Ambient Air Quality and Hospital Admissions in Gjakova: A Time Series Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Admissions Data
2.2. Environmental Data
2.3. Statistical Analysis
3. Results
3.1. Impact of Air Pollution
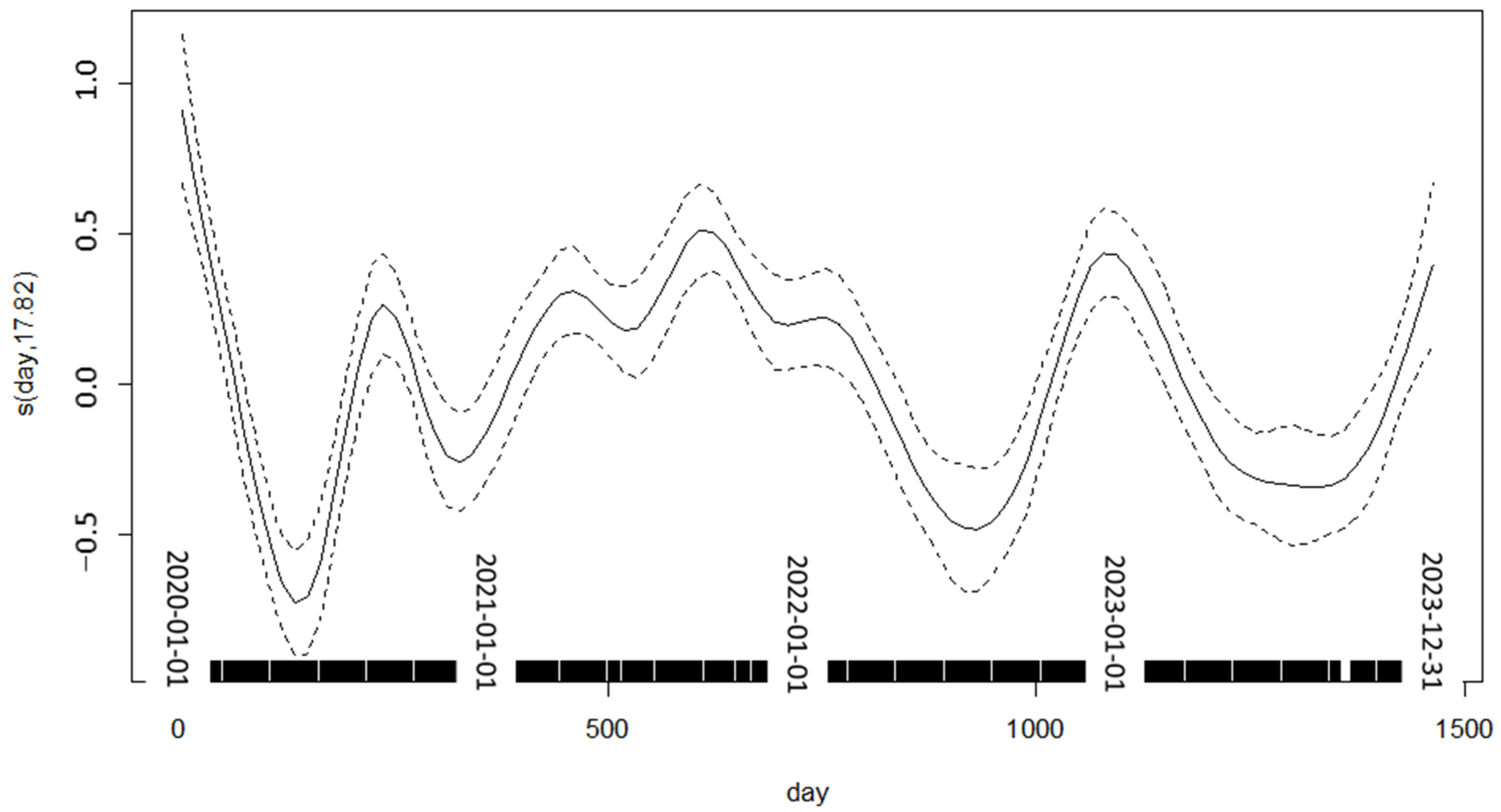
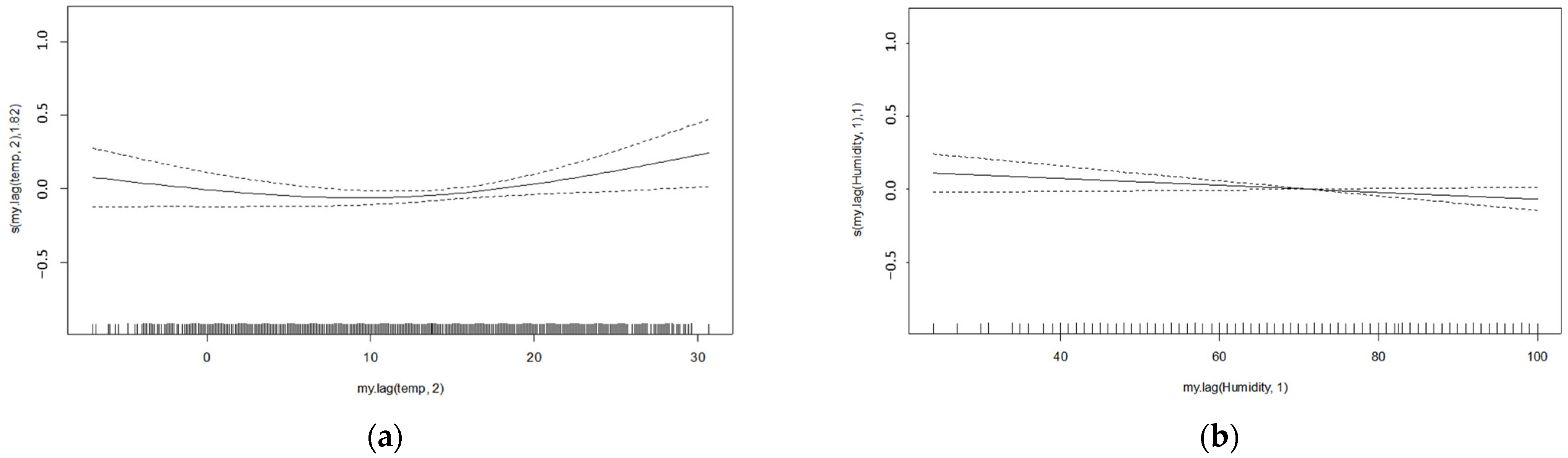
3.1.1. General Additive Model (GAM)
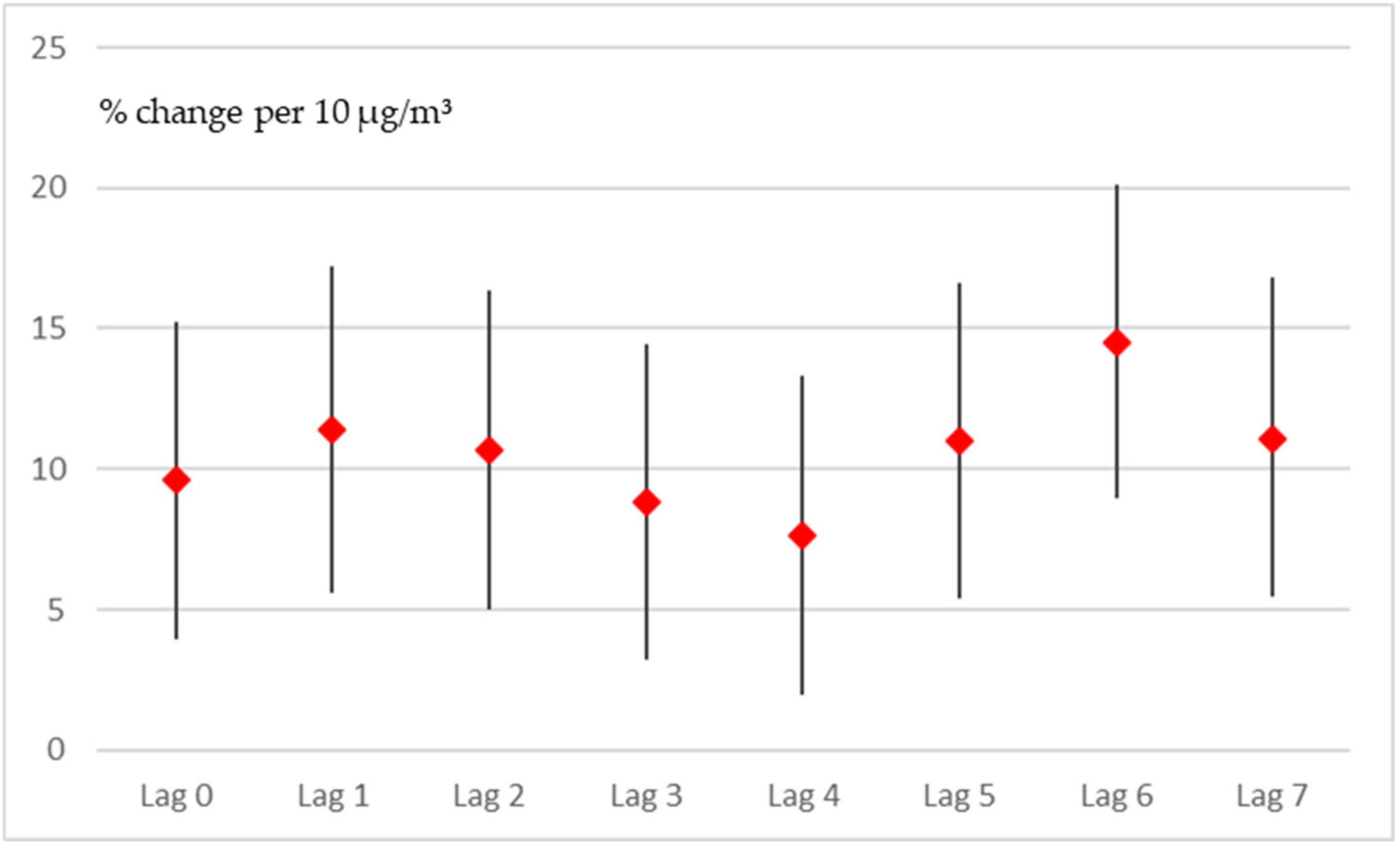
3.1.2. Quasi-Poisson Model
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| PM | Particulate matter |
| PM2.5 | Particulate matter smaller than 2.5 µm in diameter |
| PM10 | Particulate matter smaller than 10 µm in diameter |
| NO2 | Nitrogen dioxide |
| SO2 | Sulphur dioxide |
| O3 | Ozone |
| CO | Carbon oxide |
| ICD-10 | International Classification of Diseases, version 10 |
| KHMI | Kosovo Hydro-Meteorological Institute |
| EEA | European Environment Agency |
| GAM | General Additive Model |
Appendix A
| Pollutant | Pearson’s R | p-Value |
|---|---|---|
| PM10 | 0.8535 | <0.001 |
| PM2.5 | 0.8650 | <0.001 |
| NO2 | 0.7514 | <0.001 |
| O3 | 0.7347 | <0.001 |
| CO | 0.6927 | <0.001 |
| SO2 | 0.3875 | <0.001 |
| Pollutant | PM2.5 | NO2 | O3 | CO | SO2 |
|---|---|---|---|---|---|
| PM10 | 0.9780, <0.001 | 0.7835, <0.001 | −0.4997, <0.001 | 0.7524, <0.001 | 0.1215, <0.001 |
| PM2.5 | 0.7982, <0.001 | −0.5720, <0.001 | 0.8011, <0.001 | 0.0666, 0.0126 | |
| NO2 | −0.6792, <0.001 | 0.7086, <0.001 | 0.1756, <0.001 | ||
| O3 | −0.6189, <0.001 | 0.0418, 0.1193 | |||
| CO | −0.0639, 0.0169 |
| Lag | PM10 | PM2.5 | O3 | CO | SO2 |
|---|---|---|---|---|---|
| Lag 0 | 0.000 (0.974) | 0.005 (0.699) | 0.001 (0.957) | 0.0235 (0.506) | 0.037 (0.419) |
| Lag 1 | 0.005 (0.656) | 0.010 (0.435) | −0.016 (0.241) | −0.0088 (0.814) | 0.063 (0.173) |
| Lag 2 | 0.009 (0.392) | 0.016 (0.197) | −0.018 (0.150) | 0.0290 (0.419) | 0.019 (0.681) |
| Lag 3 | −0.003 (0.731) | −0.004 (0.718) | −0.009 (0.433) | −0.0224 (0.524) | 0.101 (0.027) |
| Lag 4 | −0.003 (0.7899) | −0.003 (0.816) | 0.003 (0.809) | −0.0088 (0.803) | −0.021 (0.647) |
| Lag 5 | 0.014 (0.140) | 0.016 (0.164) | −0.002 (0.867) | 0.0397 (0.246) | 0.040 (0.373) |
| Lag 6 | 0.018 (0.060) | 0.020 (0.087) | 0.005 (0.688) | 0.0634 (0.057) | −0.017 (0.703) |
| Lag 7 | 0.012 (0.241) | 0.011 (0.370) | 0.017 (0.137) | 0.0449 (0.195) | 0.000 (0.994) |
| Knots | Coefficient | Lower | Upper |
|---|---|---|---|
| per 10 µg/m3 | 95% confidence interval | ||
| 10 | 0.114 | 0.070 | 0.159 |
| 15 | 0.108 | 0.062 | 0.154 |
| 20 | 0.115 | 0.068 | 0.161 |
| 25 | 0.091 | 0.043 | 0.138 |
| 30 | 0.062 | 0.015 | 0.110 |
| 35 | 0.066 | 0.017 | 0.115 |
| 40 | 0.065 | 0.016 | 0.114 |
References
- World Health Organization (WHO). WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM 10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO: Geneva, Switzerland, 2021; Available online: https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf?sequence=1 (accessed on 5 March 2025).
- United Nations Environment Programme (UNEP). Towards A Pollution-Free Planet Background Report; UNEP: Nairobi, Kenya, 2017; Available online: http://wedocs.unep.org/bitstream/handle/20.500.11822/21800/UNEA_towardspollution_long%20version_Web.pdf?sequence=1&isAllowed=y (accessed on 5 March 2025).
- United Nations Environment Programme (UNEP). Air Pollution and Human Health: The Case of the Western Balkans; UNEP: Nairobi, Kenya, 2019; Available online: https://www.developmentaid.org/api/frontend/cms/file/2019/06/Air-Quality-and-Human-Health-Report_Case-of-Western-Balkans_preliminary_results.pdf (accessed on 5 March 2025).
- The World Bank. Air Pollution Deaths Cost Global Economy US$225 Billion; World Bank: Washington, DC, USA, 2013; Available online: https://www.worldbank.org/en/news/press-release/2016/09/08/air-pollution-deaths-cost-global-economy-225-billion (accessed on 5 March 2025).
- World Health Organization (WHO). The Global Health Observatory. Air Pollution Data Portal. Available online: https://www.who.int/data/gho/data/themes/air-pollution (accessed on 8 May 2025).
- Statista. Annual Number of Deaths from Select Risk Factors Worldwide in 2021. Available online: https://www.statista.com/statistics/1169367/worldwide-number-deaths-risk-factor/ (accessed on 8 May 2025).
- IQAir. 2019 World Air Quality Report. Region & City PM2.5 Ranking; IQAir: Steinach, Switzerland, 2020; Available online: https://www.iqair.com/dl/pdf-reports/2019-World-Air-Report-V8-20200318.pdf (accessed on 5 March 2025).
- Solomon, P.A.; Costantini, M.; Grahame, T.J.; Gerlofs-Nijland, M.E.; Cassee, F.R.; Russell, A.G.; Brook, J.R.; Hopke, P.K.; Hidy, G.; Phalen, R.F.; et al. Air pollution and health: Bridging the gap from sources to health outcomes: Conference summary. Air Qual. Atmos. Health 2012, 5, 9–62. [Google Scholar] [CrossRef]
- Di Blasi, C.; Nobile, F.; Settembrini, A.M.; Stafoggia, M.; Davoli, M.; Michelozzi, P.; Renzi, M.; Mannucci, P.M. Association between long-term exposure to air pollution and incidence of peripheral artery disease: Evidence from a longitudinal study. Eur. J. Intern. Med. 2025, 132, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Braun, D.; Christidis, T.; Cork, M.; Rodopoulou, S.; Samoli, E.; Stafoggia, M.; Wolf, K.; Wu, X.; Yuchi, W.; et al. Long-Term Exposure to Low-Level PM2.5 and Mortality: Investigation of Heterogeneity by Harmonizing Analyses in Large Cohort Studies in Canada, United States, and Europe. Environ. Health Perspect. 2023, 131, 127003. [Google Scholar] [CrossRef]
- Slama, A.; Śliwczyński, A.; Woźnica, J.; Zdrolik, M.; Wiśnicki, B.; Kubajek, J.; Turżańska-Wieczorek, O.; Gozdowski, D.; Wierzba, W.; Franek, E. Impact of air pollution on hospital admissions with a focus on respiratory diseases: A time-series multi-city analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 16998–17009. [Google Scholar] [CrossRef]
- Brunekreef, B.; Strak, M.; Chen, J.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Boutron, M.-C.; Brandt, J.; Carey, I.; et al. Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project. Res. Rep. (Health Eff. Inst.) 2021, 208, 1–127. [Google Scholar]
- Feng, S.; Li, C.; Jin, Y.; Wang, H.; Wang, R.; Mohamed, Z.A.; Zhang, Y.; Yao, Y. Air pollution and adult hospital admissions for ischemic stroke: A time-series analysis in Inner Mongolia, China. Environ. Health Prev. Med. 2025, 30, 29. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, J.; Zhou, W.; Neophytou, A.M. Interactive Effect of Air Temperature and Fine Particulate Matter on the Hospital Admissions for Stroke in Shenzhen, China. J. Am. Heart Assoc. 2025, 14, e037329. [Google Scholar] [CrossRef]
- Zama, D.; Paccapelo, A.; Betti, L.; Manieri, E.; Paglione, M.; Rinaldi, M.; Dondi, A.; Battelli, E.; Biagi, C.; Rizzolli, C.M.; et al. The influence of air pollutants on the risk of emergency department presentations of infants with bronchiolitis in an European air quality hotspot. Pediatr. Allergy Immunol. 2025, 36, e70077. [Google Scholar] [CrossRef]
- Hales, S.; Atkinson, J.; Metcalfe, J.; Kuschel, G.; Woodward, A. Long term exposure to air pollution, mortality and morbidity in New Zealand: Cohort study. Sci Total Environ. 2021, 801, 149660. [Google Scholar] [CrossRef]
- Gyaase, S.; Nyame, S.; Klipstein-Grobusch, K.; Asante, K.P.; Downward, G.S. Climate, Air Quality and Their Contribution to Cardiovascular Disease Morbidity and Mortality in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Glob. Heart 2025, 20, 35. [Google Scholar] [CrossRef]
- Lin, F.; Li, G.; Wang, Y.; Dong, P.; Yang, K.; Liu, H.; Xie, N.; Liu, J.; Chen, H.; Liu, X.; et al. Impacts of air pollutions on cardiovascular and cerebrovascular diseases through inflammation: A comprehensive analysis of one million Chinese and half million UK individuals. J. Transl. Med. 2025, 23, 469. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Chen, Y.; Sun, X.; Dong, X.; He, G.; Pu, Y.; Fan, J.; Zhong, X.; Chen, Z.; Lin, Z.; et al. Long-term exposure to ambient ozone and cardiovascular diseases: Evidence from two national cohort studies in China. J. Adv. Res. 2024, 62, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Li, P.; Xu, Y.; Zhou, X.; Qiu, J.; Tang, X.; Ding, Z.; Xu, M.; Wang, C. Associations between short-term exposure to air pollution and acute exacerbation of chronic bronchitis: A time-stratified case-crossover study. Prev. Med. 2025, 191, 108217. [Google Scholar] [CrossRef] [PubMed]
- Chi, G.C.; Hajat, A.; Bird, C.E.; Cullen, M.R.; Griffin, B.A.; Miller, K.A.; Shih, R.A.; Stefanick, M.L.; Vedal, S.; Whitsel, E.A.; et al. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ. Health Perspect. 2016, 124, 1840–1847. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality, Energy and Health. Health Impact. Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts (accessed on 5 March 2025).
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. The effects of air pollution on the incidence of myocardial infarction—A systematic review. Heart 2009, 95, 1746–1759. [Google Scholar] [CrossRef]
- Medina-Ramón, M.; Zanobetti, A.; Schwartz, J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: A national multicity study. Am. J. Epidemiol. 2006, 163, 579–588. [Google Scholar] [CrossRef]
- Zare Sakhvidi, M.J.; Lequy, E.; Goldberg, M.; Jacquemin, B. Air Pollution Exposure and Bladder, Kidney and Urinary Tract Cancer Risk: A Systematic Review. Environ. Pollut. 2020, 267, 115328. [Google Scholar] [CrossRef]
- Bekkar, B.; Pacheco, S.; Basu, R.; DeNicola, N. Association of Air Pollution and Heat Exposure with Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw. Open 2020, 3, e208243. [Google Scholar] [CrossRef]
- Alves, C.A.; Scotto, M.G.; do Carmo Freitas, M. Air Pollution and Emergency Admissions for Cardiorespiratory Diseases in Lisbon (Portugal). Quím Nova 2010, 33, 337–344. [Google Scholar] [CrossRef]
- Health Effects Institute. State of Global Air 2024. Special Report; Health Effects Institute: Boston, MA, USA, 2024; Available online: https://www.stateofglobalair.org/sites/default/files/documents/2024-06/soga-2024-report_0.pdf (accessed on 5 March 2025).
- Wjst, M.; Reitmeir, P.; Dold, S.; Nicolai, T.; Von Loeffelholz Colberg, E.; Von Mutius, E. Road traffic and adverse effects on respiratory health in children. BMJ 1993, 307, 596–600. [Google Scholar] [CrossRef]
- Oosterlee, A.; Drijver, M.; Lebret, E.; Brunekreef, B. Chronic respiratory symptoms of children and adults living along streets with high traffic density. Occup. Environ. Med. 1996, 53, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Sato, T.; Nakai, S.; Maeda, K.; Aoki, S.; Ono, M. Respiratory health associated with exposure to automobile exhaust. I: Results of cross-sectional studies in 1979, 1982 and 1983. Arch. Environ. Health 1993, 48, 53–58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Healthy Environments for Healthier People; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; Available online: https://iris.who.int/bitstream/handle/10665/346127/WHO-EURO-2018-3004-42762-59655-eng.pdf?sequence=3 (accessed on 5 March 2025).
- Neuberger, M.; Rabczenko, D.; Moshammer, H. Extended effects of air pollution on cardiopulmonary mortality in Vienna. Atmos. Environ. 2007, 41, 8549–8556. [Google Scholar] [CrossRef]
- Neuberger, M.; Moshammer, H.; Rabczenko, D. Acute and Subacute Effects of Urban Air Pollution on Cardiopulmonary Emergencies and Mortality: Time Series Studies in Austrian Cities. Int. J. Environ. Res. Public Health 2013, 10, 4728–4751. [Google Scholar] [CrossRef]
- Yao, C.; Wang, Y.; Williams, C.; Xu, C.; Kartsonaki, C.; Lin, Y.; Zhang, P.; Yin, P.; Lam, K.B.H. The association between high particulate matter pollution and daily cause-specific hospital admissions: A time-series study in Yichang, China. Environ. Sci. Pollut. Res. Int. 2020, 27, 5240–5250. [Google Scholar] [CrossRef]
- Gu, J.; Shi, Y.; Zhu, Y.; Chen, N.; Wang, H.; Zhang, Z.; Chen, T. Ambient air pollution and cause-specific risk of hospital admission in China: A nationwide time-series study. PLoS Med. 2020, 17, e1003188. [Google Scholar] [CrossRef]
- Manan, N.A.; Aizuddin, A.N.; Hod, R. Effect of Air Pollution and Hospital Admission: A Systematic Review. Ann. Glob. Health 2018, 84, 670–678. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Ramachandran, S.; Nguyen, D.; Roper, C. Investigating the acute effects of black carbon, PM2.5 exposure, and temperature on asthma and respiratory-related emergency department visits and hospitalizations in Mississippi. Environ. Pollut. 2025, 373, 126150. [Google Scholar] [CrossRef]
- Linares, C.; Díaz, J.; Navas, M.A.; Ruiz-Páez, R.; Saez, M.; Barceló, M.A.; López-Bueno, J.A. How air pollution and extreme temperatures affect emergency hospital admissions due to various respiratory causes in Spain, by age group: A nationwide study. Int. J. Hyg. Environ. Health 2025, 266, 114570. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, J.; Kang, N.; Zhang, J.; Xu, Y.; Zhang, J.; Tang, X.; Yuchi, Y.; Xu, M.; Wang, C. Association of short-term exposure to PM2.5 and its components with hospital admission for asthma in Shanghai: A time-stratified case-crossover study. J. Asthma 2025, 1–10. [Google Scholar] [CrossRef]
- The World Bank. Air Pollution Management in Kosovo; World Bank: Washington, DC, USA, 2019; Available online: https://openknowledge.worldbank.org/handle/10986/33041. (accessed on 5 March 2025).
- Liu, J.; Clark, L.P.; Bechle, M.J.; Hajat, A.; Kim, S.Y.; Robinson, A.L.; Sheppard, L.; Szpiro, A.A.; Marshall, J.D. Disparities in Air Pollution Exposure in the United States by Race/Ethnicity and Income, 1990–2010. Environ. Health Perspect. 2021, 129, 127005. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Air Q+: Burden of Disease Due to Air Pollution Manual; WHO Regional Office for Europe: Copenhagen, Denmark, 2020. [Google Scholar]
- United Nations Environment Programme (UNEP). Healthier Kosovo (HK 2). Available online: https://www.undp.org/kosovo/projects/healthier-kosovo-hk-2 (accessed on 5 March 2025).
- Rees, N. Danger in the Air: How Air Pollution May be Affecting the Brain Development of Young Children Around the World; United Nations Children’s Fund (UNICEF): New York, NY, USA, 2017; Available online: https://www.unicef.org/sites/default/files/press-releases/glo-media-Danger_in_the_Air.pdf (accessed on 5 March 2025).
- Dreshaj, A.; Millaku, B.; Shala, S.; Selimaj, A.; Shabani, H. Sources of air pollution, environmental impacts and exploitation of natural resources in Kosovo. CBU Int. Conf. Proc. 2017, 5, 1275. [Google Scholar] [CrossRef][Green Version]
- Agjencioni për Mbrojtjen e Mjedisit të Kosovës (AMMK). Raporti Vjetor për Gjendjen e Ajrit 2023. 2024. Available online: https://ammk-rks.net/assets/cms/uploads/files/Raporti%20%20vjetor%20per%20cilesi%20te%20ajrti%202023%20-final%20alb.pdf (accessed on 15 April 2025).
- World Bank. Kosovo Country Environmental Analysis. Cost Assessment of Environmental Degradation, Institutional Review, and Public Environmental Expenditure Review. January 2013. Available online: https://documents1.worldbank.org/curated/en/282361468047686579/pdf/750290ESW0P1310LIC00Kosovo0CEA0Rprt.pdf (accessed on 15 April 2025).
- Agjencioni i Statistikës së Kosovës (ASK). Regjistrimi i Popullësis, Ekonomive Familjare, dhe Banesave në Kosovë. Rezultatet Parafinale 2024. Available online: https://ask.rks-gov.net/Rekos (accessed on 6 May 2025).
- European Environment Agency (EEA). Air Quality e-Reporting (AQ e-Reporting); EEA: Copenhagen, Denmark, 2021. Available online: https://www.eea.europa.eu/data-and-maps/data/aqereporting-9 (accessed on 30 June 2022).
- KHMI. Data from Monitoring Stations. 2022. Available online: https://airqualitykosova.rks-gov.net/en/reports-for-themonitoring-stations/ (accessed on 30 June 2022).
- Vjetari Hidrometeorologjik. Available online: https://ihmk-rks.net (accessed on 30 June 2022).
- Gudziunaite, S.; Shabani, Z.; Weitensfelder, L.; Moshammer, H. Time series analysis in environmental epidemiology: Challenges and considerations. Int. J. Occup. Med. Environ. Health 2023, 36, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Schwartz, J.; Wojtyniak, B.; Touloumi, G.; Spix, C.; Balducci, F.; Medina, S.; Rossi, G.; Sunyer, J.; Bacharova, L.; et al. 2001. Investigating regional differences in short-term effects of air pollution on daily mortality in the APHEA project: A sensitivity analysis for controlling long-term trends and seasonality. Environ. Health Perspect. 2001, 109, 349–353. [Google Scholar] [CrossRef]
- Shabani Isenaj, Z.; Berisha, M.; Gjorgjev, D.; Dimovska, M.; Moshammer, H.; Ukëhaxhaj, A. Air Pollution in Kosovo: Short Term Effects on Hospital Visits of Children Due to Respiratory Health Diagnoses. Int. J. Environ. Res. Public Health 2022, 19, 10141. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Schwartz, J.; Spix, C.; Touloumi, G.; Zmirou, D.; Zanobetti, A.; Wojtyniak, B.; Vonk, J.M.; Tobias, A.; Pönkä, A.; et al. Short term effects of air pollution on health: A European approach using epidemiologic time series data: The APHEA protocol. J. Epidemiol. Community Health 1996, 50, S12–S18. [Google Scholar] [CrossRef]
- Friza, H.; Lax, F.; Neuberger, M. SO2—Ein kommunales Risiko in Wien? Untersuchungen über Beziehungen zwischen SO2 und Mortalität an Atemwegserkrankungen [SO2—A municipal risk in Vienna? Studies on the relationship between SO2 and mortality from respiratory diseases]. Forum Städte-Hyg. 1986, 37, 250–252. (In German) [Google Scholar]
- Neuberger, M.; Moshammer, H. Schwebstaub und Lungengesundheit [Suspended particulates and lung health]. Wien. Klin. Wochenschr. 2004, 116, S8–S12. (In German) [Google Scholar]
- Umweltbundesamt: Dashboard Luftschadstoff-Emissionen und Luftqualität in Österreich. Available online: https://www.umweltbundesamt.at/umweltthemen/luft/luftschadstoffe/dashboard (accessed on 4 March 2025).
- Moshammer, H.; Poteser, M.; Kundi, M.; Lemmerer, K.; Weitensfelder, L.; Wallner, P.; Hutter, H.-P. Nitrogen-Dioxide Remains a Valid Air Quality Indicator. Int. J. Environ. Res. Public Health 2020, 17, 3733. [Google Scholar] [CrossRef]
- Sofia, D.; Lotrecchiano, N.; Giuliano, A.; Barletta, D.; Poletto, M. Optimization of Number and Location of Sampling Points of an Air Quality Monitoring Network in an Urban Contest. Chem. Eng. Trans. 2019, 74, 277–282. [Google Scholar] [CrossRef]
- Bergmann, M.L.; Andersen, Z.J.; Massling, A.; Kindler, P.A.; Loft, S.; Amini, H.; Cole-Hunter, T.; Guo, Y.; Maric, M.; Nordstrøm, C.; et al. Short-term exposure to ultrafine particles and mortality and hospital admissions due to respiratory and cardiovascular diseases in Copenhagen, Denmark. Environ. Pollut. 2023, 336, 122396. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ryan, I.; Paul, S.; Deng, X.; Zhang, W.; Luo, G.; Dong, G.H.; Nair, A.; Yu, F. Particle surface area, ultrafine particle number concentration, and cardiovascular hospitalizations. Environ. Pollut. 2022, 310, 119795. [Google Scholar] [CrossRef] [PubMed]

| Variable | Observation (Days) | Mean | Minimum | Maximum |
|---|---|---|---|---|
| PM10 (µg/m3) | 1437 | 22.5 | 2.6 | 156.9 |
| PM2.5 (µg/m3) | 1437 | 16.4 | 1.5 | 130.0 |
| NO2 (µg/m3) | 1437 | 17.6 | 2.5 | 66.7 |
| O3 (µg/m3) | 1430 | 57.9 | 6.3 | 121.1 |
| SO2 (µg/m3) | 1437 | 7.4 | 0 | 30.2 |
| CO (mg/m3) | 1437 | 0.6 | 0 | 6.6 |
| Temperature (°C) | 1461 | 12.6 | −7 | 30.6 |
| Humidity (%) | 1461 | 71.3 | 24 | 100 |
| Pediatric cases | 1461 | 1.29 | 0 | 10 |
| Adult cases | 1461 | 1.54 | 0 | 15 |
| All cases | 1461 | 2.82 | 0 | 18 |
| Variable | Total Number | Mean Age | Minimum | Maximum |
| Pediatric cases | 1881 | 3.2 | 0 | 21 |
| Pediatric male | 1120 | 3.1 | 0 | 19 |
| Pediatric female | 761 | 3.2 | 0 | 21 |
| Adult cases | 2246 | 63.4 | 0 | 94 |
| Adult male | 1196 | 62.7 | 16 | 94 |
| Adult female | 1050 | 64.2 | 0 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukëhaxhaj, A.; Xhiha, R.; Hoxha, F.T.; Terziqi, H.; Moshammer, H. Ambient Air Quality and Hospital Admissions in Gjakova: A Time Series Analysis. Environments 2025, 12, 162. https://doi.org/10.3390/environments12050162
Ukëhaxhaj A, Xhiha R, Hoxha FT, Terziqi H, Moshammer H. Ambient Air Quality and Hospital Admissions in Gjakova: A Time Series Analysis. Environments. 2025; 12(5):162. https://doi.org/10.3390/environments12050162
Chicago/Turabian StyleUkëhaxhaj, Antigona, Rita Xhiha, Faton T. Hoxha, Hasime Terziqi, and Hanns Moshammer. 2025. "Ambient Air Quality and Hospital Admissions in Gjakova: A Time Series Analysis" Environments 12, no. 5: 162. https://doi.org/10.3390/environments12050162
APA StyleUkëhaxhaj, A., Xhiha, R., Hoxha, F. T., Terziqi, H., & Moshammer, H. (2025). Ambient Air Quality and Hospital Admissions in Gjakova: A Time Series Analysis. Environments, 12(5), 162. https://doi.org/10.3390/environments12050162









