Integration of Mosses (Funaria hygrometrica) and Lichens (Xanthoria parietina) as Native Bioindicators of Atmospheric Pollution by Trace Metal Elements in Mediterranean Forest Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites in Mediterranean Forest Plantations
2.2. Determination of Lichen Diversity and the Air Quality Index
2.3. Determination of Bioaccumulation Potential of Toxic Metals in the Lichen Xanthoria parietina and the Moss Funaria hygrometrica
2.3.1. Sample Collection and Preparation
2.3.2. Concentrations of Toxic Metals
2.3.3. Metal Accumulation Index (MAI)
2.4. Statistical Analyses
3. Results
3.1. Lichen Diversity and (AQI)
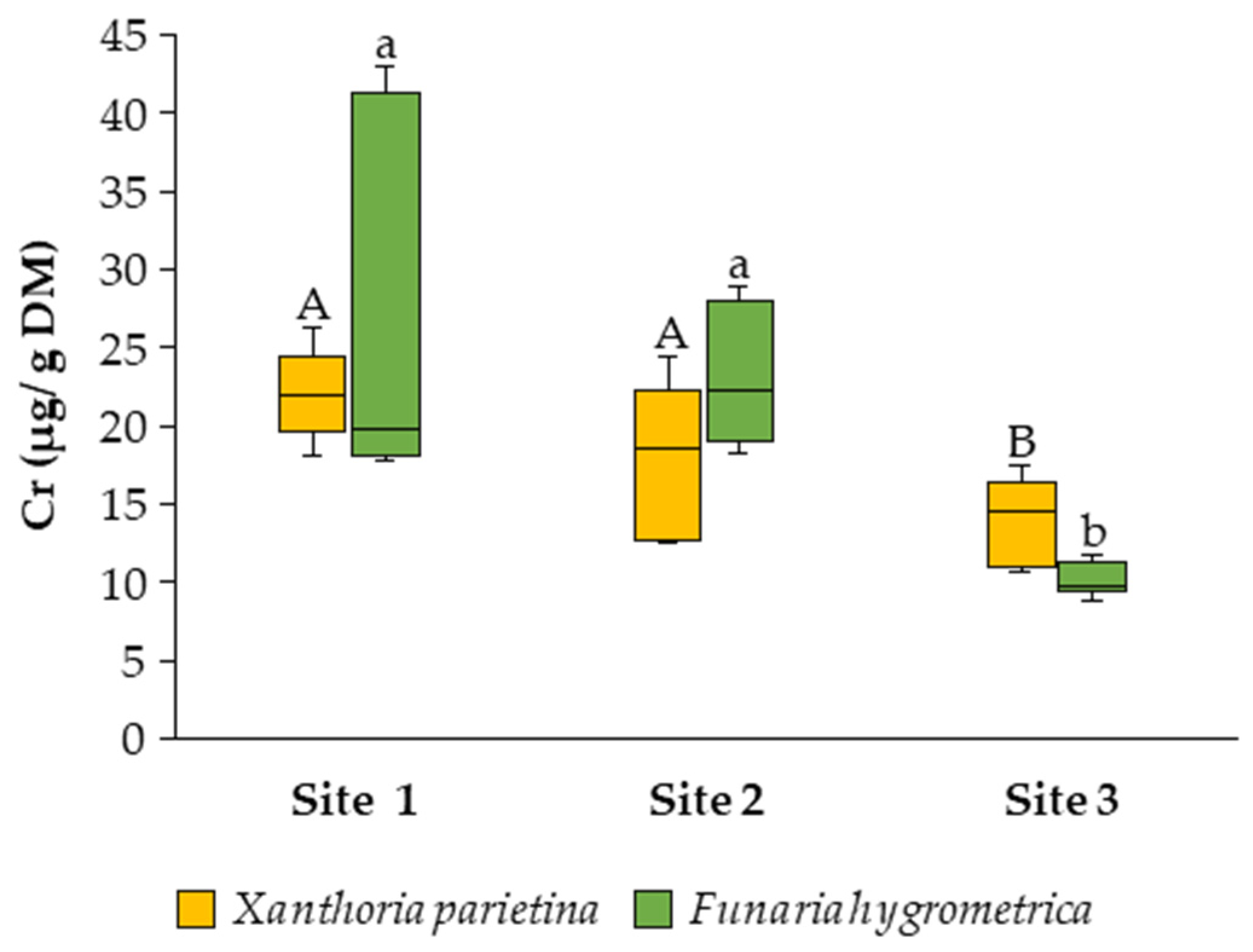
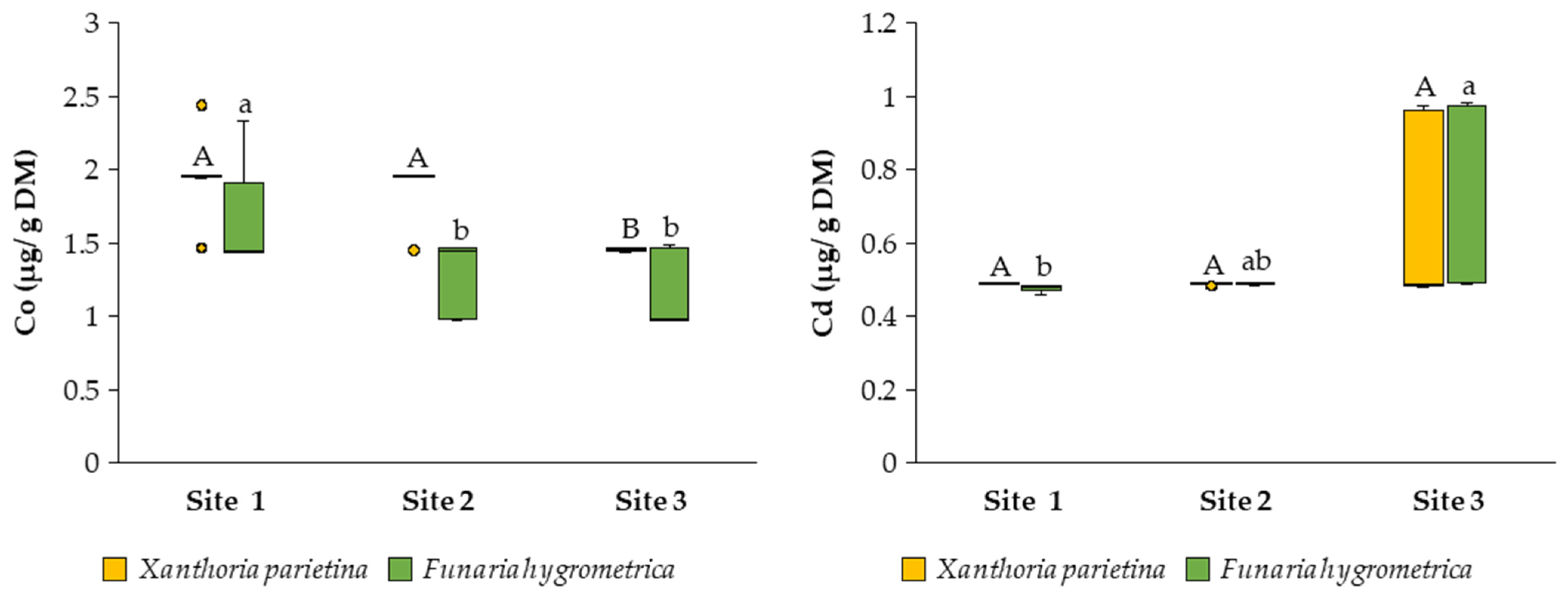
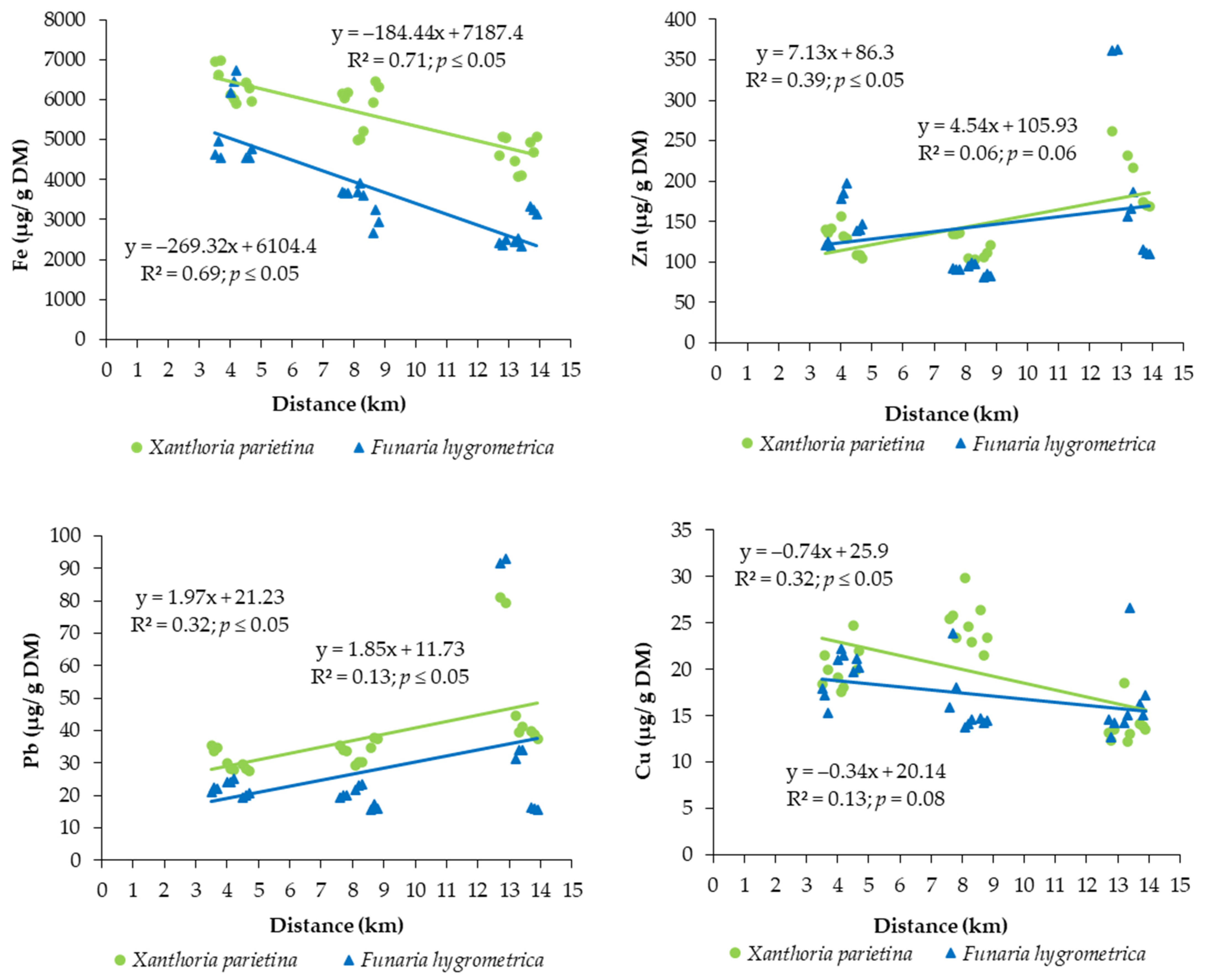
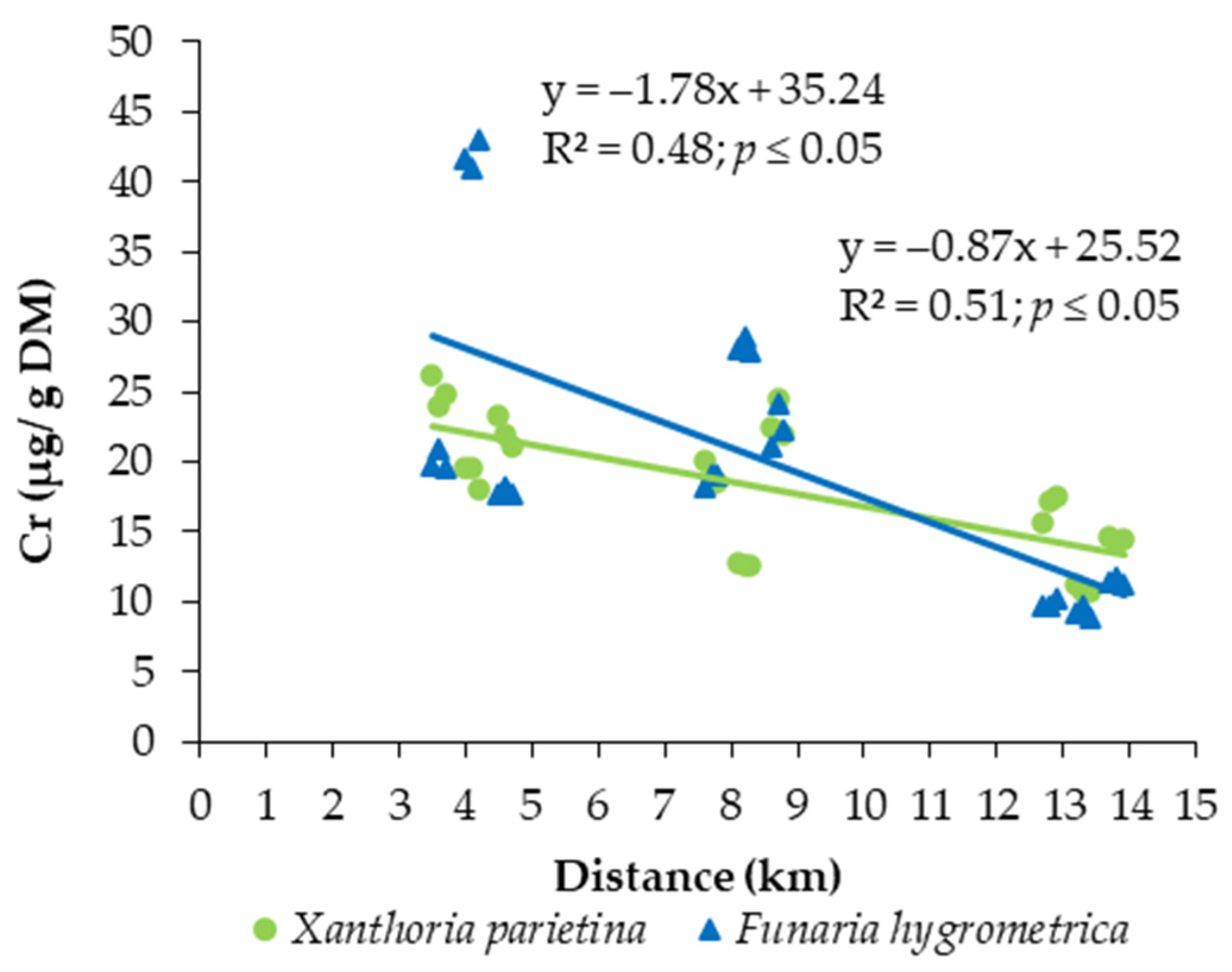
3.2. Bioaccumulation Potential of Toxic Metals
4. Discussion
5. Conclusions and Research Needs
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swisłowski, P.; Vergel, K.; Zinicovscaia, I.; Rajfur, M.; Waclawek, M. Mosses as a biomonitor to identify elements released into the air as a result of car workshop activities. Ecol. Indic. 2022, 138, 108849. [Google Scholar] [CrossRef]
- Phaenark, C.; Niamsuthi, A.; Paejaroen, P.; Chunchob, S.; Cronberg, N.; Sawangproh, W. Comparative Toxicity of Heavy Metals Cd, Pb, and Zn to Three Acrocarpous Moss Species using Chlorophyll Contents. Trends Sci. 2022, 20, 4287. [Google Scholar] [CrossRef]
- Chaligava, O.; Shetekauri, S.H.; Badawy, W.M.; Frontasyeva, M.V.; Zinicovscaia, I.; Shetekauri, T.; Kvlividze, A.; Vergel, K.; Yushin, N. Characterization of Trace Elements in Atmospheric Deposition Studied by Moss Biomonitoring in Georgia. Arch. Environ. Contam. Toxicol. 2020, 80, 350–367. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Stankovic, J.D.; Sabovljevic, A.D.; Sabovljevic, M.S. Bryophytes and heavy metals: A review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Gribacheva, N.; Gecheva, G.; Zhiyanski, M.; Pavlova-Traykova, E.; Yaneva, R. Active and passive moss monitoring of trace elements in urban and mountain areas, Bulgaria. For. Ideas 2021, 27, 309–317. [Google Scholar]
- Lazo, P.; Kika, A.; Qarri, F.; Bekteshi, L.; Allajbeu, S.; Stafilov, T. Air Quality Assessment by Moss Biomonitoring and Trace Metals Atmospheric Deposition. Aerosol Air Qual. Res. 2022, 22, 220008. [Google Scholar] [CrossRef]
- Stafilov, T.; Šajn, R.; Veličkovski-Simonović, S.; Tănăselia, C. Moss biomonitoring of air pollution with potentially toxic elements in the Kumanovo Region, North Macedonia. J. Environ. Sci. Health 2022, 57, 694–708. [Google Scholar] [CrossRef]
- Wolterbeek, B. Biomonitoring of trace element air pollution: Principles, possibilities and perspectives. Environ. Pollut. 2002, 120, 11–21. [Google Scholar] [CrossRef]
- Hasheminejad, S.; Moradi, H.; Soleimani, M. Potential of Pinus eldarica Medw. tree bark for biomonitoring polycyclic aromatic hydrocarbons in ambient air. Sci. Rep. 2024, 14, 6259. [Google Scholar] [CrossRef]
- Sakila, V.; Manohar, S. Real-time air quality monitoring in Bull Trench Kiln-based Brick industry by calibrating sensor readings and utilizing the Serverless Computing. Expert. Syst. Appl. 2024, 237, 121397. [Google Scholar] [CrossRef]
- Al-Alam, J.; Millet, M.; Khoury, D.; Rodrigues, A.; Akoury, E.; Tokajian, S.; Wazne, M. Biomonitoring of PAHs and PCBs in industrial, suburban, and rural areas using snails as sentinel organisms. Environ. Sci. Pollut. Res. 2024, 31, 4970–4984. [Google Scholar] [CrossRef] [PubMed]
- Cavazzin, B.; MacDonell, C.; Green, N.; Rothwell, J.J. Air pollution biomonitoring in an urban-industrial setting (Taranto, Italy) using Mediterranean plant species. Atmos. Pollut. Res. 2024, 15, 102105. [Google Scholar] [CrossRef]
- Frontasyeva, M.; Harmens, H.; Uzhinskiy, A.; Chaligava, O. Mosses as Biomonitors of Air Pollution: 2015/2016 Survey on Heavy Metals, Nitrogen and POPs in Europe and Beyond; PatriNat: Paris, France, 2020; p. 136. [Google Scholar]
- Zinicovscaia, I.; Chaligava, O.; Yushin, N.; Konstantin Vergel, G.; Hramco, C. Moss Biomonitoring of Atmospheric Trace Element Pollution in the Republic of Moldova. Arch. Environ. Contam. Toxicol. 2022, 82, 355–366. [Google Scholar] [CrossRef]
- Calas, A.; Schreck, E.; Viers, J.; Avellan, A.; Pages, A.; Dias-Alves, M.; Gardrat, E.; Behra, P.; Pont, V. Air quality, metalloid sources identification and environmental assessment using (bio)monitoring in the former mining district of Salsigne (Orbiel Valley, France). Chemosphere 2024, 357, 141974. [Google Scholar] [CrossRef]
- Tremper, A.H.; Agneta, M.; Burton, S.; Higgs, D.E.B. Field and Laboratory Exposures of Two Moss Species to Low Level Metal Pollution. J. Atmos. Chem. 2004, 49, 111–120. [Google Scholar] [CrossRef]
- Nascimbene, R.; Benesperi, P.; Giordani, M.; Grube, L.; Marini, C.; Mayrhofer, H. Les lichens des cheveux des forêts d’altitude peuvent-ils aider à détecter l’impact du changement global dans les Alpes? Diversité 2019, 11, 45. [Google Scholar] [CrossRef]
- Ravera, S.; Benesperi, R.; Bianchi, E.; Brunialti, G.; Di Nuzzo, L.; Frati, L.; Giordani, P.; Isocrono, D.; Nascimbene, J.; Vallese, C.; et al. Lobaria pulmonaria (L.) Hoffm.: The Multifaceted Suitability of the Lung Lichen to Monitor Forest Ecosystems. Forests 2023, 14, 2113. [Google Scholar] [CrossRef]
- Barandovski, L.; Stafilov, T.; Šajn, R.; Bačeva Andonovska, K.; Frontasyeva, M.; Zinicovscaia, I. Assessment of Atmospheric Deposition of Potentially Toxic Elements in Macedonia Using a Moss Biomonitoring Technique. Sustainability 2024, 16, 748. [Google Scholar] [CrossRef]
- Rajfur, M.; Stoica, A.L.; Swisłowski, P.; Stach, W.; Ziegenbalg, F.; Mattausch, E.M. Assessment of Atmospheric Pollution by Selected Elements and PAHs During 12-Month Active Biomonitoring of Terrestrial Mosses. Atmosphere 2024, 15, 102. [Google Scholar] [CrossRef]
- Lamano Ferreira, M.; Portela Ribeiro, A.; Rakauskas, F.; Bollamann, H.A.; Theophilo, C.Y.S.; Moreira, E.G.; Aranha, S.; Santos, C.J.; Giannico, V.; Elia, M.; et al. Spatiotemporal monitoring of subtropical urban forests in mitigating air pollution: Policy implications for nature-based solutions. Ecol. Indic. 2024, 158, 111386. [Google Scholar] [CrossRef]
- Takano, A.; Rybak, J.; Veras, M.M. Bioindicators and human biomarkers as alternative approaches for cost-effective assessment of air pollution exposure. Front. Environ. Eng. 2024, 3, 1346863. [Google Scholar] [CrossRef]
- Crisan, F. The significance of epiphytic lichens as bioindicators of air pollution for human health. Proc. Rom. Acad. 2023, 25, 167–174. [Google Scholar]
- Lin, D.; Meng, J.W.; Li, M.N.; Wu, Q.F.; Wang, L.P.; Li, X.J.; Song, J.J.; Zhao, L.C.; Xu, P.; Xia, Y.; et al. Active biomonitoring of atmospheric element deposition using Evernia mesomorpha in Tangshang, China. Ecol. Environ. Res. 2023, 22, 1191–1205. [Google Scholar] [CrossRef]
- Abas, A.; Sulaiman, N.; Adnan, N.R.; Aziz, S.A.; Nawang, W.N. Utilisation du lichen (Dirinaria sp.) Comme bioindicateur de métaux lourds en suspension dans l’air dans certaines zones industrielles en Malaisie. Environ. Asia 2019, 12, 85–90. [Google Scholar]
- Frati, L.; Brunialti, G. Recent Trends and Future Challenges for Lichen Biomonitoring in Forests. Forests 2023, 14, 647. [Google Scholar] [CrossRef]
- Allajbeu, S.; Yushin, N.S.; Qarri, F.; Duliu, P.L.; Frontasyeva, M.V. Atmospheric deposition of rare earth elements in Albania studied by the moss biomonitoring technique, neutron activation analysis and GIS technology. Environ. Sci. Pollut. Res. 2016, 23, 14087–14101. [Google Scholar] [CrossRef]
- Abas, A. A systematic review on biomonitoring using lichen as the biological indicator: A decade of practices, progress, and challenges. Ecol. Indic. 2021, 121, 107197. [Google Scholar] [CrossRef]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Di Toppi, L.S. A Cd/Fe/Zn-Responsive Phytochelatin Synthase is Constitutively Present in the Ancient Liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef]
- Agnan, Y.; Probst, A.; Séjalon-Delmas, N. Evaluation of lichen species resistance to atmospheric metal pollution by coupling diversity and bioaccumulation approaches: A new bioindication scale for French forested areas. Ecol. Indic. 2017, 72, 99–110. [Google Scholar] [CrossRef]
- Correa-Ochoa, M.A.; Vélez-Monsalve, L.C.; Saldarriaga-Molina, J.C.; Jaramillo-Ciro, M.M. Evaluation of the Index of Atmospheric Purity in an American tropical valley through the sampling of corticulous lichens in different phorophyte species. Ecol. Indic. 2020, 115, 106355. [Google Scholar] [CrossRef]
- Adie, P.A.; Kor, A.A.; Oklo, A.D.; Ikese, O.C. Funaria hygrometrica moss as Bio-indicator of Atmospheric Pollution of Polycyclic Aromatic Hydrocarbons (PAHs) in Makurdi-Nigeria: Occurrence and Sources. Int. J. Res. Sci. Innov. 2021, 8, 29–35. [Google Scholar] [CrossRef]
- Benítez, Á.; Medina, J.; Vásquez, C.; Loaiza, T.; Luzuriaga, Y.; Calva, J. Lichens and Bromeliads as Bioindicators of Heavy Metal Deposition in Ecuador. Diversity 2019, 11, 28. [Google Scholar] [CrossRef]
- Ancora, S.; Dei, R.; Rota, E.; Mariotti, G.; Bianchi, N.; Bargagli, R. Altitudinal variation of trace elements deposition in forest ecosystems along the NW side of Mt. Amiata (central Italy): Evidence from topsoil, mosses and epiphytic lichens. Atmos. Pollut. Res. 2021, 12, 101200. [Google Scholar] [CrossRef]
- Carrillo, W.; Calva, J.; Benítez, Á. The Use of Bryophytes, Lichens and Bromeliads for Evaluating Air and Water Pollution in an Andean City. Forests 2022, 13, 1607. [Google Scholar] [CrossRef]
- General Directorate of Forests. National Forest Inventory—Summary Report; Ministry of Agriculture: Tunis, Tunisia, 1998; p. 120. [Google Scholar]
- Arif, A. National Institute of Meteorology 1990–2015. Available online: http://www.meteo.tn (accessed on 5 June 2025).
- Barandovski, L.; Stafilov, T.; Šajn, R.; Frontasyeva, M.; Bačeva Andonovska, K. Atmospheric Heavy Metal Deposition in North Macedonia from 2002 to 2010 Studied by Moss Biomonitoring Technique. Atmosphere 2020, 11, 929. [Google Scholar] [CrossRef]
- Agnan, Y.; Séjalon-Delmas, N.; Probst, A. Comparing early twentieth century and present-day atmospheric pollution in SW France: A story of lichens. Environ. Pollut. 2013, 172, 139–148. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kirschbaum, U.; Wirth, V. Les Lichens Bio-Indicateurs: Les Reconnaître, Évaluer la Qualité de L’Air; Editions Ulmer: Paris, France, 1997; p. 128. [Google Scholar]
- Poličnik, H.; Simončič, P.; Batič, F. Monitoring air quality with lichens: A comparison between mapping in forest sites and in open areas. Environ. Pollut. 2008, 151, 395–400. [Google Scholar] [CrossRef]
- Koroleva, Y.; Abdo, S.; Kaniki, A.; Kanda, J.M.; Alleman, L.Y. Mapping and Spatial Prediction of Atmospheric Deposition in Moss Samples: A Study in the Kaliningrad Region, Russia. Arab. J. Geosci. 2023, 16, 651. [Google Scholar]
- Clauzade, J.; Roux, J.P. La végétation de la région méditerranéenne. Ecol. Mediterr. 1985, 11, 1–20. [Google Scholar]
- Tiévant, M. Flore et végétation des Alpes françaises. Ecol. Mediterr. 2001, 27, 33–47. [Google Scholar]
- Pichler, G.; Muggia, L.; Candotto Carniel, F.; Grube, M.; Kranner, I. How to build a lichen: From metabolite release to symbiotic interplay. New Phytol. 2023, 238, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Norme No. VDI-Richtlinien 3799, Blatt 1; Verein Deutscher Ingenieure (VDI). Messen von Immissionswirkungen—Ermittlung und Beurteilung Phytotoxischer Wirkungen von Immissionen mit Flechten—Flechtenkartierung zur Ermittlung des Luftgütewertes (LGW). Engl. VDI/DIN-Kommission Reinhaltung der Luft (KRdL)—Normenausschuss: Berlin, Germany, 1995.
- El Rhzaoui, G.E.; Divakar, P.K.; Crespo, A.M.; Tahiri, H.; El Alaoui-Faris, F.E. Xanthoria parietina as a biomonitor of airborne heavy metal pollution in forest sites in the North East of Morocco. Lazaroa 2015, 36, 31–41. [Google Scholar] [CrossRef]
- Occelli, F.; Cuny, M.A.; Devred, I. Étude de l’imprégnation de l’environnement de trois bassins de vie de la région Nord-Pas-de-Calais par les éléments Traces Métalliques. Pollut. Atmosphérique 2013, 220, 15–25. [Google Scholar] [CrossRef]
- Yushin, N.; Chaligava, O.; Zinicovscaia, I.; Vergel, K.; Grozdov, D. Mosses as Bioindicators of Heavy Metal Air Pollution in the Lockdown Period Adopted to Cope with the COVID-19 Pandemic. Atmosphere 2020, 11, 1194. [Google Scholar] [CrossRef]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P.A. (Eds.) Monitoring with Lichens—Monitoring Lichens (NATO Science Series: IV—Earth and Environmental Sciences); Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 7, p. 408. ISBN 1-4020-0429-X. [Google Scholar]
- Agilent Technologies. Agilent 5100 and 5110 ICP-OES User’s Guide, 6th ed.; Agilent Technologies: Santa Clara, CA, USA, 2018. [Google Scholar]
- Centre D’expertise en Analyses Environnementale du Québec. Détermination des Métaux Assimilables et du Phosphore: Méthode par Spectrométrie de Masse à Source Ionisante au Plasma D’argon, MA. 200—Mét-P ass. 1.0, Rév. 2; Ministère du Développement Durable, de l’Environnement et de la Lutte Contre les Changements Climatiques du Québec: Québec, QC, Canada, 2014; p. 15.
- Liu, Y.J.; Zhu, Y.G.; Ding, H. Lead and cadmium in leaf of deciduous trees in Beijing, China: Development of a metal accumulation index (MAI). Environ. Pollut. 2007, 145, 387–390. [Google Scholar] [CrossRef]
- Loppi, S. Les lichens en tant que sentinelles de la pollution atmosphérique dans les régions alpines reculées (Italie). Environ. Sci. Pollut. Res. 2014, 21, 2563–2571. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Calderisi, M.; Malaspina, P.; Frati, L. Diversité bêta et similitude des communautés de lichens comme un signe des temps. Lichenologist 2018, 50, 371–383. [Google Scholar] [CrossRef]
- Brunialti, G.L.; Frati, C.; Malegori, P.; Giordani, P.; Malaspina, P. Des équipes différentes produisent-elles des résultats différents en matière de biosurveillance à long terme des lichens? Diversité 2019, 11, 43. [Google Scholar] [CrossRef]
- Daillant, O. Lichens et accumulation des métaux lourds. Taureau. Informer. Cul. Fr. Lichénologie 2003, 28, 31–43. [Google Scholar]
- Van Haluwyn, C.; Lerond, M. Les lichens et la qualité de l’air: Évolution méthodologique et limites. Bulletin de la Société Botanique de France. Actual. Bot. 1986, 133, 81–112. [Google Scholar]
- Seed, L.; Wolseley, P.; Gosling, L.; Davies, L.; Power, S.A. Modelling relationships between lichen bioindicators, air quality and climate on a national scale: Results from the UK OPAL air survey. Environ. Pollut. 2013, 182, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Nowak, A.; Rajfur, M. Comparison of Exposure Techniques and Vitality Assessment of Mosses in Active Biomonitoring for Their Suitability in Assessing Heavy Metal Pollution in Atmospheric Aerosol. Environ. Toxicol. Chem. 2022, 41, 1429–1438. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Granata, A.; Vergara, A.; Alberico, M.; Rossi, M.; Spagnuolo, V.; Giordano, S. Optimizing Moss and Lichen Transplants as Biomonitors of Airborne Anthropogenic Microfibers. Biology 2023, 12, 1278. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L. Biomonitoring of nine elements by the lichen Xanthoria parietina in Adriatic Italy: A retrospective study over a 7-year time span. Sci. Total Environ. 2007, 387, 289–300. [Google Scholar] [CrossRef]
- Scerbo, R.; Ristori, T.; Possenti, L.; Lampugnani, L.; Barale, R.; Barghigiani, C. Lichen (Xanthoria parietina) biomonitoring of trace element contamination and air quality assessment in Pisa Province (Tuscany, Italy). Sci. Total Environ. 2002, 286, 27–40. [Google Scholar] [CrossRef]
- Çobanoğlu, G.; Kaan, T. Biomonitoring of Atmospheric heavy metals in native lichen Xanthoria parietina around Salda Lake (Burdur-Turkey), a special environmental protection area. Air Qual. Atmos. Health 2024, 17, 2789–2800. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Identification of Carcinogenic Hazards to Humans: Arsenic and Arsenic Compounds. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Arsenic-Metals-Fibres-And-Dusts-2012 (accessed on 16 April 2025).
- Boonpeng, C.; Polyiam, W.; Sriviboon, C.; Jhampasri, T.; Watthana, S.; Sangvichien, E.; Boonpeng, K. Accumulation of inorganic polluants and photosynthetic responses of transplanted lichens at distances away from an industrial complex. Thai J. Bot. 2017, 9, 181–191. [Google Scholar]
- Shvetsova, M.S.; Kamanina, I.Z.; Frontasyeva, M.V.; Madadzada, A.I.; Zinicovscaia, I.I.; Pavlov, S.S.; Vergel, K.N.; Yushin, N.S. Active Moss Biomonitoring Using the “Moss Bag Technique” in the Park of Moscow. Phys. Part. Nucl. Lett. 2019, 16, 994–1003. [Google Scholar] [CrossRef]
- Han, Y.; Du, P.; Cao, J.; Posmentier, E.S.; Wang, Q.; Li, T.; Zhang, R. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci. Total Environ. 2021, 355, 176–186. [Google Scholar]
- Cardoso-Gustavson, P.; Fernandes, F.F.; Alves, E.S.; Pereira Victorio, M.; Baesso Moura, B.; Domingos, M.; Albuquerque Rodrigues, C.; Portella Ribeiro, A.; Carvalho Nievola, C.; G Figueiredo, A.M. Tillandsia usneoides: A successful alternative for biomonitoring changes in air quality due to a new highway in São Paulo, Brazil. Environ. Sci. Pollut. Res. 2016, 23, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Chrastný, V.; Šillerová, H.; Vítková, M.; Francová, A.; Jehlička, J.; Kocourková, J.; Aspholm, P.E.; Nilsson, L.O.; Berglen, T.F.; Jensen, H.K.B.; et al. Unleaded gasoline as a significant source of Pb emissions in the Subarctic. Chemosphere 2018, 193, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Cincinelli, A.; Martellini, T.; Katsoyiannis, A. Atmospheric deposition of trace metals in coastal urban environments: Sources, transport and trends. Environ. Pollut. 2017, 222, 240–250. [Google Scholar] [CrossRef]
- Di Palma, A.; Capozzi, F.; Spagnuolo, V.; Giordano, S.; Adamo, P. Atmospheric particulate matter intercepted by moss-bags: Relations to moss trace element uptake and land use. Chemosphere 2017, 176, 361–368. [Google Scholar] [CrossRef]
- Vitali, M.; Antonucci, A.M.; Owczarek, M.; Guidotti, M.; Astolfi, M.L.; Manigrasso, M.; Protano, C. Évaluation de la qualité de l’air dans différents scénarios environnementaux par la détermination de métaux lourds typiques et de polluants organiques persistants chez le lichen indigène Xanthoria parietina. Pollut. Environ. 2019, 254, 113013. [Google Scholar] [CrossRef]
- Marié, D.C.; Martino, L.; Chaparro, M.A.; D’Angelo, C.; Lavornia, J.M.; Böhnel, H.N. A moss species for magnetic biomonitoring the airborne particle pollution. Boletín Soc. Geológica Mex. 2024, 76, A040324. [Google Scholar]
- Salo, H.; Bućko, M.S.; Vaahtovuo, E.; Limo, J.; Mäkinen, J.; Pesonen, L.J. Biomonitoring of air pollution in SW Finland by magnetic and chemical measurements of moss bags and lichens. Geochem. Explor. 2012, 115, 69–81. [Google Scholar] [CrossRef]
- Kłos, A.; Ziembik, Z.; Rajfur, M.; Dołhańczuk-Śródka, A.; Bochenek, Z.; Bjerke, J.W.; Tømmervik, H.; Zagajewski, B.; Ziółkowski, D.; Jerz, D.; et al. Using moss and lichens in biomonitoring of heavy-metal contamination of forest areas in southern and north-eastern Poland. Sci. Total Environ. 2018, 627, 438–449. [Google Scholar] [CrossRef]
- Integrated Risk Information System (IRIS). Available online: https://www.epa.gov/iris (accessed on 19 March 2025).
- Kasongo, J.; Alleman, L.Y.; Kanda, J.M.; Kaniki, A.; Riffault, V. Metal-bearing airborne particles from mining activities: A review on their characteristics, impacts and research perspectives. Sci. Total Environ. 2024, 951, 175426. [Google Scholar] [CrossRef]





| Lichen Species | Thallus Type | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| Bacidia rubella (Hoffm.) A. Massal. | Crustose | 1 | 1 | 1 |
| Bactrospora patellarioides (Nyl.) Almq. | Crustose | 1 | 0 | 0 |
| Caloplaca cerina Ehrh. ex Hedw. | Crustose | 1 | 1 | 0 |
| Caloplaca ferruginea Huds. | Crustose | 0 | 0 | 1 |
| Chrysothrix candelaris (L.) J.R. Laundon | Leprose | 0 | 1 | 1 |
| Dendrographa decolorans (Turner & Borrer) Ertz &Tehler. | Crustose | 1 | 1 | 1 |
| Diploicia canescens (Dicks.) A. Massal. | Crustose | 0 | 1 | 1 |
| Dirina ceratoniae (Ach.) Fr. | Crustose | 1 | 1 | 1 |
| Evernia prunastri (L.) Ach. | Fruticose | 0 | 0 | 1 |
| Flavoparmelia caperata (L.) Hale | Foliose | 0 | 0 | 1 |
| Hyperphyscia adglutinata (Flörke) H. Mayrhofer & Poelt | Crustose | 0 | 1 | 1 |
| Lecania naegeli (Hepp.) Diedrich & van den Boom | Crustose | 1 | 1 | 1 |
| Lecanora argentata (Ach.) Malme | Crustose | 0 | 1 | 0 |
| Lecanora expallens Ach. | Crustose | 0 | 1 | 1 |
| Lecanora chlarotera Nyl. | Crustose | 1 | 1 | 1 |
| Lecanora compallens Herk & Aptroot | Leprose | 0 | 1 | 1 |
| Lecanora horiza (Ach.) Linds. | Crustose | 0 | 1 | 1 |
| Lecanora lividocinerea Bagl. | Crustose | 0 | 1 | 1 |
| Lecanora strobilina (Spreng). kieff. | Crustose | 0 | 1 | 1 |
| Lecidella elaoechroma (Ach.) M. Choisy | Crustose | 1 | 1 | 1 |
| Micarea prasina Fr. | Crustose | 1 | 1 | 1 |
| Ocellomma picconianum (Bagl.) Ertz & Tehler | Crustose | 1 | 1 | 0 |
| Opegrapha atra Pers. | Crustose | 0 | 1 | 1 |
| Opegrapha celtidicola Jatta | Crustose | 1 | 0 | 0 |
| Opegrapha herbarum Mont. | Crustose | 0 | 1 | 1 |
| Opegrapha niveoatra (Borrer) J.R.Laundon | Crustose | 1 | 1 | 1 |
| Opegrapha vulgata (Ach.) Ach. | Crustose | 1 | 1 | 1 |
| Parmelina tiliacea (Hoffm.) Hale | Foliose | 0 | 0 | 1 |
| Parmotrema hypoleucinum (J. Steiner) Hale | Foliose | 0 | 0 | 1 |
| Parmotrema perlatum (Huds.) M.Choisy | Foliose | 0 | 0 | 1 |
| Physcia adscendens H. Olivier | Foliose | 1 | 1 | 1 |
| Physcia tenella (Scop.) DC. | Foliose | 1 | 1 | 1 |
| Pyrrhospora quernea (Dicks.) Krộb. | Crustose | 0 | 1 | 1 |
| Ramalina farinacea (L.) Ach. | Fruticose | 0 | 0 | 1 |
| Ramalina lacera (With.) J.R. Laundon | Fruticose | 0 | 1 | 1 |
| Rinodina pruinella Bagl. | Crustose | 0 | 1 | 1 |
| Thelopsis isiaca Stizenb. | Crustose | 1 | 1 | 0 |
| Xanthoria parietina (L.) Th.Fr. | Foliose | 1 | 1 | 1 |
| Specific richness | 17 | 29 | 32 | |
| Species abundance | 3.65 | 4.82 | 7.66 | |
| Shannon index (H’) | 3.76 | 3.88 | 4.7 | |
| AQI | 36.5 | 44 | 76.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousbih, M.; Lamhamedi, M.S.; Abassi, M.; Khasa, D.P.; Bejaoui, Z. Integration of Mosses (Funaria hygrometrica) and Lichens (Xanthoria parietina) as Native Bioindicators of Atmospheric Pollution by Trace Metal Elements in Mediterranean Forest Plantations. Environments 2025, 12, 191. https://doi.org/10.3390/environments12060191
Bousbih M, Lamhamedi MS, Abassi M, Khasa DP, Bejaoui Z. Integration of Mosses (Funaria hygrometrica) and Lichens (Xanthoria parietina) as Native Bioindicators of Atmospheric Pollution by Trace Metal Elements in Mediterranean Forest Plantations. Environments. 2025; 12(6):191. https://doi.org/10.3390/environments12060191
Chicago/Turabian StyleBousbih, Malek, Mohammed S. Lamhamedi, Mejda Abassi, Damase P. Khasa, and Zoubeir Bejaoui. 2025. "Integration of Mosses (Funaria hygrometrica) and Lichens (Xanthoria parietina) as Native Bioindicators of Atmospheric Pollution by Trace Metal Elements in Mediterranean Forest Plantations" Environments 12, no. 6: 191. https://doi.org/10.3390/environments12060191
APA StyleBousbih, M., Lamhamedi, M. S., Abassi, M., Khasa, D. P., & Bejaoui, Z. (2025). Integration of Mosses (Funaria hygrometrica) and Lichens (Xanthoria parietina) as Native Bioindicators of Atmospheric Pollution by Trace Metal Elements in Mediterranean Forest Plantations. Environments, 12(6), 191. https://doi.org/10.3390/environments12060191








