Mineral Condition Changes in Amended Soils and Woody Vegetation Installed on a Polluted Soil with Trace Metals in Lubumbashi (DR Congo): Results of a Four-Year Trial
Abstract
1. Introduction
2. Materials and Methods
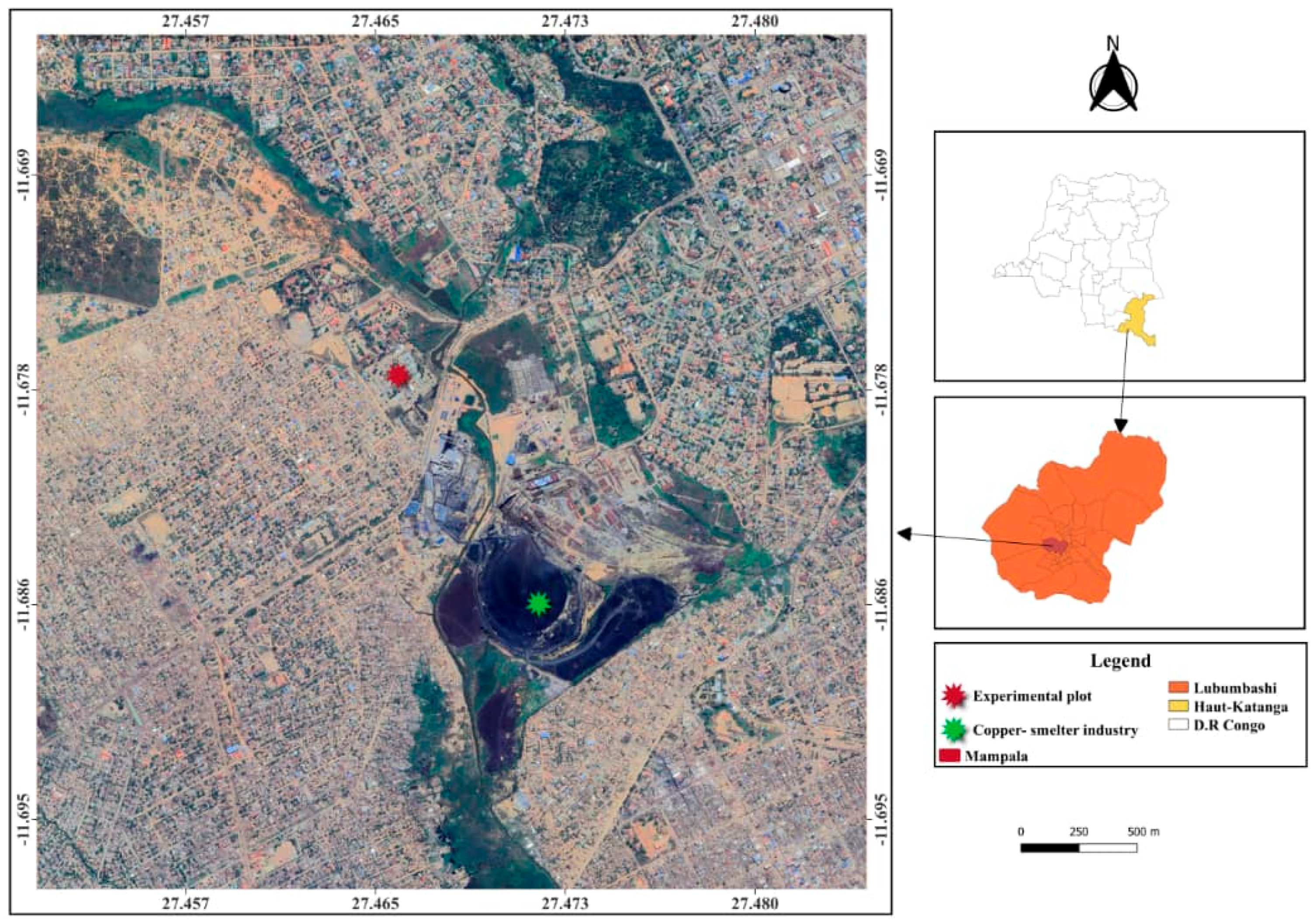
2.1. Study Site
2.2. Plant Material
2.3. Experimental Plot
2.4. Plantation Monitoring and Species Inventory
2.5. Chemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Dynamics of Mineral Composition in Soils over Four Years
3.2. Vegetation Dynamics Between 2019 and 2024
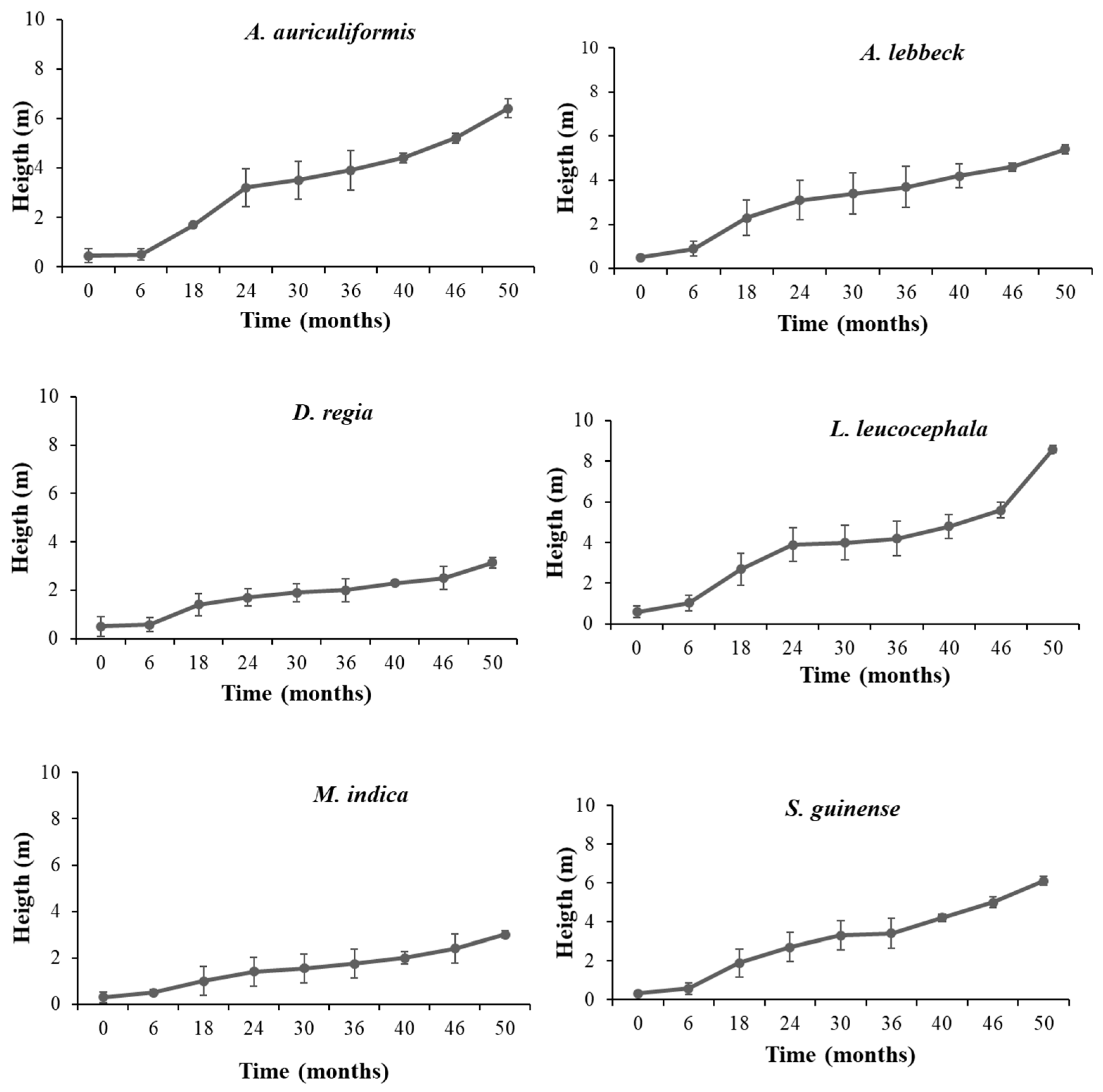
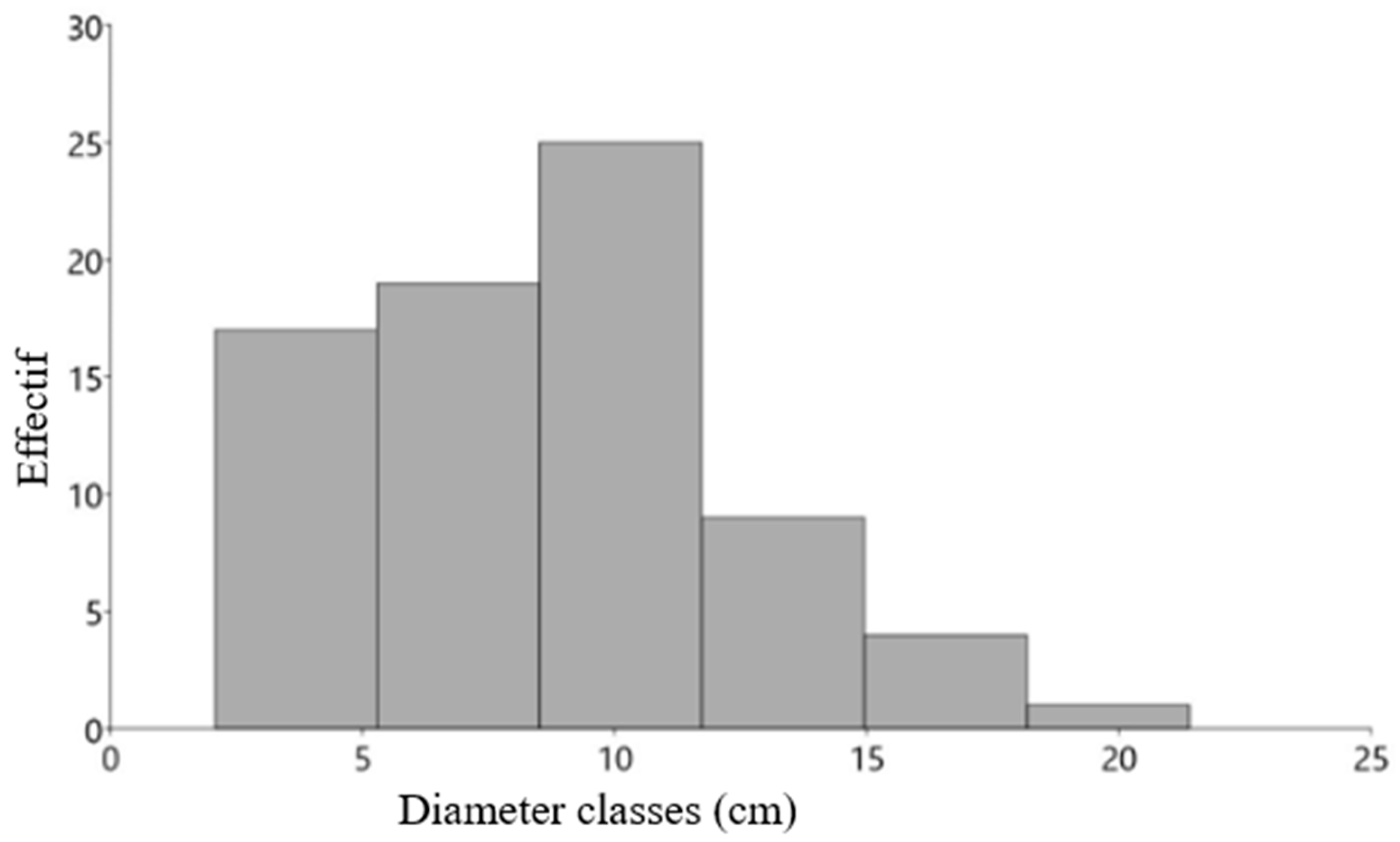
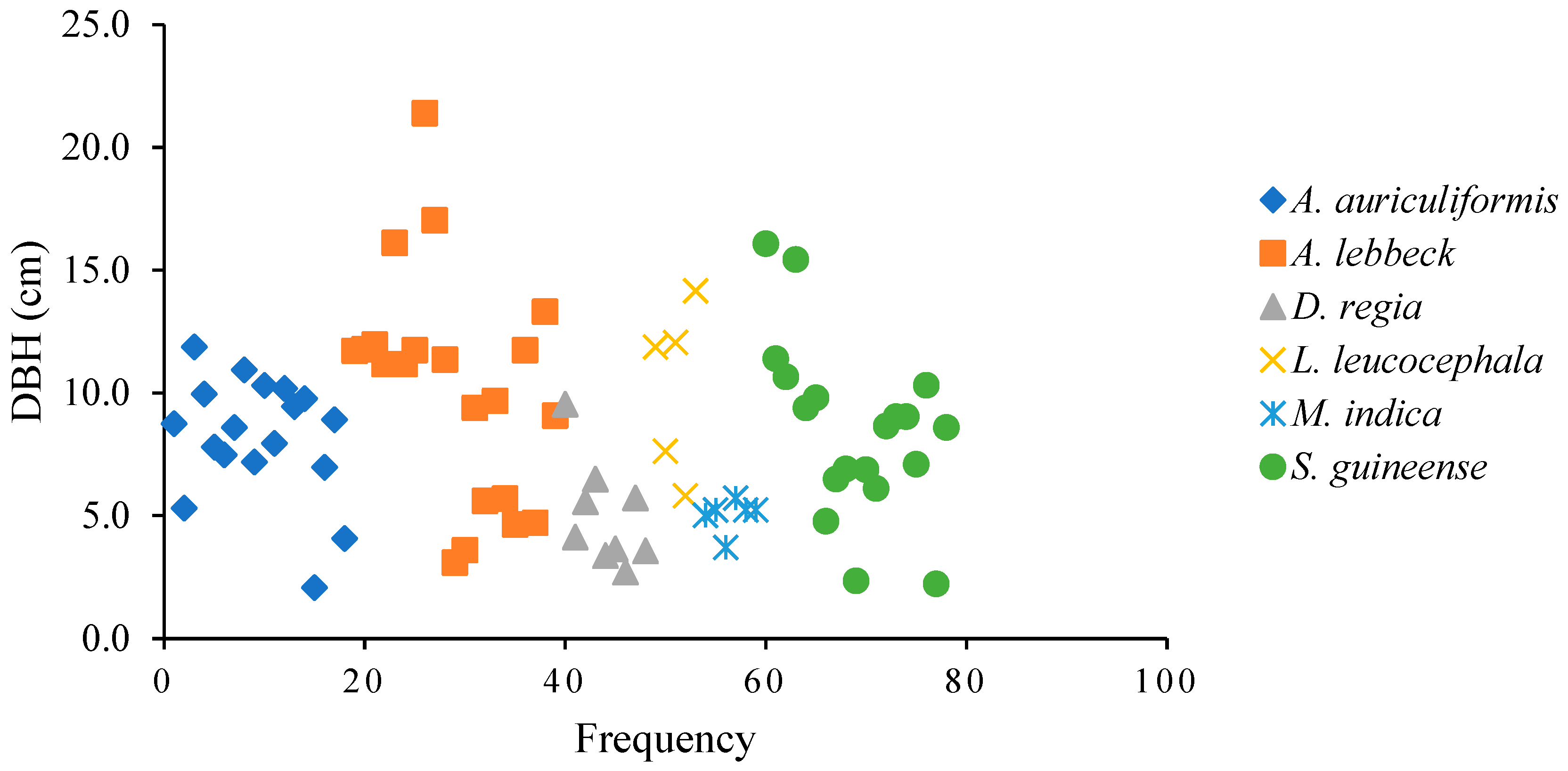
3.2.1. Survival Dynamics, Growth, Reproduction, and Regeneration of Woody Species
3.2.2. Dynamics of the Herbaceous Layer
4. Discussion
4.1. Dynamics of Mineral Composition in the Soil Around Trees over Time
4.2. Medium-Term Plantation Dynamics
4.3. Implications for the Remediation of Soil Polluted by Trace Metals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarenga, P.; Gonçalves, A.P.; Fernandes, R.M.; De Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Organic residues as immobilizing agents in aided phytostabilization: (I) Effects on soil chemical characteristics. Chemosphere 2009, 74, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, J.A.; Hursthouse, A.S.; Marinho Reis, P.A.; Stewart, A.G. Metalliferous Mine Dust: Human Health Impacts and the Potential Determinants of Disease in Mining Communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef]
- Doumett, S.; Lamperi, L.; Checchini, L.; Azzarello, E.; Mugnai, S.; Mancuso, S.; Petruzzelli, G.; Del Bubba, M. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: Influence of different complexing agents. Chemosphere 2008, 72, 1481–1490. [Google Scholar] [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Squadrone, S.; Burioli, E.; Monaco, G.; Koya, M.K.; Prearo, M.; Gennero, S.; Dominici, A.; Abete, M.C. Human exposure to metals due to consumption of fish from an artificial lake basin close to an active mining area in Katanga (D.R. Congo). Sci. Total Environ. 2016, 568, 679–684. [Google Scholar] [CrossRef]
- Van Brusselen, D.; Kayembe-Kitenge, T.; Mbuyi-Musanzayi, S.; Lubala Kasole, T.; Kabamba Ngombe, L.; Musa Obadia, P.; Kyanika wa Mukoma, D.; Van Herck, K.; Avonts, D.; Devriendt, K.; et al. Metal mining and birth defects: A case-control study in Lubumbashi, Democratic Republic of the Congo. Lancet Planet Health 2020, 4, e158–e167. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements of Group 12 (Previously Group IIb). In Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 283–319. [Google Scholar] [CrossRef]
- Duvigneaud, P.; Denaeyer-De Smet, S. Cuivre et végétation au Katanga. Bull. Société R. Bot. Belg. 1963, 96, 93–231. [Google Scholar]
- Faucon, M.-P.; Colinet, G.; Mahy, G.; Ngongo Luhembwe, M.; Verbruggen, N.; Meerts, P. Soil influence on Cu and Co uptake and plant size in the cuprophytes Crepidorhopalon perennis and C. tenuis (Scrophulariaceae) in SC Africa. Plant Soil 2009, 317, 201–212. [Google Scholar] [CrossRef]
- Leblanc, M.; Malaisse, F. Lubumbashi, un Écosystème Urbain Tropical; Centre International de Sémiologie, Université Nationale du Zaïre: Lubumbashi, République du Zaïre, 1978; pp. 95–96. [Google Scholar]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Narendrula, R.; Nkongolo, K.K.; Beckett, P. Comparative Soil Metal Analyses in Sudbury (Ontario, Canada) and Lubumbashi (Katanga, DR-Congo). Bull. Environ. Contam. Toxicol. 2012, 88, 187–192. [Google Scholar] [CrossRef]
- Pourret, O.; Lange, B.; Bonhoure, J.; Colinet, G.; Decrée, S.; Mahy, G.; Séleck, M.; Shutcha, M.; Faucon, M.-P. Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). Appl. Geochem. 2016, 64, 43–55. [Google Scholar] [CrossRef]
- Mpinda, M.T.; Mujinya, B.B.; Mees, F.; Kasangij, P.K.; Van Ranst, E. Patterns and forms of copper and cobalt in Macrotermes falciger mounds of the Lubumbashi area, DR Congo. J. Geochem. Explor. 2022, 238, 107002. [Google Scholar] [CrossRef]
- Atibu, E.K.; Devarajan, N.; Thevenon, F.; Mwanamoki, P.M.; Tshibanda, J.B.; Mpiana, P.T.; Prabakar, K.; Mubedi, J.I.; Wildi, W.; Poté, J. Concentration of metals in surface water and sediment of Luilu and Musonoie Rivers, Kolwezi-Katanga, Democratic Republic of Congo. Appl. Geochem. 2013, 39, 26–32. [Google Scholar] [CrossRef]
- Mudimbi, K.D.; Kabamba, T.A.; Kodondi, K.F.; Luboya, N.O.; Kasongo, B.C.; Kabundi, K.D.; Kisunka, B.Y.; Musola, C.H.; Longanga, O.A.; Lukumwena, K.Z. Impact of mining on water of the rivers Shinkolobwe, Lwisha and Kansonga in the province of Katanga (DRC). J. Med. Res. 2017, 3, 71–73. [Google Scholar]
- Banza, C.L.N.; Nawrot, T.S.; Haufroid, V.; Decrée, S.; De Putter, T.; Smolders, E.; Kabyla, B.I.; Luboya, O.N.; Ilunga, A.N.; Mutombo, A.M.; et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ. Res. 2009, 109, 745–752. [Google Scholar] [CrossRef]
- Cheyns, K.; Banza Lubaba Nkulu, C.; Ngombe, L.K.; Asosa, J.N.; Haufroid, V.; De Putter, T.; Nawrot, T.; Kimpanga, C.M.; Numbi, O.L.; Ilunga, B.K.; et al. Pathways of human exposure to cobalt in Katanga a mining area of the D.R. Congo. Sci. Total Environ. 2014, 490, 313–321. [Google Scholar] [CrossRef]
- Mukendi, R.-A.-M.; Banza, C.L.N.; Mukeng, C.-A.-K.; Ngwe, J.T.M.; Mwembo, A.N.-A.-N.; Kalenga, P.M.K. Human exposure to metallic traced elements and sperm alteration: A study conducted in the mining areas of Haut-Katanga in the Democratic Republic of Congo. Pan Afr. Med. J. 2018, 30, 35. [Google Scholar] [CrossRef]
- Gerwing, T.G.; Hawkes, V.C.; Gann, G.D.; Murphy, S.D. Restoration, reclamation, and rehabilitation: On the need for, and positing a definition of, ecological reclamation. Restor. Ecol. 2022, 30, e13461. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.; Liu, R.; Wang, L.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Huslina, F.; Khudur, L.S.; Shah, K.; Surapaneni, A.; Netherway, P.; Ball, A.S. Mine Site Restoration: The Phytoremediation of Arsenic-Contaminated Soils. Environments 2024, 11, 99. [Google Scholar] [CrossRef]
- Pinho, R.C.; Miller, R.P.; Alfaia, S.S. Agroforestry and the Improvement of Soil Fertility: A View from Amazonia. Appl. Environ. Soil Sci. 2012, 2012, 616383. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J.M. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ. 2022, 45, 719–736. [Google Scholar] [CrossRef]
- Bedair, H.; Ghosh, S.; Abdelsalam, I.M.; Keerio, A.A.; AlKafaas, S.S. Potential implementation of trees to remediate contaminated soil in Egypt. Environ. Sci. Pollut. Res. 2022, 29, 78132–78151. [Google Scholar] [CrossRef]
- Kidd, P.; Barceló, J.; Bernal, M.P.; Navari-Izzo, F.; Poschenrieder, C.; Shilev, S.; Clemente, R.; Monterroso, C. Trace element behaviour at the root–soil interface: Implications in phytoremediation. Environ. Exp. Bot. 2009, 67, 243–259. [Google Scholar] [CrossRef]
- Munyemba, K.; Bamba, I.; Djibu, K.J.P.; Amisi, M.; Veroustraete, F.; Ngongo, L.M.; Bogaert, J. Occupation des sols Dans le Cône de Pollution à Lubumbashi; Presses Universitaires de Lubumbashi: Lubumbashi, Democratic Republic of the Congo, 2008; Volume 1, pp. 19–22. [Google Scholar]
- Mpundu, M.M.; Useni, S.Y.; Nyembo, K.L.; Colinet, G. Effets d’amendements carbonatés et organiques sur la culture de deux légumes sur sol contaminé à Lubumbashi (RD Congo). BASE 2014, 18, 367–375. [Google Scholar]
- Mwanasomwe, K.J. Amélioration du Procédé de Phytostabilisation Avec les Espèces Ligneuses Pour la Production des Services Écosystémiques en Milieux Pollués Urbains et Périurbains de L’arc Cuprifère Katangais. Ph.D. Thesis, Université de Liège Gembloux-Agro Bio Tech, Gembloux, Belgium, 2022. [Google Scholar]
- Langunu, S.; Imabo, P.M.I.; Bibi Fwanda, B.; Kilela Mwanasomwe, J.; Colinet, G.; Ngoy Shutcha, M. Accumulation of Trace Metals in Fruits from Mango and Syzygium guineense Growing in Residential Households from a Contaminated District of Lubumbashi (DR Congo): Is Fruit Consumption at Risk? Toxics 2023, 11, 620. [Google Scholar] [CrossRef]
- Mwanasomwe, J.K.; Langunu, S.; Shutcha, M.N.; Colinet, G. Effects of 15-Year-Old Plantation on Soil Conditions, Spontaneous Vegetation, and the Trace Metal Content in Wood Products at Kipushi Tailings Dam. Front. Soil Sci. 2022, 2, 934491. [Google Scholar] [CrossRef]
- Drew, D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D. Modelling day-to-day stem diameter variation and annual growth of balsam fir (Abies balsamea (L.) Mill.) from daily climate. For. Ecol. Manag. 2011, 262, 863–872. [Google Scholar] [CrossRef]
- Ngongo, M.L.; Van Ranst, E.; Baert, G.; Kasongo, E.L.; Verdoodt, A.; Mujinya, B.B.; Mukalay, J.M.; Guide des Sols en, R.D. Etude et Gestion; Congo Tome I; UGent, UNILU: Lubumbashi, Democratic Republic of the Congo, 2009; p. 262. [Google Scholar]
- Shutcha, M.N.; Mukobo, R.P.; Muyumba, K.D.; Mpundu, M.M.; Faucon, M.P.; Lubalega, K.T.; Ludovic, A.; Annabelle, J.; Vandenheede, N.; Pourret, O.; et al. Pedogeochemical background and mapping of soil pollution in Lubumbashi. In Anthropisation des paysages Katangais; Bogaert, J., Gilles, C., Gregory, M., Eds.; Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2018; pp. 215–228. [Google Scholar]
- Malaisse, F. How to Live and Survive in Zambezian Open Forest (Miombo Ecoregion); Presses Agronomiques de Gembloux: Gembloux, Belgium, 2010. [Google Scholar]
- Kalombo, K.D. Caractérisation de la répartition temporelle des précipitations à Lubumbashi (Sud-Est de la RDC) sur la période 1970–2014. In Proceedings of the XXIII Colloque de l’Association Internationale de Climatologie, Liège, Belgium, 12–13 July 2015; pp. 531–536. [Google Scholar]
- Meeinkuirt, W.; Pokethitiyook, P.; Kruatrachue, M.; Tanhan, P.; Chaiyarat, R. Phytostabilization of a pb-contaminated mine tailing by various tree species in pot and field trial experiments. Int. J. Phytoremediat. 2012, 14, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Kambing’a, M.K.; Syampungani, S. Performance des espèces d’arbres poussant sur les sols des barrages de résidus en Zambie: Une base pour la sélection des espèces pour la revégétalisation des barrages de résidus. J. Environ. Sci. Eng. 2012, 1, 827. [Google Scholar]
- Mpundu, M.M.; Amandine, L.; Shutcha, N.M.; Michel, N.L.; Gilles, C. Phytostabilisation des sols contaminés au Katanga: Résultats d’expérimentations sur la sélection d’espèces ligneuses combinée à des doses croissantes d’amendements. In Anthropisation des Paysages Katangais; Bogaert, J., Gilles, C., Gregory, M., Eds.; Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2018; pp. 177–191. [Google Scholar]
- Mwanasomwe, J.K.; Langunu, S.; Nkulu, S.N.; Shutcha, M.N.; Colinet, G. Effect of Organic Amendment on the Physicochemical Characteristics of Tailings Dam Soil and Root Development of Tree Species, Fifteen Years After Planting. Front. Soil Sci. 2022, 2, 934999. [Google Scholar] [CrossRef]
- Meerts, P. An annotated checklist to the trees and shrubs of the Upper Katanga (D. R. Congo). Phytotaxa 2016, 258, 201–250. [Google Scholar] [CrossRef][Green Version]
- Useni, Y.S.; Malaisse, F.; Yona, J.M.; Mwamba, T.M.; Bogaert, J. Diversity, use and management of household-located fruit trees in two rapidly developing towns in Southeastern D.R. Congo. Urban For. Urban Green. 2021, 63, 127220. [Google Scholar] [CrossRef]
- Mwanasomwe, J.K. Sélection des Espèces Ligneuses Pour la Phytostabilisation et la Valorisation des Sols Contaminés en ETMs de Gécamines/Penga Penga. Master’s Thesis, Université de Lubumbashi, Lubumbashi, République Démocratique du Congo, 2012; p. 56. [Google Scholar]
- Rondeux, J. La Mesure des Arbres et des Peuplements Forestiers, 3rd ed.; Les Presses Universitaires de Liège, Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2021; 738p, Available online: http://hdl.handle.net/2268/262622 (accessed on 20 February 2025).
- Ameja, L.G.; Ribeiro, N.; Sitoe, A.A.; Guillot, B. Regeneration and Restoration Status of Miombo Woodland Following Land Use Land Cover Changes at the Buffer Zone of Gile National Park, Central Mozambique. Trees For. People 2022, 9, 100290. [Google Scholar] [CrossRef]
- Furman, B.T.; Leone, E.H.; Bell, S.S.; Durako, M.J.; Hall, M.O. Braun-Blanquet data in ANOVA designs: Comparisons with percent cover and transformations using simulated data. Mar. Ecol. Prog. Ser. 2018, 597, 13–22. [Google Scholar] [CrossRef]
- Nsielolo, K.R.; Kiyulu, B.J.; Ndungu, M.R. Inventaire floristique de la forêt sacrée de Wuya dans la province du Kongo-central en République Démocratique du Congo. Afr. Sci. 2020, 16, 218–225. [Google Scholar]
- Liénard, A.; Colinet, G. Assessment of vertical contamination of Cd, Pb and Zn in soils around a former ore smelter in Wallonia, Belgium. Environ. Earth Sci. 2016, 75, 1322. [Google Scholar] [CrossRef]
- Sastre, J.; Sahuquillo, A.; Vidal, M.; Rauret, G. Determination of Cd, Cu, Pb and Zn in environmental samples: Microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal. Chim. Acta 2002, 1, 59–72. [Google Scholar] [CrossRef]
- Gnahoua, G.; Nguessan, K.; Balle, P. Les jachères de légumineuses arborescentes: Sources potentielles de bois énergie et de service en Côte d’Ivoire. J. Appl. Biosci. 2014, 1, 7290. [Google Scholar] [CrossRef]
- Shutcha, M.N.; Faucon, M.-P.; Kamengwa Kissi, C.; Colinet, G.; Mahy, G.; Ngongo Luhembwe, M.; Visser, M.; Meerts, P. Three years of phytostabilisation experiment of bare acidic soil extremely contaminated by copper smelting using plant biodiversity of metal-rich soils in tropical Africa (Katanga, DR Congo). Ecol. Eng. 2015, 82, 81–90. [Google Scholar] [CrossRef]
- Mpinda, M.T.; Kisimba, T.N.; Mwamba, T.M.; Kasongo, E.L.M.; Kaniki, A.T.; Mujinya, B.B. Baseline Concentrations of 11 Elements as a Function of Land uses in Surface Soils of the Katangese Copperbelt Area (D.R. Congo). Am. J. Environ. Sci. 2021, 17, 125–135. [Google Scholar] [CrossRef]
- de Oliveira, V.P.; Martins, W.B.R.; Rodrigues, J.I.d.M.; Silva, A.R.; Lopes, J.D.C.A.; Neto, J.F.d.L.; Schwartz, G. Are liming and pit size determining for tree species establishment in degraded areas by kaolin mining? Ecol. Eng. 2022, 178, 106599. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Cao, Y.; Wang, J.; Tang, M.; Ban, Y. Comparisons of Soil Properties, Enzyme Activities and Microbial Communities in Heavy Metal Contaminated Bulk and Rhizosphere Soils of Robinia pseudoacacia L. in the Northern Foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Ayari, F.; Hamdi, H.; Jedidi, N.; Gharbi, N.; Kossai, R. Heavy metal distribution in soil and plant in municipal solid waste compost amended plots. Int. J. Environ. Sci. Technol. 2010, 7, 465–472. [Google Scholar] [CrossRef]
- Abd-Elhalim, B.T.; Gideon, M.; Anton, K.; Boyi, M.O. Impact of Dumpsite-Derived Compost on Heavy Metal Accumulation in Cultivated Maize and Spinach. BMC Res. Notes 2025, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, Z.; Li, D.; Zheng, M.; Nie, X.; Liao, Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Langunu, S. Evaluation de L’efficacité de la Phytoremédiation de sols Pollués en Métaux Traces dans L’arc Cuprifère Katangais (République Démocratique du Congo). Ph.D. Thesis, Université de Liège—Gembloux Agro-Bio Tech, Gembloux, Belgium, 2025; 271p. [Google Scholar]
- Legrand, P.; Turmel, M.-C.; Sauvé, S.; Courchesne, F. Speciation and bioavailability of trace metals (Cd, Cu, Ni, Pb, Zn) in the rhizosphere of contaminated soils. In Biogeochemistry of Trace Elements in the Rhizosphere; Elsevier: Amsterdam, The Netherlands, 2005; pp. 261–299. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Aryal, M. Phytoremediation strategies for mitigating environmental toxicants. Heliyon 2024, 10, e38683. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization potential of Jatropha curcas L. in polymetallic acid mine tailings. Int. J. Phytoremediat. 2011, 13, 788–804. [Google Scholar] [CrossRef]
- Olaniran, A.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Madejón, P.; Marañón, T.; Navarro-Fernández, C.M.; Domínguez, M.T.; Alegre, J.M.; Robinson, B.; Murillo, J.M.; Paz-Ferreiro, J. Potential of Eucalyptus camaldulensis for phytostabilization and biomonitoring of trace-element contaminated soils. PLoS ONE 2017, 12, e0180240. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Hnini, M.; Rabeh, K.; Oubohssaine, M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: A systematic review. Plant Stress 2024, 11, 100391. [Google Scholar] [CrossRef]
- Paniagua-López, M.; Silva-Castro, G.A.; Romero-Freire, A.; Martín-Peinado, F.J.; Sierra-Aragón, M.; García-Romera, I. Integrating waste valorization and symbiotic microorganisms for sustainable bioremediation of metal(loid)-polluted soils. Sci. Total Environ. 2024, 945, 174030. [Google Scholar] [CrossRef]
- ADEME. Phytotechnologies appliquées aux sites pollués. In Proceedings of the Journée Technique Nationale, Recueil des Interventions, Paris, France, 17 October 2012; p. 115. [Google Scholar]
- Lee, S.-H.; Ji, W.; Lee, W.-S.; Koo, N.; Koh, I.H.; Kim, M.-S.; Park, J.-S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef]
- Labidi, S.; Firmin, S.; Verdin, A.; Bidar, G.; Laruelle, F.; Douay, F.; Shirali, P.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Nature of fly ash amendments differently influences oxidative stress alleviation in four forest tree species and metal trace element phytostabilization in aged contaminated soil: A long-term field experiment. Ecotoxicol. Environ. Saf. 2017, 138, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W. Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 2009, 321, 385–408. [Google Scholar] [CrossRef]
- Gupta, N.; Fischer, A.R.H.; Frewer, L.J. Socio-psychological determinants of public acceptance of technologies: A review. Public Underst. Sci. 2012, 21, 782–795. [Google Scholar] [CrossRef]
- Bolia, N.E.; Bosanza, J.B.Z.; Mongeke, M.M.; Ngbolua, K.T.N. Études Dendrométrique et Floristique des Forêts à Gilbertiodendron Dewevrei d’une Concession Forestière en République Démocratique du Congo. 2019. Available online: https://www.agrimaroc.org/index.php/Actes_IAVH2/article/view/677#google_vignette (accessed on 20 February 2025).
- Ewango, C.; Maindo, A.; Shaumba, J.P.; Kyanga, M.; Macqueen, D. Options for Sustainable Community Forestry Business Incubation in the Democratic Republic of Congo (DRC); IIED: London, UK, 2019; Available online: https://www.iied.org/13613iied (accessed on 20 February 2025).
- Bisiaux, F.; Peltier, R.; Muliele, J.C. Plantations industrielles et agroforesterie au service des populations des plateaux Batéké, Mampu, en République démocratique du Congo. Bois For. Des. Trop. 2009, 301, 21–32. [Google Scholar] [CrossRef]
- Proces, P.; Dubiez, E.; Bisiaux, F.; Péroches, A.; Fayolle, A. Production d’Acacia auriculiformis dans le système agroforestier de Mampu, plateau Batéké, République démocratique du Congo. Bois For. Des. Trop. 2018, 334, 23. [Google Scholar] [CrossRef]
- Kaboneka, S.; Ndayishimiye, J.; Nkurunziza, C.; Ndorere, V.; Nyengayenge, D.; Ndayisaba, D. Adaptation et croissance des acacias australiens introduits au Burundi. Rev. L’université Burundi (Sci. Exactes Nat.) 2020, 29, 45–55. [Google Scholar]
- Wang, F.; Li, Z.; Xia, H.; Zou, B.; Li, N.; Liu, J.; Zhu, W. Effects of nitrogen-fixing and non-nitrogen-fixing tree species on soil properties and nitrogen transformation during forest restoration in southern China. Soil Sci. Plant Nutr. 2010, 56, 297–306. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Albert, K.M. Role of revegetation in restoring fertility of degraded mined soils in Ghana: A review. Int. J. Biodivers. Conserv. 2015, 7, 57–80. [Google Scholar] [CrossRef]
- Antwi-Boasiako, A.; Amponsah, P.; Adoma Opoku, J.; Coulibaly, D.; Mintah, P. Increasing Mango Production Efficiency under the Fast-Changing Climate. In Abiotic Stress in Crop Plants—Ecophysiological Responses and Molecular Approaches; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Vospernik, S.; Heym, M.; Pretzsch, H.; Pach, M.; Steckel, M.; Aldea, J.; Brazaitis, G.; Bravo-Oviedo, A.; Del Rio, M.; Löf, M.; et al. Tree species growth response to climate in mixtures of Quercus robur/Quercus petraea and Pinus sylvestris across Europe—A dynamic, sensitive equilibrium. For. Ecol. Manag. 2023, 530, 120753. [Google Scholar] [CrossRef]
- Conesa, H.M.; Faz, Á. Metal Uptake by Spontaneous Vegetation in Acidic Mine Tailings from a Semiarid Area in South Spain: Implications for Revegetation and Land Management. Water Air Soil Pollut. 2011, 215, 221–227. [Google Scholar] [CrossRef]
- Schleicher, J.; Meyer, K.M.; Wiegand, K.; Schurr, F.M.; Ward, D. Disentangling facilitation and seed dispersal from environmental heterogeneity as mechanisms generating associations between savanna plants: Cause of spatial associations of savanna plant species. J. Veg. Sci. 2011, 22, 1038–1048. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Padullés Cubino, J.; Balfour, K.; Zhu, Z.-X.; Wang, H.-F. Drivers of spontaneous and cultivated species diversity in the tropical city of Zhanjiang, China. Urban For. Urban Green. 2022, 67, 127428. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Mi, J.; Liu, R.; Hou, H.; Zhang, S. Effects of Vegetation Pattern and Spontaneous Succession on Remediation of Potential Toxic Metal-Polluted Soil in Mine Dumps. Sustainability 2019, 11, 397. [Google Scholar] [CrossRef]
- Boisson, S.; Collignon, J.; Langunu, S.; Lebrun, J.; Shutcha, M.N.; Mahy, G. Reconciling phytostabilisation of polluted soils with conservation of cupro-cobaltic flora with a novel strategy to enhance extreme ecosystems? In Territoires Périurbains. Développement, Enjeux et Perspectives dans les Pays du Sud; Bogaert, J., Halleux, J.-M., Eds.; Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2015; pp. 127–138. [Google Scholar]
- Bolan, N.; Naidu, R.; Choppala, G.; Park, J.; Mora, M.L.; Budianta, D.; Panneerselvam, P. Solute Interactions in Soils about the Bioavailability and Environmental Remediation of Heavy Metals and Metalloids. Pedologist 2012, 53, 1–18. [Google Scholar]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Senila, M.; Kovacs, E. Use of diffusive gradients in thin-film technique to predict the mobility and transfer of nutrients and toxic elements from agricultural soil to crops—An overview of recent studies. Environ. Sci. Pollut. Res. 2024, 31, 34817–34838. [Google Scholar] [CrossRef]
- Lawrence, G.B.; Fernandez, I.J.; Richter, D.D.; Ross, D.S.; Hazlett, P.W.; Bailey, S.W.; Ouimet, R.; Warby, R.A.F.; Johnson, A.H.; Lin, H.; et al. Measuring Environmental Change in Forest Ecosystems by Repeated Soil Sampling: A North American Perspective. J. Environ. Qual. 2013, 42, 623–639. [Google Scholar] [CrossRef]
- El-Sharkawy, G.; Alotaibi, M.O.; Zuhair, R.; Mahmoud, E.; El Baroudy, A.; Omara, A.E.-D.; El-Sharkawy, M. Ecological Assessment of Polluted Soils: Linking Ecological Risks, Soil Quality, and Biota Diversity in Contaminated Soils. Sustainability 2025, 17, 1524. [Google Scholar] [CrossRef]
- Shutcha, M.N.; Mubemba, M.M.; Faucon, M.-P.; Luhembwe, M.N.; Visser, M.; Colinet, G.; Meerts, P. Phytostabilisation of Copper-Contaminated Soil in Katanga: An Experiment with Three Native Grasses and Two Amendments. Int. J. Phytoremediat. 2010, 12, 616–632. [Google Scholar] [CrossRef] [PubMed]



| Family | Species | Original Status | Functional Group |
|---|---|---|---|
| Fabaceae | Acacia auriculiformis A. Cunn. Ex. Benth | Ex | TM/CR/PBE/SR |
| Fabaceae | Albizia lebbeck (L.) Benth | Ex | TM/CR/PBE/SR |
| Fabaceae | Delonix regia (Bojer ex Hook.) Raf. | Ex | TM/PBE/SR |
| Fabaceae | Leucaena. Leucocephala (Lam.) de Wit | Ex | TM/CR/PBE/SR |
| Myrtaceae | * Syzygium guineense (Willd) DC. Sub macrocarpum | In | TM/CR/PBE/SR/FC |
| Anacardiceae | Mangifera indica L. | Ex | TM/SR/FC |
| Parameters | Test Soil | Municipal Compost |
|---|---|---|
| pHkcl | 5.5 (4.5–5.6) | 7.2 (7.2–7.3) |
| TOC | 1.9 (0.8–2.6) | - |
| Fe (%) | - | 5.4 (4.7–6.2) |
| P (mg kg−1) | 5,3 (2.8–8.9) | 1327 (413.3–1898) |
| K (mg kg−1) | 1.0 (0.7–1.3) | 3133 (2995.2–3238) |
| Ca (mg kg−1) | 9.5 (3.6–20.6) | 21,797 (6153–30,348) |
| Mg (mg kg−1) | 1.4 (1.1–2.0) | 2791 (1542–3457) |
| As(T) (mg kg−1) | 232.1 (254.7–396.6) | 16.2 (14.1–17.2) |
| Cd(T) (mg kg−1) | 33 (23.5–52.3) | 2.7 (4.3–1.4) |
| Cu(T) (mg kg−1) | 17,330 (1462–29,216) | 725 (707–896) |
| Co(T) (mg kg−1) | 295 (276–505) | 151 (149–187) |
| Pb(T) (mg kg−1) | 1168 (1213–1723) | 62 (35–104) |
| Zn(T) (mg kg−1) | 3189.7 (2878–5347) | 558 (173–1114) |
| Cd(s) (mg kg−1) | 2.8 (1.5–4.5) | 0.01 (0.008–0.035) |
| Cu(s) (mg kg−1) | 104.9 (39.2–199.6) | 0.709 (0.177–1.631) |
| Co(s) (mg kg−1) | 19.8 (13.3–29.3) | 0.089 (0.007–0.263) |
| Pb(s) (mg kg−1) | 3.05 (0.7–7.3) | 0.128 (0.105–0.152) |
| Zn(s) (mg kg−1) | 152.7 (124.7–195.3) | 0.470 (0.033–1.277) |
| Parameters | 2019 | 2023 |
|---|---|---|
| PHKCl | 7.2 ± 0.03 | 7.2 ± 0.2 |
| Al (%) | 3.1 ± 0.32 | 3.8 ± 0.31 |
| Fe (%) | 5.4 ± 0.61 | 5.0 ± 0.63 |
| P (mg kg−1) | 1.327 ± 688 | 516 ± 71.0 |
| K (mg kg−1) | 3133 ± 101.8 | 2497 ± 182.6 |
| Ca (mg kg−1) | 21.797 ± 10.862 | 12.262 ± 6.062 |
| Mg (mg kg−1) | 2.791 ± 871 | 3.550 ± 695 |
| Metals | 2019 | 2023 |
|---|---|---|
| As (mg kg−1) | 16.2 ± 1.4 | 95 ± 28.5 |
| Cd (mg kg−1) | 2.7 ± 1.3 | 8.7 ± 2.0 |
| Cu (mg kg−1) | 725 ± 136 | 6.141 ± 1.853 |
| Co (mg kg−1) | 151 ± 36.3 | 182 ± 113 |
| Pb (mg kg−1) | 62 ± 32 | 421 ± 160 |
| Zn (mg kg−1) | 558 ± 418 | 1098 ± 1037 |
| Species | A. auriculiformis | A. lebbeck | D. regia | L. leucocephala | M. indica | S. guineense |
|---|---|---|---|---|---|---|
| Height (m) | 14.16 | 9.45 | 5.2 | 14.53 | 5.1 | 8.76 |
| Diameter (cm) | 14.26 | 8.14 | 5.6 | 8.52 | 5.2 | 8.2 |
| Metal-contaminated Environments | ||||||
| Height (m) | 6.4 | 5.5 | 3.2 | 8.6 | 3.0 | 6.1 |
| Diameter (cm) | 8.2 | 10.2 | 4.9 | 10.3 | 4.9 | 8.4 |
| Species | Family | 2021 | 2023 | ∆R (%) |
|---|---|---|---|---|
| Bulbostylis pseudoperennis Goetgh. | Cyperaceae | 86 | 48 | −38 |
| Microchloa cupricola (Rendle) Stapf | Poaceae | 49 | 68.6 | +19 |
| Imperata cylindrica (L.) P. Beauv. | Poaceae | 12 | 19 | +7 |
| Celosia trygina L. | Amaranthaceae | 3.2 | 6 | +2.8 |
| Setaria pumila (Poir.) Roem. & Schult. | Poaceae | 2.2 | 0.2 | −2 |
| Teramnus labialis (L.f.) | Fabaceae | 2 | 9 | +7 |
| Ageratum conyzoides L. | Asteraceae | 1.2 | 5.8 | +4.6 |
| Euphorbia hirta L. | Euphorbiaceae | 0.4 | 5 | +4.6 |
| Tithonia diversifolia (Hemsl.) | Asteraceae | 0.2 | 1 | +0.8 |
| Crassocephalum rubens (Jus. Ex Jacq.) | Asteraceae | 0 | 0.4 | +0.4 |
| Cynodon dactylon (L.) Pers. | Poaceae | 0 | 1.2 | +1.2 |
| Nicandra physaloïdes (L.) | Solanaceae | 0 | 3 | +3 |
| Panicum maximum Jacq. | Poaceae | 0 | 0.2 | +0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langunu, S.; Kilela Mwanasomwe, J.; Nghonda, D.-d.N.; Colinet, G.; Ngoy Shutcha, M. Mineral Condition Changes in Amended Soils and Woody Vegetation Installed on a Polluted Soil with Trace Metals in Lubumbashi (DR Congo): Results of a Four-Year Trial. Environments 2025, 12, 224. https://doi.org/10.3390/environments12070224
Langunu S, Kilela Mwanasomwe J, Nghonda D-dN, Colinet G, Ngoy Shutcha M. Mineral Condition Changes in Amended Soils and Woody Vegetation Installed on a Polluted Soil with Trace Metals in Lubumbashi (DR Congo): Results of a Four-Year Trial. Environments. 2025; 12(7):224. https://doi.org/10.3390/environments12070224
Chicago/Turabian StyleLangunu, Serge, Jacques Kilela Mwanasomwe, Dieu-donné N’Tambwe Nghonda, Gilles Colinet, and Mylor Ngoy Shutcha. 2025. "Mineral Condition Changes in Amended Soils and Woody Vegetation Installed on a Polluted Soil with Trace Metals in Lubumbashi (DR Congo): Results of a Four-Year Trial" Environments 12, no. 7: 224. https://doi.org/10.3390/environments12070224
APA StyleLangunu, S., Kilela Mwanasomwe, J., Nghonda, D.-d. N., Colinet, G., & Ngoy Shutcha, M. (2025). Mineral Condition Changes in Amended Soils and Woody Vegetation Installed on a Polluted Soil with Trace Metals in Lubumbashi (DR Congo): Results of a Four-Year Trial. Environments, 12(7), 224. https://doi.org/10.3390/environments12070224








