 |
| Coastal area | 6.79 × 10−13 | 2.52 × 10−6 | 1.72 × 10−12 | 1.10 × 10−10 | 3.23 × 10−9 | 2.52 × 10−6 |
| Rural area | 1.63 × 10−13 | 6.09 × 10−7 | 8.10 × 10−13 | 5.61 × 10−11 | 4.80 × 10−10 | 6.10 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmin, S.; Wang, Q.; Islam, M.R.; Isobe, Y.; Enyoh, C.E.; Shangrong, W. Unveiling Heavy Metal Distribution in Different Agricultural Soils and Associated Health Risks Among Farming Communities of Bangladesh. Environments 2025, 12, 198. https://doi.org/10.3390/environments12060198
Sharmin S, Wang Q, Islam MR, Isobe Y, Enyoh CE, Shangrong W. Unveiling Heavy Metal Distribution in Different Agricultural Soils and Associated Health Risks Among Farming Communities of Bangladesh. Environments. 2025; 12(6):198. https://doi.org/10.3390/environments12060198
Chicago/Turabian StyleSharmin, Sumaya, Qingyue Wang, Md. Rezwanul Islam, Yogo Isobe, Christian Ebere Enyoh, and Wu Shangrong. 2025. "Unveiling Heavy Metal Distribution in Different Agricultural Soils and Associated Health Risks Among Farming Communities of Bangladesh" Environments 12, no. 6: 198. https://doi.org/10.3390/environments12060198
APA StyleSharmin, S., Wang, Q., Islam, M. R., Isobe, Y., Enyoh, C. E., & Shangrong, W. (2025). Unveiling Heavy Metal Distribution in Different Agricultural Soils and Associated Health Risks Among Farming Communities of Bangladesh. Environments, 12(6), 198. https://doi.org/10.3390/environments12060198








