Association of PFAS and Metals with Cardiovascular Disease Risk: Exploring the Mediating Effect of Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Calculation of Dietary Inflammatory Index (DII) Scores
2.3. Statistical Analysis
2.3.1. Descriptive Statistics
2.3.2. Bayesian Kernel Machine Regression
2.3.3. Mediation Analysis
- Their exposure (A) to pollutants like PFAS and metals.
- Their mediator (M), in this case, diet, could influence the relationship between exposure and health.
- Their outcome (Y), such as cardiovascular risk, is what we are ultimately interested in understanding.
- What would an individual’s life look like if they were exposed to lower levels of PFAS and metals?
- Would their cardiovascular risk be lower?
- How much of this effect would be due to dietary changes?
- Ya: The counterfactual outcome Y if exposure A were set to level a.
- Ma: The counterfactual mediator M that would have been observed if exposure A were set to a.
- YaMa*: The counterfactual outcome when exposure is set to a and the mediator is set to the level it would have taken under exposure a*.
3. Results
3.1. Descriptive Statistics of Study Sample
3.2. Linear Regression of Cardiovascular-Related Markers and Key Study Variables
3.3. Spearman Correlation Analysis of Key Study Variables
3.4. Bayesian Kernel Machine Regression Causal Mediation Analysis
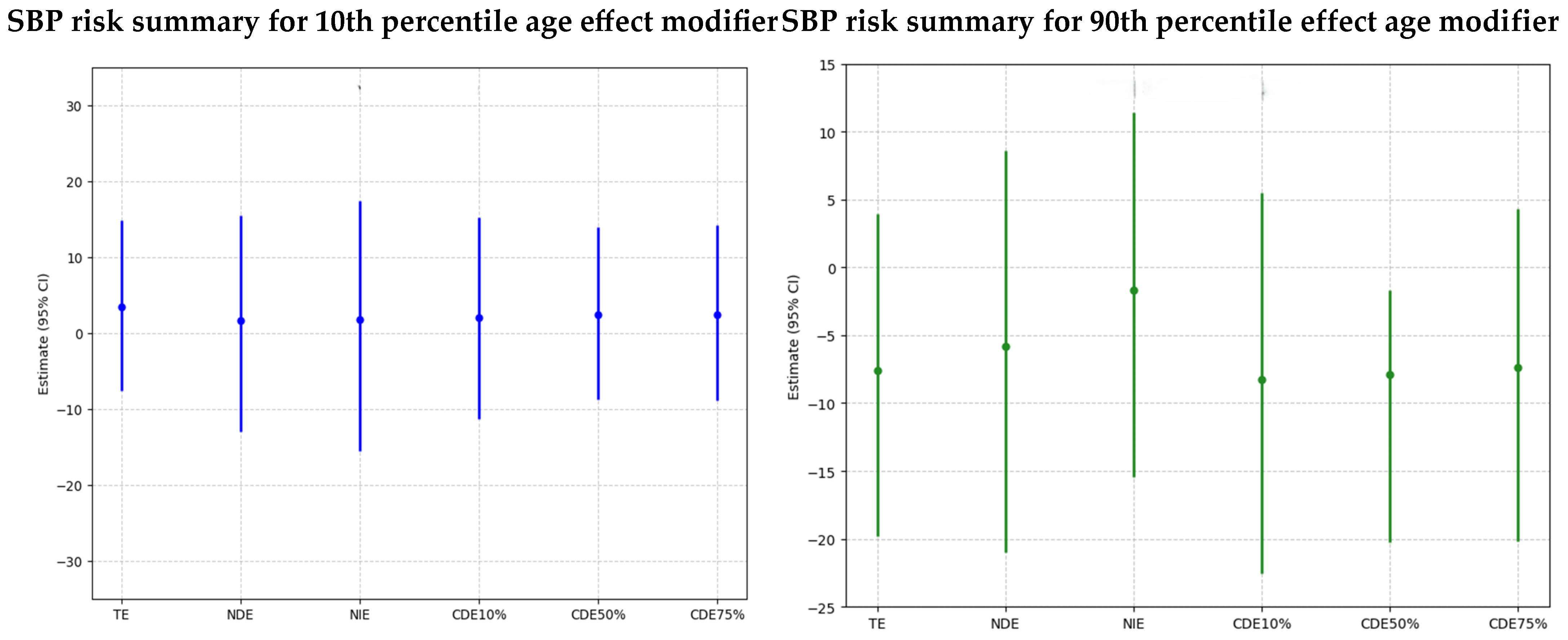
Systolic Blood Pressure
- Younger Age Group (10th Percentile): For younger individuals, the combined exposure to PFAS and metals has varying effects that become more positive at higher exposure levels for younger individuals. The plot shows an increasing trend from negative to slightly positive values across quantiles, but large credible intervals that cross zero emphasize the uncertainty in the results.
- Older Age Group (90th Percentile): For older individuals, the combined exposure to PFAS and metals shows a consistent negative association with SBP at higher exposure levels for older individuals suggesting the negative effects are at lower quantiles of exposure. The wide credible intervals that cross zero indicate an elevated level of uncertainty in this group.
- TE (Total Effect): The total effect, combining both direct and mediated pathways, shows a minor positive effect in SBP associated with exposure in younger individuals, but this effect is not strong, with the large credible interval crossing zero, emphasizing the uncertainty in the results.
- NDE (Natural Direct Effect): This reflects the direct impact of PFAS and metals on SBP, independent of the DII. The slightly positive estimate suggests a direct association between PFAS/metals and SBP, though the effect is weak and uncertain due to an overlapping credible interval that crosses zero.
- NIE (Natural Indirect Effect): This shows the impact of PFAS and metals on SBP that is mediated through the DII. The estimate is close to zero, suggesting that, for younger individuals, the inflammatory diet does not mediate the relationship between PFAS/metals and SBP.
- CDEs at Different Quantiles of DII (10%, 50%, 75%): The Controlled Direct Effects remain close to zero or slightly positive, indicating that, even when controlling for specific levels of DII, PFAS, and metals, they do not have a strong impact on SBP in younger individuals. Additionally, the large, credible intervals, which also cross zero, highlight the uncertainty in the results.
- TE (Total Effect): The total effect of PFAS and metals on SBP is negative, suggesting a potential reduction in SBP with exposure. Nevertheless, the credible interval crosses zero, highlighting the uncertainty in the results.
- NDE (Natural Direct Effect): The negative direct effect estimate suggests a higher PFAS/metals lower SBP. The slightly larger magnitude of NDE indicates that the observed effect might be due to the direct pathway. The credible interval is large and crosses zero, highlighting the results’ uncertainty.
- NIE (Natural Indirect Effect): The indirect effect is negative, showing that DII negatively and minimally mediates the impact of PFAS and metals on SBP for older individuals. Additionally, the credible interval is large and crosses zero, highlighting the results’ uncertainty.
- CDEs at Different Quantiles of DII (10%, 50%, 75%): The Controlled Direct Effects show a negative trend, with effects being slightly negative, indicating that exposure effects on SBP are negative in older individuals across PFAS/metal exposure levels. Additionally, the credible intervals are large and cross zero, highlighting the uncertainty in the results.
4. Discussion
4.1. The Mediating Role of Diet
4.2. Limitations
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharya, S.; Prescott, G.; Iversen, L.; Campbell, D.M.; Smith, W.C.; Hannaford, P.C. Hypertensive Disorders of Pregnancy and Future Health and Mortality: A Record Linkage Study. Pregnancy Hypertens. 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jafri, K.; Bartels, C.M.; Shin, D.B.; Gelfand, J.M.; Ogdie, A. Incidence and Management of Cardiovascular Risk Factors in Psoriatic Arthritis and Rheumatoid Arthritis: A Population-Based Study. Arthritis Care Res. 2016, 69, 51–57. [Google Scholar] [CrossRef]
- Violán, C.; Bejarano-Rivera, N.; Foguet-Boreu, Q.; Roso-Llorach, A.; Pons-Vigués, M.; Mateo, M.M.; Pujol-Ribera, E. The Burden of Cardiovascular Morbidity in a European Mediterranean Population with Multimorbidity: A Cross-Sectional Study. BMC Fam. Pract. 2016, 17, 150. [Google Scholar] [CrossRef]
- Szuba, A.; Martynowicz, H.; Zatońska, K.; Ilow, R.; Regulska−Ilow, B.; Różańska, D.; Wołyniec, M.; Połtyn–Zaradna, K.; Zatoński, W. Prevalence of Hypertension in Polish Population of PURE Poland Study. J. Health Inequal. 2016, 2, 157–162. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, X.; Li, T.; Lyu, C.; Li, J.; Liu, H. Clinical Characteristics and Predictors for Cardiovascular System Involvement in Patients with Behçet’s Disease. Clin. Exp. Rheumatol. 2022, 41, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Chen, G.; Walker, R.L.; Wielgosz, A.; Dai, S.; Tu, K.; Campbell, N.R.; Hemmelgarn, B.R.; Hill, M.D.; Johansen, H.; et al. Incidence, Cardiovascular Complications and Mortality of Hypertension by Sex and Ethnicity. Heart 2013, 99, 715–721. [Google Scholar] [CrossRef]
- Ghoreishian, H.; Tohidi, M.; Derakhshan, A.; Hajsheikholeslami, F.; Azizi, F.; Kazempour-Ardebili, S.; Hadaegh, F. Presence of Hypertension Modifies the Impact of Insulin Resistance on Incident Cardiovascular Disease in a Middle Eastern Population: The Tehran Lipid and Glucose Study. Diabet. Med. 2015, 32, 1311–1318. [Google Scholar] [CrossRef]
- Boafo, Y.S.; Mostafa, S.; Obeng-Gyasi, E. Association of Combined Metals and PFAS with Cardiovascular Disease Risk. Toxics 2023, 11, 979. [Google Scholar] [CrossRef]
- Feng, X.; Long, G.; Zeng, G.; Zhang, Q.; Song, B.; Wu, K.-H. Association of increased risk of cardiovascular diseases with higher levels of perfluoroalkylated substances in the serum of adults. Environ. Sci. Pollut. Res. 2022, 29, 89081–89092. [Google Scholar] [CrossRef]
- Liu, F.; Chen, X.; Liu, Y.; Niu, Z.; Tang, H.; Mao, S.; Li, N.; Chen, G.; Xiang, H. Serum Cardiovascular-Related Metabolites Disturbance Exposed to Different Heavy Metal Exposure Scenarios. J. Hazard. Mater. 2021, 415, 125590. [Google Scholar] [CrossRef]
- Planchart, A.; Green, A.; Hoyo, C.; Mattingly, J. Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies. Curr. Environ. Health Rep. 2018, 5, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.K.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental Toxic Metal Contaminants and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Gong, T.; Liang, P. Heavy metal exposure and cardiovascular disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.; He, Y.; Huang, Y.-H.; Stecker, E.C.; Seixas, A.; Thosar, S.S. Cardiovascular Disease Risk: It Is Complicated, but Race and Ethnicity Are Key, a Bayesian Network Analysis. Front. Public Health 2024, 12, 1364730. [Google Scholar] [CrossRef]
- Kim, I.-G.; Hong, S.; Yim, S.; Jeong, J.-H.; Choi, K.; Lee, J.H.; Hong, Y.S.; Eom, S.Y.; Kim, H.; Kim, Y.D. Sex-Specific Effects of Combined Heavy Metal Exposure on Blood Pressure: A Bayesian Kernel Machine Regression Analysis. Atmosphere 2024, 15, 1157. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Guo, S.-F.; Ma, Y.; Zhang, Y. Associations Between Patterns of Blood Heavy Metal Exposure and Health Outcomes: Insights from NHANES 2011–2016. BMC Public Health 2024, 24, 558. [Google Scholar] [CrossRef]
- Cousins, I.T.; Ng, C.A.; Wang, Z.; Scheringer, M. Why is high persistence alone a major cause of concern? Environ. Sci. Process. Impacts 2019, 21, 781–792. [Google Scholar] [CrossRef]
- Chisolm, J.J. Poisoning from heavy metals (mercury, lead, and cadmium). Pediatr. Ann. 1980, 9, 28–42. [Google Scholar] [CrossRef]
- Zhang, M.; Qing, W.; Xin, S.; Mukhtiar, A.; Zhiwen, T.; Xin, L.; Zhang, Z.; Shurong, M.; Jinhui, B.; Zhongyuan, L. Co-occurrence of per-and polyfluoroalkyl substances, heavy metals and polycyclic aromatic hydrocarbons and their composite impacts on microbial consortium in soil: A field study. Pedosphere 2024, 34, 736–748. [Google Scholar] [CrossRef]
- Cai, W.; Navarro, D.A.; Du, J.; Srivastava, P.; Cao, Z.; Ying, G.; Kookana, R.S. Effect of heavy metal co-contaminants on the sorption of thirteen anionic per-and poly-fluoroalkyl substances (PFAS) in soils. Sci. Total Environ. 2023, 905, 167188. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Brown, H.M.; Thompson, N.I.; Matthew, E.A. The Effect of Heavy Metal Contaminants in Some Herbal Cosmetics on Cardiovascular Risk Indices of Rabbits. J. Adv. Med. Pharm. Sci. 2023, 25, 32–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, G.; Sang, N. Heavy Metals Bound to Fine Particulate Matter from Northern China Induce Season-Dependent Health Risks: A Study Based on Myocardial Toxicity. Environ. Pollut. 2016, 216, 380–390. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Oh, H.; Kim, M.S. Effects of Antioxidant Vitamins, Curry Consumption, and Heavy Metal Levels on Metabolic Syndrome with Comorbidities: A Korean Community-Based Cross-Sectional Study. Antioxidants 2021, 10, 808. [Google Scholar] [CrossRef]
- Pizzino, G.; Bitto, A.; Interdonato, M.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; Luca, F.D.; Minutoli, L.; et al. Oxidative Stress and DNA Repair and Detoxification Gene Expression in Adolescents Exposed to Heavy Metals Living in the Milazzo-Valle Del Mela Area (Sicily, Italy). Redox Biol. 2014, 2, 686–693. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality—A Meta-Analysis. Nutrients 2018, 10, 200. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Dailey, A.B.; Kearney, G.D.; Talbott, E.O.; Cook, R.L. Potential Health Impacts of Heavy Metals on HIV-Infected Population in USA. PLoS ONE 2013, 8, e74288. [Google Scholar] [CrossRef]
- Labaronne, E.; Pinteur, C.; Vega, N.; Pesenti, S.; Julien, B.; Meugnier-Fouilloux, E.; Vidal, H.; Naville, D.; Le Magueresse-Battistoni, B. Low-dose pollutant mixture triggers metabolic disturbances in female mice leading to common and specific features as compared to a high-fat diet. J. Nutr. Biochem. 2017, 45, 83–93. [Google Scholar] [CrossRef]
- Centers-for-Disease-Control-and-Prevention-(NHANES). NHANES 2017–2018 Laboratory Methods. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2017 (accessed on 26 May 2025).
- Hebert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Devick, K.L.; Bobb, J.F.; Mazumdar, M.; Claus Henn, B.; Bellinger, D.C.; Christiani, D.C.; Wright, R.O.; Williams, P.L.; Coull, B.A.; Valeri, L. Bayesian kernel machine regression-causal mediation analysis. Stat. Med. 2022, 41, 860–876. [Google Scholar] [CrossRef]
- Houston, M.C. The Role of Mercury in Cardiovascular Disease. J. Cardiovasc. Dis. Diagn. 2014, 2, 170. [Google Scholar] [CrossRef]
- Pan, X.-F.; Marklund, M.; Wu, J.H. Fish consumption for cardiovascular health: Benefits from long-chain omega-3 fatty acids versus potential harms due to mercury. Heart 2019, 105, 1384–1385. [Google Scholar] [CrossRef]
- Zhou, R.; Peng, J.; Zhang, L.; Sun, Y.; Yan, J.; Jiang, H. Association between the dietary inflammatory index and serum perfluoroalkyl and polyfluoroalkyl substance concentrations: Evidence from NANHES 2007–2018. Food Funct. 2024, 15, 7375–7386. [Google Scholar] [CrossRef]
- Omoike, O.E.; Pack, R.P.; Mamudu, H.M.; Liu, Y.; Strasser, S.; Zheng, S.; Okoro, J.; Wang, L. Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ. Res. 2021, 196, 110361. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E.; Obeng-Gyasi, B. Association of combined lead, cadmium, and mercury with systemic inflammation. Front. Public Health 2024, 12, 1385500. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Rathod, D.; Ho, W.-C.; Caffrey, J.J. Cadmium Exposure Is Associated with Elevated Blood C-Reactive Protein and Fibrinogen in the U. S. Population: The Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). Ann. Epidemiol. 2009, 19, 592–596. [Google Scholar] [CrossRef]
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a mixture of heavy metals in blood and urine and all-cause, cardiovascular disease and cancer mortality: A population-based cohort study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hebert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Buhari, O.; Dayyab, F.; Igbinoba, O.; Atanda, A.; Medhane, F.; Faillace, R. The association between heavy metal and serum cholesterol levels in the US population: National Health and Nutrition Examination Survey 2009–2012. Hum. Exp. Toxicol. 2020, 39, 355–364. [Google Scholar] [CrossRef]
- Kim, D.-w.; Ock, J.; Moon, K.-W.; Park, C.-H. Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017. Int. J. Environ. Res. Public Health 2022, 19, 3181. [Google Scholar] [CrossRef]
- De Toni, L.; Radu, C.M.; Sabovic, I.; Di Nisio, A.; Dall’Acqua, S.; Guidolin, D.; Spampinato, S.; Campello, E.; Simioni, P.; Foresta, C. Increased Cardiovascular Risk Associated with Chemical Sensitivity to Perfluoro–Octanoic Acid: Role of Impaired Platelet Aggregation. Int. J. Mol. Sci. 2020, 21, 399. [Google Scholar] [CrossRef]
- Jia, C.; Anderson, J.L.C.; Gruppen, E.G.; Lei, Y.; Bakker, S.J.L.; Dullaart, R.P.F.; Tietge, U.J.F. High-Density Lipoprotein Anti-Inflammatory Capacity and Incident Cardiovascular Events. Circulation 2021, 143, 1935–1945. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Shi, P.; Morris, J.S.; Spiegelman, D.; Grandjean, P.; Siscovick, D.S.; Willett, W.C.; Rimm, E.B. Mercury Exposure and Risk of Cardiovascular Disease in Two U.S. Cohorts. N. Engl. J. Med. 2011, 364, 1116–1125. [Google Scholar] [CrossRef]
- Turunen, A.W.; Jula, A.; Suominen, A.L.; Männistö, S.; Marniemi, J.; Kiviranta, H.; Tiittanen, P.; Karanko, H.; Moilanen, L.; Nieminen, M.S.; et al. Fish consumption, omega-3 fatty acids, and environmental contaminants in relation to low-grade inflammation and early atherosclerosis. Environ. Res. 2013, 120, 43–54. [Google Scholar] [CrossRef]
- Daniele, N.D.; Petramala, L.; Renzo, L.D.; Sarlo, F.; Rocca, D.G.D.; Rizzo, M.; Fondacaro, V.; Iacopino, L.; Pepine, C.J.; Lorenzo, A.D. Body Composition Changes and Cardiometabolic Benefits of a Balanced Italian Mediterranean Diet in Obese Patients with Metabolic Syndrome. Acta Diabetol. 2012, 50, 409–416. [Google Scholar] [CrossRef]
- Steinman, B.A.; Vasunilashorn, S.M. Biological Risk of Older Adults with Visual Impairments. J. Nutr. Health Aging 2011, 15, 296–302. [Google Scholar] [CrossRef]
- Nunes, V.S.; da Silva, E.J.; Ferreira, G.d.S.; Assis, S.I.S.d.; Cazita, P.M.; Nakandakare, E.R.; Zago, V.H.d.S.; de Faria, E.C.; Quintão, E.C.R. The Plasma Distribution of Non-cholesterol Sterol Precursors and Products of Cholesterol Synthesis and Phytosterols Depend on HDL Concentration. Front. Nutr. 2022, 9, 723555. [Google Scholar] [CrossRef]
- Stadler, J.T.; Mangge, H.; Rani, A.; Curcic, P.; Herrmann, M.; Prüller, F.; Marsche, G. Low HDL Cholesterol Efflux Capacity Indicates a Fatal Course of COVID-19. Antioxidants 2022, 11, 1858. [Google Scholar] [CrossRef]
- Elshorbagy, H.H.; Ghoname, I.A. High-Sensitivity C-Reactive Protein as a Marker of Cardiovascular Risk in Obese Children and Adolescents. Health 2010, 2, 1078–1084. [Google Scholar] [CrossRef]
- Stavnsbo, M.; Skrede, T.; Aadland, E.; Aadland, K.N.; Chinapaw, M.J.M.; Anderssen, S.A.; Andersen, L.B.; Resaland, G.K. Cardiometabolic Risk Factor Levels in Norwegian Children Compared to International Reference Values: The ASK Study. PLoS ONE 2019, 14, e0220239. [Google Scholar] [CrossRef]
- Odediran, A.; Obeng-Gyasi, E. Association Between Combined Metals and PFAS Exposure with Dietary Patterns: A Preliminary Study. Environments 2024, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; McKay, S.A. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Gaye, B.; Naji, N.B.; Sims, M.; Cuffee, Y.; Ogungbe, O.; Michos, E.D.; Lassale, C.; Sabouret, P.; Jouven, X. Deep Diving Into the Cardiovascular Health Paradox: A Journey Towards Personalized Prevention. Public Health Rev. 2024, 45, 1606879. [Google Scholar] [CrossRef]
- Ozorio, C.P.; Guiloski, I.C.; Helena Cristina Silva de, A.; Martins, C.C.; Sandrini-Neto, L.; Lana, P.d.C. Oxidative Stress and Neurotoxicity in Scolelepis Goodbodyi (Polychaeta, Spionidae) After an Experimental Oil Spill in a Dissipative Sandy Beach. Ocean Coast. Res. 2024, 72, e24012. [Google Scholar] [CrossRef]
- Kannan, A.; Dávila, J.; Gao, L.; Rattan, S.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Maternal High-Fat Diet During Pregnancy with Concurrent Phthalate Exposure Leads to Abnormal Placentation. Sci. Rep. 2021, 11, 16602. [Google Scholar] [CrossRef]


| Variable | Participants (n) | Mean | Standard Deviation (SD) |
|---|---|---|---|
| Age (Years) | 660 | 49.33 | 18.51 |
| BMI (kg/m2) | 660 | 29.80 | 7.79 |
| Lead (µg/dL) | 660 | 1.21 | 1.27 |
| Cadmium (µg/L) | 660 | 0.45 | 0.47 |
| Mercury (µg/L) | 660 | 1.24 | 1.83 |
| PFOA (ng/mL) | 660 | 1.69 | 1.21 |
| PFOS (mg/mL) | 660 | 6.94 | 8.25 |
| DII | 660 | 1.50 | 1.68 |
| SBP (mm Hg) | 660 | 125.01 | 19.15 |
| DBP (mm Hg) | 660 | 71.86 | 12.27 |
| Total Cholesterol (mg/dL) | 660 | 184.00 | 42.19 |
| Triglycerides (mg/dL) | 660 | 103.32 | 62.74 |
| C-Reactive Protein (mg/L) | 660 | 4.48 | 10.65 |
| LDL Cholesterol (mg/dL) | 660 | 109.40 | 36.73 |
| HDL Cholesterol (mg/dL) | 660 | 53.96 | 16.31 |
| SBP | Coefficient | Std. Error | p-Value | 95% Confidence Interval |
|---|---|---|---|---|
| Lead (µg/dL) | 0.604 | 0.530 | 0.255 | −0.436, 1.644 |
| Cadmium (µg/L) | −1.541 | 1.424 | 0.279 | −4.338, 1.255 |
| Mercury (µg/L) | −0.948 | 0.395 | 0.017 | −1.723, −0.172 |
| PFOA (ng/mL) | 1.025 | 0.612 | 0.095 | −0.178, 0.0934 |
| PFOS (mg/mL) | −0.0845 | 0.0906 | 0.352 | −0.262, 0.0934 |
| DII | 0.223 | 0.395 | 0.572 | −0.552, 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odediran, A.; Bollen, K.; Obeng-Gyasi, E. Association of PFAS and Metals with Cardiovascular Disease Risk: Exploring the Mediating Effect of Diet. Environments 2025, 12, 178. https://doi.org/10.3390/environments12060178
Odediran A, Bollen K, Obeng-Gyasi E. Association of PFAS and Metals with Cardiovascular Disease Risk: Exploring the Mediating Effect of Diet. Environments. 2025; 12(6):178. https://doi.org/10.3390/environments12060178
Chicago/Turabian StyleOdediran, Augustina, Kenneth Bollen, and Emmanuel Obeng-Gyasi. 2025. "Association of PFAS and Metals with Cardiovascular Disease Risk: Exploring the Mediating Effect of Diet" Environments 12, no. 6: 178. https://doi.org/10.3390/environments12060178
APA StyleOdediran, A., Bollen, K., & Obeng-Gyasi, E. (2025). Association of PFAS and Metals with Cardiovascular Disease Risk: Exploring the Mediating Effect of Diet. Environments, 12(6), 178. https://doi.org/10.3390/environments12060178







