Reduced Graphene Oxide Modulates Physiological Responses of Lemna minor Under Environmental Heavy Metal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Reduced Graphene Oxide (rGO)
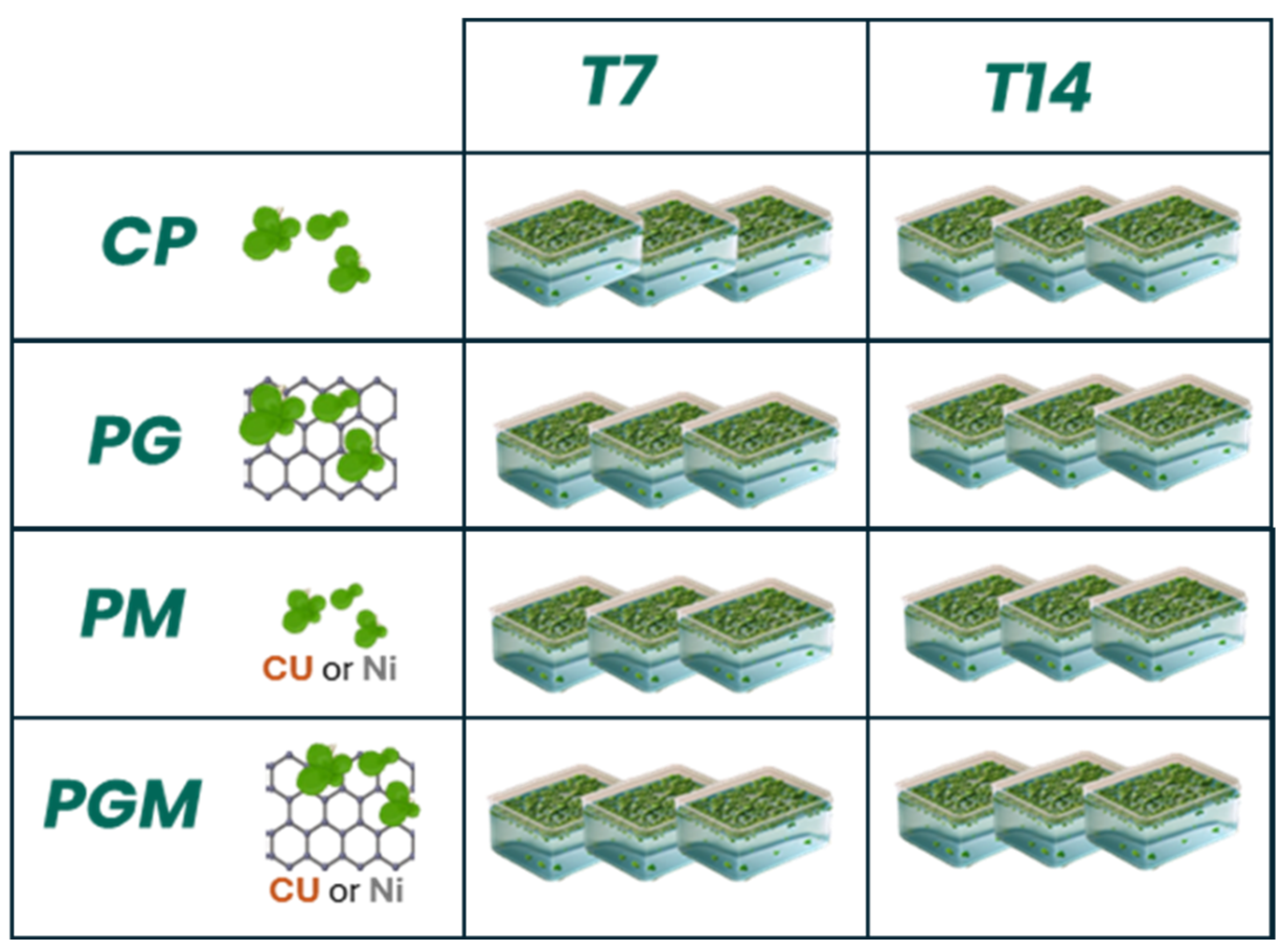
2.2. Plant Material and Experimental Design
2.3. Accumulation of Nickel (Ni) and Copper (Cu)
2.4. Bioconcentration Factor of Nickel and Copper
2.5. Plant Growth Analyses
2.6. Determination of Pigment Content
2.7. Measurement of Lipid Peroxidation Level
2.8. Biochemical Assays of Antioxidant Enzyme Activities
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Reduced Graphene Oxide and Nickel to L. minor
3.1.1. Growth Analysis
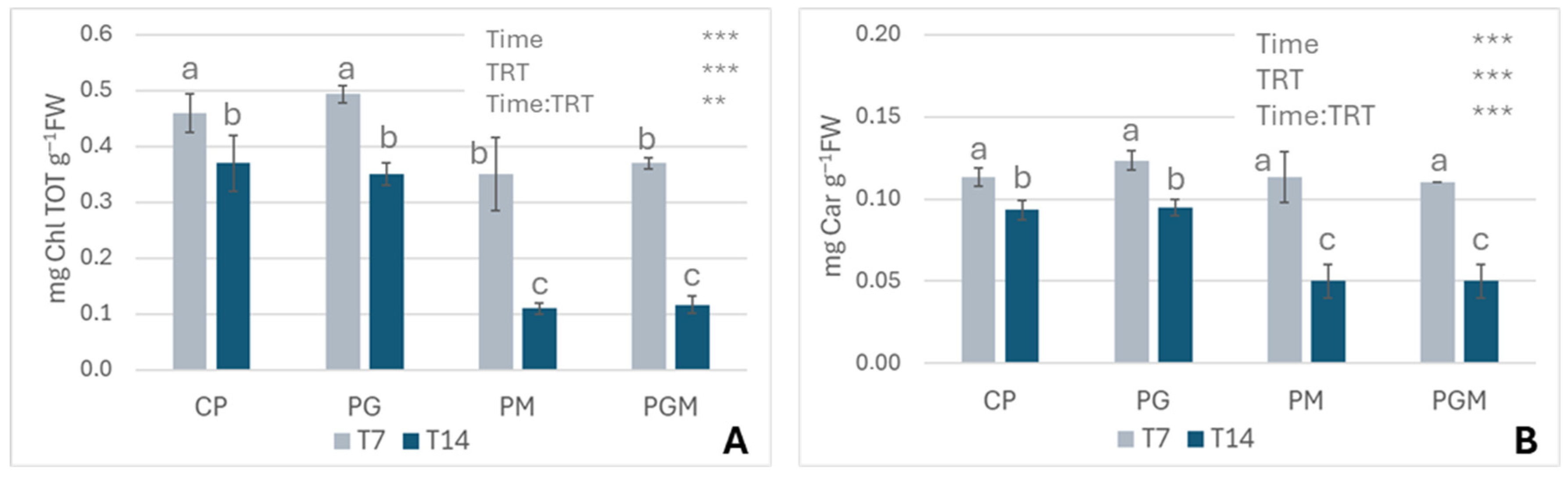
3.1.2. Pigment Contents
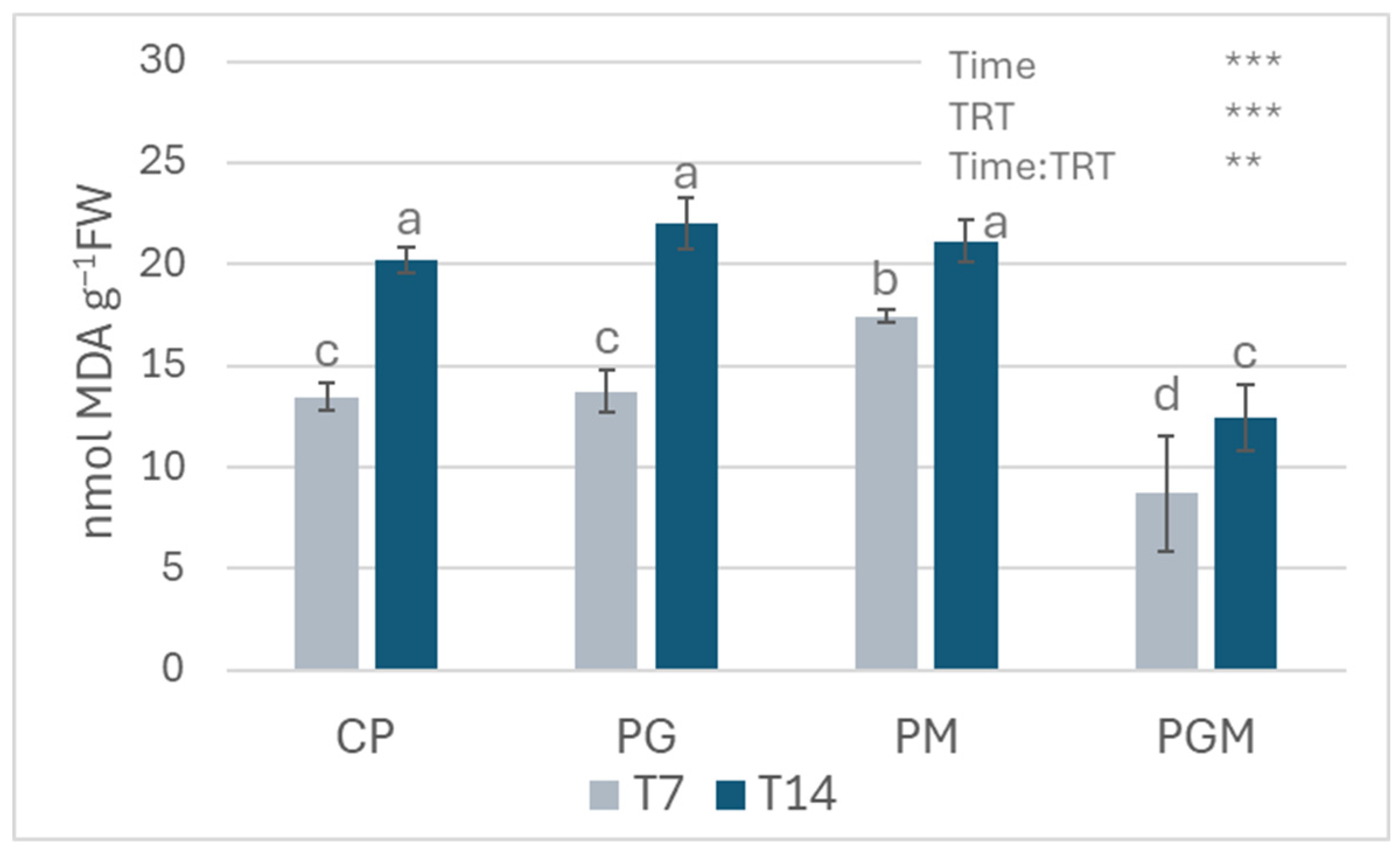
3.1.3. Lipid Peroxidation Measurements
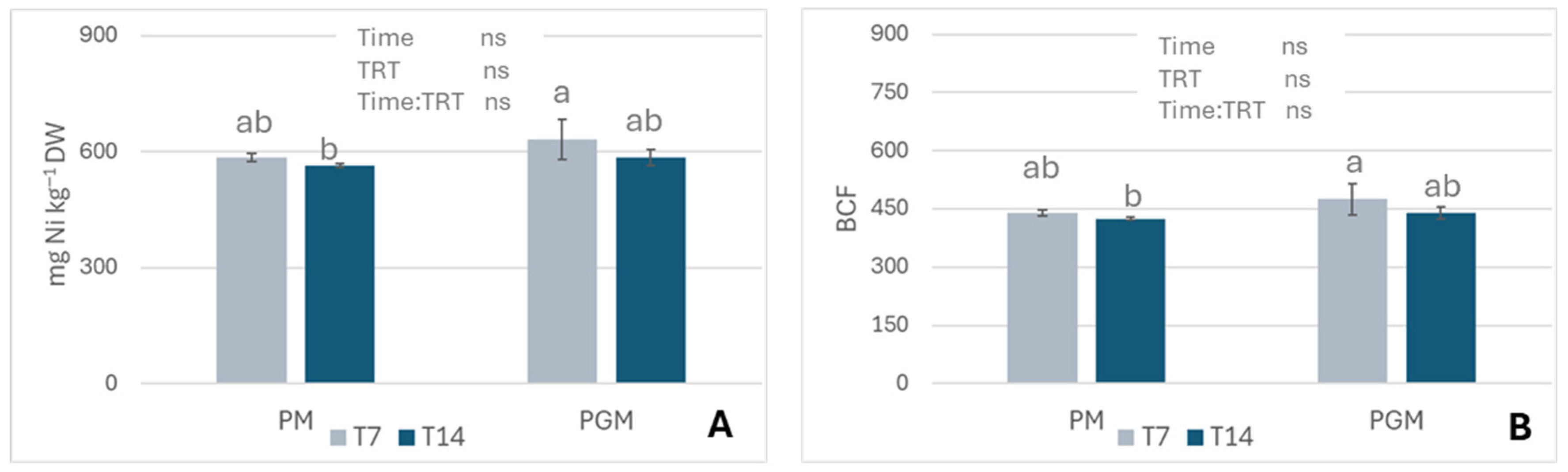
3.1.4. Nickel Uptake and Bioconcentration Factor (BCF)
3.2. Effects of Reduced Graphene Oxide and Copper on L. minor
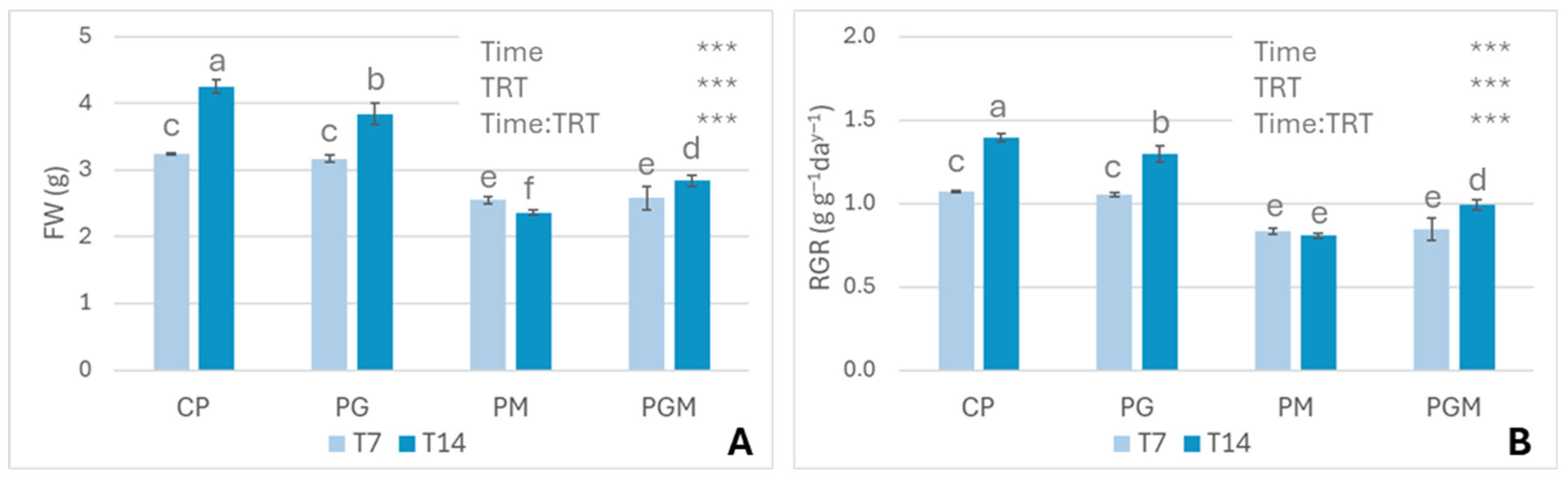
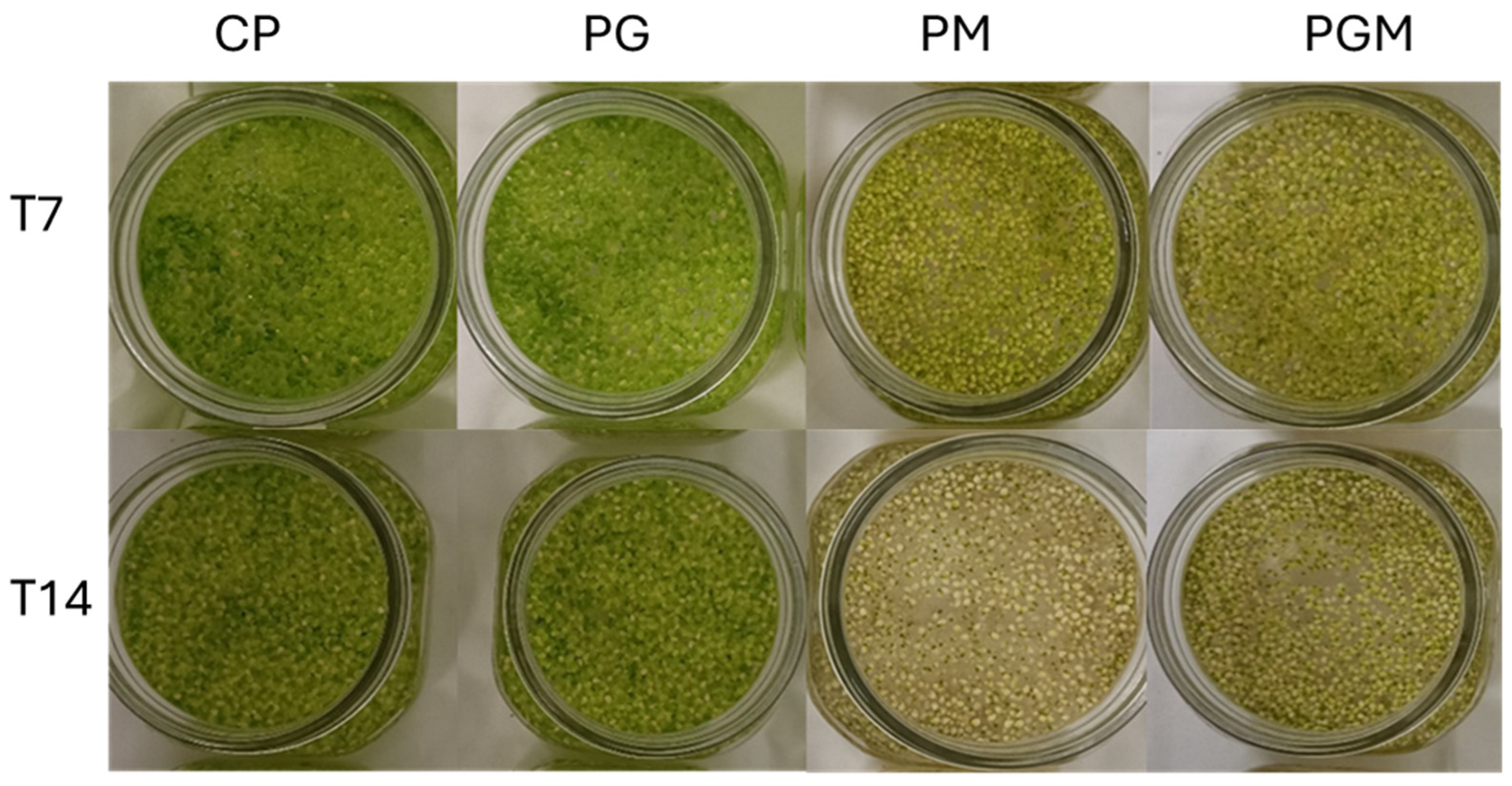
3.2.1. Growth Analysis
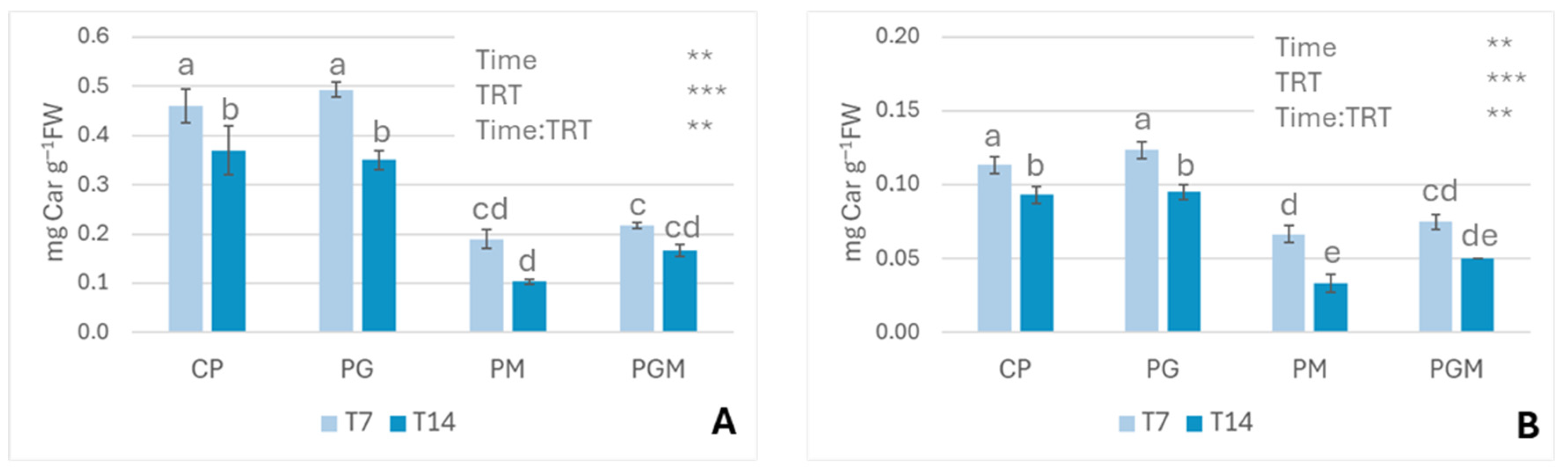
3.2.2. Pigment Contents
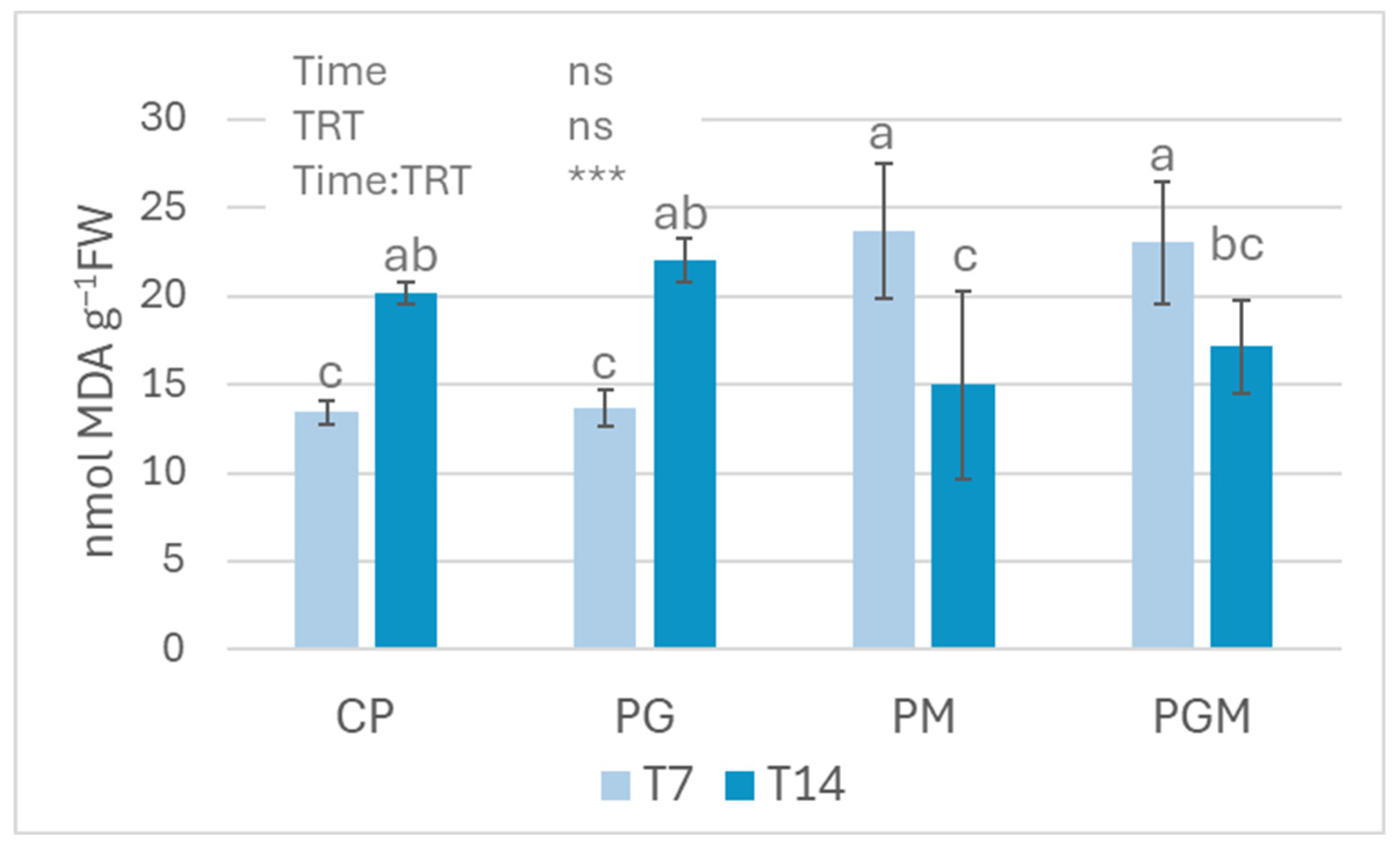
3.2.3. Lipid Peroxidation Measurements
3.2.4. Copper Uptake and Bioconcentration Factor (BCF)
3.2.5. Oxidative Stress Response to Reduced Graphene Oxide and Copper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Singh, A.; Young, S.-J.; Gupta, V.; Singh, M.; Arya, S. Synthesis Techniques and Advances in Sensing Applications of Reduced Graphene Oxide (rGO) Composites: A Review. Compos. Part Appl. Sci. Manuf. 2023, 165, 107373. [Google Scholar] [CrossRef]
- Liu, F.; Choi, K.S.; Park, T.J.; Lee, S.Y.; Seo, T.S. Graphene-Based Electrochemical Biosensor for Pathogenic Virus Detection. BioChip J. 2011, 5, 123–128. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Liu, X.; Qian, J.; Zuo, P.; Zhuang, Q. Non-Covalently Modified Graphene@poly (Ionic Liquid) Nanocomposite with High-Temperature Resistance and Enhanced Dielectric Properties. Compos. Part Appl. Sci. Manuf. 2022, 154, 106800. [Google Scholar] [CrossRef]
- Sharma, V.; Mitlin, D.; Datta, D. Understanding the Strength of the Selenium–Graphene Interfaces for Energy Storage Systems. Langmuir 2021, 37, 2029–2039. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, J.-C.; Zhou, H.; Liu, Y.-Q.; Han, D.-D.; Sun, H.-B. Electro-Responsive Actuators Based on Graphene. Innovation 2021, 2, 100168. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, X.; Zhang, W.; Zhou, L.; Xue, W.; Tang, M.; Sun, S. The Aggregation Behaviour and Mechanism of Commercial Graphene Oxide in Surface Aquatic Environments. Sci. Total Environ. 2022, 806, 150942. [Google Scholar] [CrossRef] [PubMed]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya-Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable One-Step Wet-Spinning of Graphene Fibers and Yarns from Liquid Crystalline Dispersions of Graphene Oxide: Towards Multifunctional Textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive Removal of Heavy Metal Ions Using Graphene-Based Nanomaterials: Toxicity, Roles of Functional Groups and Mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Sun, B.; Liu, X.; Zhang, T.; Ma, Y.; Zhao, S. Concentration-Dependent Effects of Humic Acid and Protein on the Stability of Hematite Nanoparticles in an Aqueous Environment. J. Nanoparticle Res. 2023, 25, 109. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Hong, H.; Nowack, B. Form-Specific Prospective Environmental Risk Assessment of Graphene-Based Materials in European Freshwater. Environ. Sci. Technol. 2024, 58, 21750–21759. [Google Scholar] [CrossRef]
- Graphene-Based Materials Functionalization with Natural Polymeric Biomolecules|IntechOpen. Available online: https://www.intechopen.com/chapters/51598 (accessed on 31 July 2025).
- Sarmiento, V.; Lockett, M.; Sumbarda-Ramos, E.G.; Vázquez-Mena, O. Effective Removal of Metal Ion and Organic Compounds by Non-Functionalized rGO. Molecules 2023, 28, 649. [Google Scholar] [CrossRef]
- Kumar, P.; Das, S. Efficient Adsorption of Toxic Heavy Metal Ions on the Surface Engineered Violuric Acid-Reduced Graphene Oxide Nanomaterial. ACS Appl. Eng. Mater. 2023, 1, 1343–1355. [Google Scholar] [CrossRef]
- Yang, X.; Liu, P.; Yu, H. Adsorption of Heavy Metals from Wastewater Using Reduced Graphene Oxide@titanate Hybrids in Batch and Fixed Bed Systems. BMC Chem. 2025, 19, 72. [Google Scholar] [CrossRef]
- Song, C.; Yang, C.-M.; Sun, X.-F.; Xia, P.-F.; Qin, J.; Guo, B.-B.; Wang, S.-G. Influences of Graphene Oxide on Biofilm Formation of Gram-Negative and Gram-Positive Bacteria. Environ. Sci. Pollut. Res. 2018, 25, 2853–2860. [Google Scholar] [CrossRef]
- Yang, H.; Feng, S.; Ma, Q.; Ming, Z.; Bai, Y.; Chen, L.; Yang, S.-T. Influence of Reduced Graphene Oxide on the Growth, Structure and Decomposition Activity of White-Rot Fungus Phanerochaete chrysosporium. RSC Adv. 2018, 8, 5026–5033. [Google Scholar] [CrossRef]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and Toxicity of Radio-Labeled Few Layer Graphene in Mice after Intratracheal Instillation. Part. Fibre Toxicol. 2016, 13, 7. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Graphene Phytotoxicity in the Seedling Stage of Cabbage, Tomato, Red Spinach, and Lettuce. Carbon 2011, 49, 3907–3919. [Google Scholar] [CrossRef]
- Christudoss, A.C.; Sah, K.K.; Vikram, R.; Giri, S.; Viswanathan, D.; Dimkpa, C.O.; Mukherjee, A. Tailoring the Synthesis Route of Reduced Graphene Oxide and Its Toxicological Effects on Allium cepa L. ACS Omega 2025, 10, 20771–20783. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Liu, L.; Chen, C.; Sha, A.; Li, J. Effects of Three Graphene-Based Materials on the Growth and Photosynthesis of Brassica napus L. Ecotoxicol. Environ. Saf. 2022, 234, 113383. [Google Scholar] [CrossRef]
- Du, S.; Zhang, P.; Zhang, R.; Lu, Q.; Liu, L.; Bao, X.; Liu, H. Reduced Graphene Oxide Induces Cytotoxicity and Inhibits Photosynthetic Performance of the Green Alga Scenedesmus obliquus. Chemosphere 2016, 164, 499–507. [Google Scholar] [CrossRef]
- Gamoń, F.; Ziembińska-Buczyńska, A.; Łukowiec, D.; Tomaszewski, M. Ecotoxicity of Selected Carbon-Based Nanomaterials. Int. J. Environ. Sci. Technol. 2023, 20, 10153–10162. [Google Scholar] [CrossRef]
- Zhan, Q.; Ahmad, A.; Arshad, H.; Yang, B.; Chaudhari, S.K.; Batool, S.; Hasan, M.; Feng, G.; Mustafa, G.; Hatami, M. The Role of Reduced Graphene Oxide on Mitigation of Lead Phytotoxicity in Triticum aestivum L. Plants at Morphological and Physiological Levels. Plant Physiol. Biochem. 2024, 211, 108719. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Bianconi, D.; Massacci, A.; Iannelli, M.A. Combined Effects of Elevated CO2 and Cd-Contaminated Water on Growth, Photosynthetic Response, Cd Accumulation and Thiolic Components Status in Lemna minor L. J. Hazard. Mater. 2016, 309, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Wani, G.A.; Majid, H.; Farooq, F.U.; Reshi, Z.A.; Husaini, A.M.; Shah, M.A. Differential Bioaccumulation of Select Heavy Metals from Wastewater by Lemna Minor. Bull. Environ. Contam. Toxicol. 2020, 105, 777–783. [Google Scholar] [CrossRef]
- Iannelli, M.A.; Bellini, A.; Venditti, I.; Casentini, B.; Battocchio, C.; Scalici, M.; Ceschin, S. Differential Phytotoxic Effect of Silver Nitrate (AgNO3) and Bifunctionalized Silver Nanoparticles (AgNPs-Cit-L-Cys) on Lemna Plants (Duckweeds). Aquat. Toxicol. 2022, 250, 106260. [Google Scholar] [CrossRef]
- Carioti, V.; Savio, S.; Fabriani, M.; Ellwood, N.T.W.; Gemin, L.; Congestri, R.; Iannelli, M.A.; Ceschin, S. Nickel Tolerance and Phytoremediation Potential of the Aquatic Plant Lemna Minuta and the Cyanobacterium Trichormus variabilis in Monoculture and Consortium. Aquat. Bot. 2025, 200, 103888. [Google Scholar] [CrossRef]
- Sasmaz, A.; Obek, E. The Accumulation of Silver and Gold in Lemna gibba L. Exposed to Secondary Effluents. Geochemistry 2012, 72, 149–152. [Google Scholar] [CrossRef]
- Domingo, G.; Bracale, M.; Vannini, C. Chapter 8—Phytotoxicity of Silver Nanoparticles to Aquatic Plants, Algae, and Microorganisms. In Nanomaterials in Plants, Algae and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–168. ISBN 978-0-12-811488-9. [Google Scholar]
- OECD 2006. Test No. 221: Lemna sp. Growth Inhibition Test. Available online: https://www.oecd.org/en/publications/test-no-221-lemna-sp-growth-inhabition-test_9789264016194-en.html (accessed on 10 October 2025).
- Li, X.; Gu, A.Z.; Zhang, Y.; Xie, B.; Li, D.; Chen, J. Sub-Lethal Concentrations of Heavy Metals Induce Antibiotic Resistance via Mutagenesis. J. Hazard. Mater. 2019, 369, 9–16. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Tong, Y.W. Chapter 2—Phytoremediation of Metals: Bioconcentration and Translocation Factors. In Current Developments in Biotechnology and Bioengineering; Sharma, P., Pandey, A., Tong, Y.W., Ngo, H.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–37. ISBN 978-0-323-99907-6. [Google Scholar]
- Radford, P.J. Growth Analysis Formulae—Their Use and Abuse. Crop Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. [51] Assays for Differentiation of Glutathione S-Transferases. In Methods in Enzymology; Detoxication and Drug Metabolism: Conjugation and Related Systems; Academic Press: Cambridge, MA, USA, 1981; Volume 77, pp. 398–405. [Google Scholar]
- Yruela, I. Copper in Plants: Acquisition, Transport and Interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Malina, T.; Lamaczová, A.; Maršálková, E.; Zbořil, R.; Maršálek, B. Graphene Oxide Interaction with Lemna Minor: Root Barrier Strong Enough to Prevent Nanoblade-Morphology-Induced Toxicity. Chemosphere 2022, 291, 132739. [Google Scholar] [CrossRef] [PubMed]
- Goswami, C.; Majumder, A. Potential of Lemna Minor in Ni and Cr Removal from Aqueous Solution. Pollution 2015, 1, 373–385. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Growth Response of the Duckweed Lemna gibba L. to Copper and Nickel Phytoaccumulation. Ecotoxicology 2010, 19, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, L.; Li, X.; Xu, Y.; Ge, Z.; Zhao, Y. Effect of Graphene Oxide on Copper Stress in Lemna minor L.: Evaluating Growth, Biochemical Responses, and Nutrient Uptake. J. Hazard. Mater. 2018, 341, 168–176. [Google Scholar] [CrossRef]
- Mukhtar, S.M.; HAQ Bhatti, H.N.; Khalid, M.; Haq, M.A.; Shahzad, S.M. Potential of Sunflower (Helianthus annuus L.) for Phytoremediation of Nickle (Ni) and Lead (Pb) Contaminated Water. Pak. J. Bot. 2010, 42, 4017–4026. [Google Scholar]
- Kazemi, N.; Khavari-Nejad, R.A.; Fahimi, H.; Saadatmand, S.; Nejad-Sattari, T. Effects of Exogenous Salicylic Acid and Nitric Oxide on Lipid Peroxidation and Antioxidant Enzyme Activities in Leaves of Brassica napus L. under Nickel Stress. Sci. Hortic. 2010, 126, 402–407. [Google Scholar] [CrossRef]
- Chen, J.; Mu, Q.; Tian, X. Phytotoxicity of Graphene Oxide on Rice Plants Is Concentration-Dependent. Mater. Express 2019, 9, 635–640. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Axtell, N.R.; Sternberg, S.P.K.; Claussen, K. Lead and Nickel Removal Using Microspora and Lemna minor. Bioresour. Technol. 2003, 89, 41–48. [Google Scholar] [CrossRef]
- Kanoun-Boulé, M.; Vicente, J.A.F.; Nabais, C.; Prasad, M.N.V.; Freitas, H. Ecophysiological Tolerance of Duckweeds Exposed to Copper. Aquat. Toxicol. 2009, 91, 1–9. [Google Scholar] [CrossRef]
- Malec, P.; Maleva, M.; Prasad, M.N.V.; Strzałka, K. Copper Toxicity in Leaves of Elodea Canadensis Michx. Bull. Environ. Contam. Toxicol. 2009, 82, 627–632. [Google Scholar] [CrossRef]
- You, Y.; Liu, L.; Wang, Y.; Li, J.; Ying, Z.; Hou, Z.; Liu, H.; Du, S. Graphene Oxide Decreases Cd Concentration in Rice Seedlings but Intensifies Growth Restriction. J. Hazard. Mater. 2021, 417, 125958. [Google Scholar] [CrossRef]
- Mottier, A.; Légnani, M.; Candaudap, F.; Flahaut, E.; Mouchet, F.; Gauthier, L.; Evariste, L. Graphene Oxide Worsens Copper-Mediated Embryo-Larval Toxicity in the Pacific Oyster While Reduced Graphene Oxide Mitigates the Effects. Chemosphere 2023, 335, 139140. [Google Scholar] [CrossRef]
- Ben Amor, N.; Ben Hamed, K.; Debez, A.; Grignon, C.; Abdelly, C. Physiological and Antioxidant Responses of the Perennial Halophyte Crithmum maritimum to Salinity. Plant Sci. 2005, 168, 889–899. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving Abiotic Stress Tolerance in Plants through Antioxidative Defense Mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shi, H.; Duan, D.; Li, H.; Lei, T.; Wang, M.; Zhao, H.; Zhao, Y. The Influence of Duckweed Species Diversity on Ecophysiological Tolerance to Copper Exposure. Aquat. Toxicol. 2015, 164, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS Signaling: The New Wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Dobritzsch, D.; Grancharov, K.; Hermsen, C.; Krauss, G.-J.; Schaumlöffel, D. Inhibitory Effect of Metals on Animal and Plant Glutathione Transferases. J. Trace Elem. Med. Biol. 2020, 57, 48–56. [Google Scholar] [CrossRef]





| CAT | GST | |||
|---|---|---|---|---|
| nmol H2O2 min−1 g−1 FW | nmol CDNB min−1 g−1 FW | |||
| T7 | T14 | T7 | T14 | |
| CP | 219.6 ± 47.7 d | 226.3 ± 22.5 d | 139.0 ± 13.9 d | 109.6 ± 14.6 d |
| PG | 180.1 ± 50.7 d | 510.2 ± 70.6 ab | 183.1 ± 35.6 bc | 64.8 ± 11.0 e |
| PM | 383.0 ± 83.1 c | 255.0 ± 33.6 d | 253.7 ± 80.3 a | 212.8 ± 63.1 ab |
| PGM | 553.7 ± 36.2 a | 423.3 ± 22.9 bc | 104.9 ± 17.4 de | 149.8 ± 34.5 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Eugenio, M.; Casentini, B.; Iannelli, M.A. Reduced Graphene Oxide Modulates Physiological Responses of Lemna minor Under Environmental Heavy Metal Stress. Environments 2025, 12, 407. https://doi.org/10.3390/environments12110407
D’Eugenio M, Casentini B, Iannelli MA. Reduced Graphene Oxide Modulates Physiological Responses of Lemna minor Under Environmental Heavy Metal Stress. Environments. 2025; 12(11):407. https://doi.org/10.3390/environments12110407
Chicago/Turabian StyleD’Eugenio, Marco, Barbara Casentini, and M. Adelaide Iannelli. 2025. "Reduced Graphene Oxide Modulates Physiological Responses of Lemna minor Under Environmental Heavy Metal Stress" Environments 12, no. 11: 407. https://doi.org/10.3390/environments12110407
APA StyleD’Eugenio, M., Casentini, B., & Iannelli, M. A. (2025). Reduced Graphene Oxide Modulates Physiological Responses of Lemna minor Under Environmental Heavy Metal Stress. Environments, 12(11), 407. https://doi.org/10.3390/environments12110407






