Arsenic in Soil: A Critical and Scoping Review of Exposure Pathways and Health Impacts
Abstract
1. Introduction
- What health impacts have been observed in relation to As in soil?
- What concentrations of As in soil are associated with adverse health effects?
- What other soil or exposure parameters are important to understanding As’s impact on human health?
- How robust are the existing studies on the health impacts of As in soil?
- What methods are used to assess As exposure, and how reliable are they?
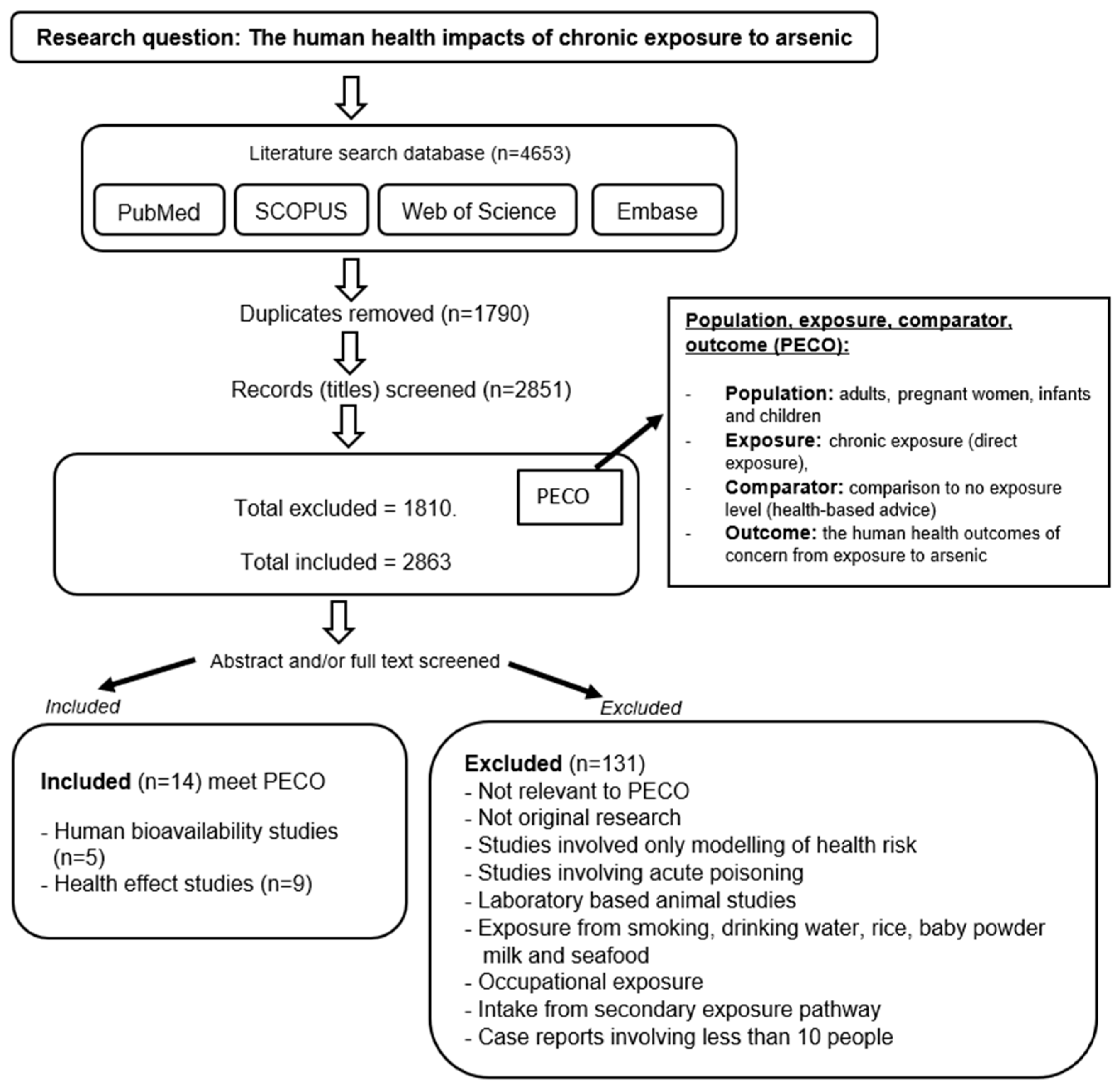
2. Method—Literature Search and Selection Strategy
3. Results
3.1. Observed Health Effects from Association with As in Soil
3.1.1. Birth Outcomes in Areas with <4 mg kg−1 As in Soil
3.1.2. Neurological Effects Reported in People Living near a Pesticide Plant
3.1.3. DNA Damage in Children Exposed to Soil As from Mine Tailings
3.1.4. Cancer Outcomes from Six Ecological Studies with Different As Impact Scenarios
3.2. Exposure to As in Soil–Human Biomarker and Bioaccessibility Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Z.; Gao, S. Upper crustal abundances of trace elements: A revision and update. Chem. Geol. 2008, 253, 205–221. [Google Scholar] [CrossRef]
- Gao, S.; Luo, T.-C.; Zhang, B.-R.; Zhang, H.-F.; Han, Y.-w.; Zhao, Z.-D.; Hu, Y.-K. Chemical composition of the continental crust as revealed by studies in East China. Geochim. Cosmochim. Acta 1998, 62, 1959–1975. [Google Scholar] [CrossRef]
- Sims, K.; Newsom, H.; Gladney, E. Chemical fractionation during formation of the Earth’s core and continental crust: Clues from As, Sb, W, and Mo. Orig. Earth 1990, 291–317. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The geochemical evolution of the continental crust. Rev. Geophys. 1995, 33, 241–265. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: London, UK, 1979. [Google Scholar]
- Vinogradov, A.P. The Geochemistry of Rare and Dispersed Chemical Elements in Soils; Consultants Bureau: New York, NY, USA, 1959; Volume 209. [Google Scholar]
- Colbourn, P.; Alloway, B.; Thornton, I. Arsenic and heavy metals in soils associated with regional geochemical anomalies in south-west England. Sci. Total Environ. 1975, 4, 359–363. [Google Scholar] [CrossRef]
- Craw, D.; Chappell, D.; Reay, A.; Walls, D. Mobilisation and attenuation of arsenic around gold mines, east Otago, New Zealand. New Zealand J. Geol. Geophys. 2000, 43, 373–383. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Zhang, C.; Pinto, L.s.S.; Patinha, C.; Reis, P. Hazard assessment on arsenic and lead in soils of Castromil gold mining area, Portugal. Appl. Geochem. 2004, 19, 887–898. [Google Scholar] [CrossRef]
- Nriagu, J.; Bhattacharya, P.; Mukherjee, A.; Bundschuh, J.; Zevenhoven, R.; Loeppert, R. Arsenic in soil and groundwater: An overview. Trace Met. Other Contam. Environ. 2007, 9, 3–60. [Google Scholar]
- Smith, E.; Smith, J.; Smith, L.; Biswas, T.; Correll, R.; Naidu, R. Arsenic in Australian environment: An overview. J. Environ. Sci. Health Part A 2003, 38, 223–239. [Google Scholar] [CrossRef]
- Walker, S.R.; Parsons, M.B.; Jamieson, H.E.; Lanzirotti, A. Arsenic mineralogy of near-surface tailings and soils: Influences on arsenic mobility and bioaccessibility in the Nova Scotia gold mining districts. Can. Mineral. 2009, 47, 533–556. [Google Scholar] [CrossRef]
- Morais, S.; Fonseca, H.M.; Oliveira, S.M.; Oliveira, H.; Gupta, V.K.; Sharma, B.; de Lourdes Pereira, M. Environmental and health hazards of chromated copper arsenate-treated wood: A review. Int. J. Environ. Res. Public Health 2021, 18, 5518. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.A. Analysis of worldwide regulatory guidance values for less frequently regulated elemental surface soil contaminants. J. Environ. Manag. 2013, 128, 561–585. [Google Scholar] [CrossRef] [PubMed]
- IARC. Arsenic, Metals, Fibres, and Dusts; IARC monographs on the evaluation of carcinogenic risks to humans; IARC: Lyon, France, 2012; Volume 100. [Google Scholar]
- IARC. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; WHO; International Agency for Research on Cancer: Geneva, Switzerland, 2004; Volume 84. [Google Scholar]
- WHO. Fact Sheet—Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 3 March 2024).
- US EPA. Guidelines for Carcinogen Risk Assessment; US EPA: Washington, DC, USA, 2005.
- National Environment Protection Council. National Environment Protection (Assessment of Site Contamination) Measure 1999; National Environment Protection Council: Canberra, Australia, 1999.
- Nurchi, V.M.; Buha Djordjevic, A.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Arsenic, Agency for Toxic Substances and Disease Registry; US Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry: Washington, DC, USA, 2007.
- Zhou, X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 86–98. [Google Scholar]
- Cheraghali, A.M.; Kobarfard, F.; Faeizy, N. Heavy metals contamination of table salt consumed in Iran. Iran. J. Pharm. Res. IJPR 2010, 9, 129. [Google Scholar]
- FSANZ. Arsenic; Food Standards Australia and New Zealand (FSANZ): Kingston, Australia, 2020.
- enHealth. Environmental Health Risk Assessment—Guidelines for Assessing Human Health Risks from Environmental Hazards. 2012. Available online: https://www.health.gov.au/resources/publications/enhealth-guidance-guidelines-for-assessing-human-health-risks-from-environmental-hazards?language=en (accessed on 22 May 2022).
- Ibañez-Del Rivero, C.; Fry, K.L.; Gillings, M.M.; Barlow, C.F.; Aelion, C.M.; Taylor, M.P. Sources, pathways and concentrations of potentially toxic trace metals in home environments. Environ. Res. 2023, 220, 115173. [Google Scholar] [CrossRef]
- Isley, C.F.; Fry, K.L.; Liu, X.; Filippelli, G.M.; Entwistle, J.A.; Martin, A.P.; Kah, M.; Meza-Figueroa, D.; Shukle, J.T.; Jabeen, K. International analysis of sources and human health risk associated with trace metal contaminants in residential indoor dust. Environ. Sci. Technol. 2021, 56, 1053–1068. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Smith, E.; Weber, J.; Naidu, R.; Rees, M.; Rofe, A.; Kuchel, T.; Sansom, L. Effect of soil ageing on in vivo arsenic bioavailability in two dissimilar soils. Chemosphere 2008, 71, 2180–2186. [Google Scholar] [CrossRef]
- Meunier, L.; Koch, I.; Reimer, K.J. Effect of particle size on arsenic bioaccessibility in gold mine tailings of Nova Scotia. Sci. Total Environ. 2011, 409, 2233–2243. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H., Jr. Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Ollson, C.J.; Smith, E.; Scheckel, K.G.; Betts, A.R.; Juhasz, A.L. Assessment of arsenic speciation and bioaccessibility in mine-impacted materials. J. Hazard. Mater. 2016, 313, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Orloff, K.; Mistry, K.; Metcalf, S. Biomonitoring for environmental exposures to arsenic. J. Toxicol. Environ. Health Part B 2009, 12, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Gerr, F.; Letz, R.; Ryan, P.; Green, R.C. Neurological effects of environmental exposure to arsenic in dust and soil among humans. Neurotoxicology 2000, 21, 475–487. [Google Scholar]
- McDermott, S.; Bao, W.; Aelion, C.M.; Cai, B.; Lawson, A.B. Does the metal content in soil around a pregnant woman’s home increase the risk of low birth weight for her infant? Environ. Geochem. Health 2014, 36, 1191–1197. [Google Scholar] [CrossRef]
- Yáñez, L.; García-Nieto, E.; Rojas, E.; Carrizales, L.; Mejía, J.; Calderón, J.; Razo, I.; Díaz-Barriga, F. DNA damage in blood cells from children exposed to arsenic and lead in a mining area. Environ. Res. 2003, 93, 231–240. [Google Scholar] [CrossRef]
- Hinwood, A.L.; Jolley, D.J.; Sim, M.R. Cancer incidence and high environmental arsenic concentrations in rural populations: Results of an ecological study. Int. J. Environ. Health Res. 1999, 9, 131–141. [Google Scholar] [CrossRef]
- Núñez, O.; Fernández-Navarro, P.; Martín-Méndez, I.; Bel-Lan, A.; Locutura, J.F.; López-Abente, G. Arsenic and chromium topsoil levels and cancer mortality in Spain. Environ. Sci. Pollut. Res. 2016, 23, 17664–17675. [Google Scholar] [CrossRef]
- Pearce, D.C.; Dowling, K.; Sim, M.R. Cancer incidence and soil arsenic exposure in a historical gold mining area in Victoria, Australia: A geospatial analysis. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 248–257. [Google Scholar] [CrossRef]
- Putila, J.J.; Guo, N.L. Association of arsenic exposure with lung cancer incidence rates in the United States. PLoS ONE 2011, 6, e25886. [Google Scholar] [CrossRef]
- Yaffee, A.Q.; Scott, B.; Kaelin, C.; Cambron, J.; Sanderson, W.; Christian, W.J.; Moran, T.P.; Chamness, J. Collaborative response to arsenic-contaminated soil in an Appalachian Kentucky neighborhood. J. Toxicol. Environ. Health Part A 2019, 82, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liao, Q.L.; Ma, Z.W.; Jin, Y.; Hua, M.; Bi, J.; Huang, L. Association of soil arsenic and nickel exposure with cancer mortality rates, a town-scale ecological study in Suzhou, China. Environ. Sci. Pollut. Res. 2015, 22, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Hinwood, A.L.; Sim, M.R.; Jolley, D.; de Klerk, N.; Bastone, E.B.; Gerostamoulos, J.; Drummer, O.H. Hair and toenail arsenic concentrations of residents living in areas with high environmental arsenic concentrations. Environ. Health Perspect. 2003, 111, 187–193. [Google Scholar] [CrossRef]
- Hinwood, A.L.; Sim, M.R.; Jolley, D.; de Klerk, N.; Bastone, E.B.; Gerostamoulos, J.; Drummer, O.H. Exposure to inorganic arsenic in soil increases urinary inorganic arsenic concentrations of residents living in old mining areas. Environ. Geochem. Health 2004, 26, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.C.; Dowling, K.; Gerson, A.R.; Sim, M.R.; Sutton, S.R.; Newville, M.; Russell, R.; McOrist, G. Arsenic microdistribution and speciation in toenail clippings of children living in a historic gold mining area. Sci. Total Environ. 2010, 408, 2590–2599. [Google Scholar] [CrossRef]
- Martin, R.; Dowling, K.; Pearce, D.; Bennett, J.; Stopic, A. Ongoing soil arsenic exposure of children living in an historical gold mining area in regional Victoria, Australia: Identifying risk factors associated with uptake. J. Asian Earth Sci. 2013, 77, 256–261. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, H.; Lin, C.; Zhang, X.; Duan, X.; Wang, Q.; Xu, D. Arsenic exposure analysis for children living in central China: From ingestion exposure to biomarkers. Chemosphere 2022, 287, 132194. [Google Scholar] [CrossRef]
- Ryan, R. Cochrane Consumers and Communication Group: Meta-Analysis. 2016. Available online: http://cccrg.cochrane.org (accessed on 23 May 2022).
- Baptist, D.L.; Leslie, N.S. Children playing with poison: Arsenic exposure from CCA-treated wood. J. Nurse Pract. 2008, 4, 48–53. [Google Scholar] [CrossRef]
- CCME. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health, Arsenic (Inorganic) 1997, Updated 2001; Canadian Council of Ministers of the Environment (CCME): Winnipeg, MB, Canada, 1999.
- EA UK. Updated Technical Background to the CLEA Model (SR3); Environment Agency, United Kingdom (EA UK): London, UK, 2009.
| Health Effects | Reference | Location | Study Type | Sample Size/Population | Environmental As Conc a | Biological As Exposure Conc b | Adverse Health Effect | Adjustment Factors |
|---|---|---|---|---|---|---|---|---|
| Birth Outcomes | [37] | South Carolina, USA | Retrospective cohort study. General metals in soil. | n = 9920 mother-child pairs c | Soil at maternal address: 2.56 mg kg−1 (IQR); soil at LBW mother’s address: 3.47 mg kg−1; soil at normal birth weight mothers address: 3.25 d | Not measured | The As variable was nonlinear in relation to LBW, and the association between higher concentrations of As with LBW was strong (p = 0.002) | Characteristics: Maternal: age, race, ethnicity, parity, tobacco use, and alcohol use. Child: sex, birthweight, and weeks of gestation. Neighborhood: density per square mile and median age of residential building. |
| Neurological Effects | [36] | Georgia, USA | Retrospective cohort study. Windblown dust and impacted soil from pesticide plant. | n = 85 adults exposed | Soil: range: 2–1845 mg kg−1; mean: 46.6 mg kg−1; standard deviation: 164.1 mg kg−1 | Not measured | Strong association between As-containing dust exposure and peripheral neuropathy in adults (OR 5.1; p = 0.004) | None mentioned |
| DNA Damage | [38] | Villa de la Paz, Mexico | Cohort study. Gold and other metal/mineral mining sites. | Exposure Villa de la Paz: n = 20 children e | Exposure Villa de la Paz: Soil: range: 141–11,930 mg kg−1; mean: 2462 mg kg−1; n = 26 dust: range: 352–9950 mg kg−1; mean: 2231 mg kg−1; n = 18 | Exposure Villa de la Paz: FVU: range: 87–323 µg g−1 geometric mean: 136; creatinine; n = 20 | 100% of the children had AsU > health guidelines f Comet study: tail length and tail moment in cells were significantly higher than the cells from the control site in Matehuala (p < 0.05) g | None mentioned |
| Control Matehuala: n = 35 children e | Control Matehuala: Soil: range: 51–6866 mg kg−1; mean: 1019 mg kg−1; n = 23 | Control Matehuala: FVU: geometric mean: 34; range: 8–60 µg g−1 creatinine; n = 35 | ||||||
| Cancer | [43] | Former lumber treatment facility; Kentucky, USA | Cross-sectional study. | n = 84 adults and children | Soil: range: 10.3–100.6. median: 64.8 mg kg−1 h | Toenail: median: 0.43 mg kg−1 (IQR: 0.12–0.9) i | Cancer incidence: no difference found for lung and bladder cancer j | Gender, age, race/ethnicity, occupational history, tobacco use, soil and gardening exposures, and well water. |
| [39] | Victoria, Australia | Ecological study. | People living in high soil As areas and cancer data from Victorian Cancer Registry data as per ICD-9 codes | Soil > 100 mg kg−1 median soil range: 1–16,800 mg kg−1 | Not measured | Cancer relative risk rate (95%CI): All cancers 1.06 (1.03–1.09) Prostate cancer 1.14 (1.05–1.23) Kidney cancer 1.16 (0.98–1.37) Melanoma 1.36 (1.24–1.48) Chronic myeloid leukemia 1.54 (1.13–2.10) Breast cancer in females 1.10 (1.03–1.18) | Gender, geographical location and As soil and water concentrations. | |
| [41] | Gold mining region; Victoria, Australia | Ecological study. | People living in the goldfields region and peripheral regions (for comparison). Victoria Cancer Registry data as per ICD-10 code | Soil range: 1.4–1857 mg kg−1 | Not measured | Cancer relative risk rate (95%CI): Males: all cancers 1.21 (1.15–1.27) Males: melanoma 1.52 (1.25–1.85) Females: all cancers 1.08 (1.03–1.14) Females: melanoma: 1.29 (1.08–1.55) | Gender and socioeconomic disadvantage. | |
| [42] | USA | Ecological study. | Individual patient cases from the Surveillance Epidemiology and End-Results (SEER) database | Soil and sediment range: <1.476 mg kg−1 to >14,525 mg kg−1 | Not measured | Cancer incidence rate: 1 mg kg−1 increase in soil As conc was associated with 1.04% increase in lung cancer rate (p < 0.0001) | Age, income, tobacco use, As exposure and residential and geographical location. | |
| [44] | Suzhou, China | Ecological study. | All resident death records from 2005 to 2010. Mortality classified using ICD-10; expressed as per 100,000 people/year | Soil range: 6.14–16.52 mg kg−1; Mean: 9.08 mg kg−1; | Not measured | Age-adjusted mortality rates: Colon cancer 1.083 (1.027, 1.142) Gastric cancer 1.111 (1.061, 1.165) Kidney, cancer 1.129 (1.039, 1.228) Lung cancer 1.050 (1.001, 1.102) Nasopharyngeal cancer 1.086 (1.028, 1.148) | Age, “heavy metals”, tobacco use, and education. | |
| [40] | Spain (including the Canary and Balearic Islands) | Ecological study. | Mortality data from National Statistics Institute for 27 tumors as per ICD-9 and ICD-10 codes | Soil range: 0.10 to 2510 mg kg−1 | Not measured | Mortality: Cancers of the stomach, pancreas, lung, and brain, and non-Hodgkin lymphomas statistically significant (RR > 1) in males and females | Geographical location, sociodemographic indicators such as urban/rural zoning, occupation, number of people in household, literacy levels, unemployment, and income. |
| Reference | Location | Study Type | Sample Size | Environmental As Conc a | Biological As Exposure Conc b |
|---|---|---|---|---|---|
| [45] | Gold mining region Victoria, Australia | Cross-sectional study | n = 153 adults and children | Soil: range: 9.1–9900 mg kg−1; geometric mean: 123.1 mg kg−1; median: 92.0 mg kg−1. Control soil: range: 1.7–80 mg kg−1; geometric mean: 4.3 mg kg−1; median: 3.3 mg kg−1. Dust: range: 12–1300 mg kg−1; geometric mean: 60.8 mg kg−1; median: 53.0 mg kg−1 | Toenail: range: 3.20–477 mg kg−1; geometric mean: 32.1 mg kg−1 (no median provided). Control (low personal): range: 1.30–7.70 mg kg−1; geometric mean: 3.35 mg kg−1 (no median provided). Hair: range: 0.4–27.3 mg kg−1; geometric mean: 3.31 mg kg−1 (no median provided). Control (low personal) hair: 0.20–4.80 mg kg−1; geometric mean: 1.27 mg kg−1 (no median provided). |
| [46] | Gold mining region Victoria, Australia | Cross-sectional study | Exposure: n = 55 adults Control: n = 52 adults | Soil: range: 9.1–9900 mg kg−1; geometric mean: 123.1 mg kg−1; median: 92.0 mg kg−1. Control soil: range: 1.7–80 mg kg−1 Dust: range: 12–1300 mg kg−1; Geometric mean: 60.8 mg kg−1; median: 53.0 mg kg−1. Control dust: range: 2.2–21 mg kg−1; geometric mean: 3.9 mg kg−1; median: 3.9 mg kg−1. | FVU: exposure: <DL-28.4 ug/L; geometric mean: 1.64 μg/L; (no median provided). Control: <DL-2.81 μg/L; geometric mean: 1.18 μg/L; (no median provided). |
| [47] | Gold mining region Victoria, Australia | Cross-sectional study | n = 29 children 5–13 yrs | Soil: range: 3.3–130 mg kg−1; geometric mean: 11.5 mg kg−1 | Toenail: range: 0.15–2.1 µg g−1; geometric mean: 0.49 mg kg−1. |
| [48] | Gold mining region Victoria, Australia | Cross-sectional study | n = 24 children 5–17 years | Soil: range: 3.4–97.1 mg kg−1; geometric mean: 9.53 mg kg−1; median: 5.93 | Toenail: range: 0.03–0.712 a mg kg−1; geometric mean, 0.173 a mg kg−1; median: 0.166. |
| [49] | Hubei Province, China | Cross-sectional study | n = 120 children 3–17 years | Soil: range: 3.1–17.2 mg kg−1; Geometric mean: 8–10.7 mg kg−1 b | Ave first morning urine: 15.5 µg/L; mean 24 h urine: 11.4 µg/L (R = 0.143, p = 0.166); Creatinine-adjusted urine: FVU: mean: 22 µg/L; 24 h: mean: 18.1 µg/L (R = 0.450, p < 0.01). |
| [43] | Lumber treatment facility c | Cross-sectional study | n = 84 adults and children | Soil: range: 10.3–100.6. mg kg−1 median: 64.8 mg kg−1 d | Toenail: median: 0.43 mg kg−1 (IQR: 0.12–0.9) e. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irwin, C.; Gudka, S.; De Meyer, S.; Dennekamp, M.; Netherway, P.; Moslehi, M.; Chaston, T.; Mikkonen, A.; Martin, J.; Taylor, M.P.; et al. Arsenic in Soil: A Critical and Scoping Review of Exposure Pathways and Health Impacts. Environments 2025, 12, 161. https://doi.org/10.3390/environments12050161
Irwin C, Gudka S, De Meyer S, Dennekamp M, Netherway P, Moslehi M, Chaston T, Mikkonen A, Martin J, Taylor MP, et al. Arsenic in Soil: A Critical and Scoping Review of Exposure Pathways and Health Impacts. Environments. 2025; 12(5):161. https://doi.org/10.3390/environments12050161
Chicago/Turabian StyleIrwin, Catherine, Sajni Gudka, Sofie De Meyer, Martine Dennekamp, Pacian Netherway, Maryam Moslehi, Timothy Chaston, Antti Mikkonen, Jen Martin, Mark Patrick Taylor, and et al. 2025. "Arsenic in Soil: A Critical and Scoping Review of Exposure Pathways and Health Impacts" Environments 12, no. 5: 161. https://doi.org/10.3390/environments12050161
APA StyleIrwin, C., Gudka, S., De Meyer, S., Dennekamp, M., Netherway, P., Moslehi, M., Chaston, T., Mikkonen, A., Martin, J., Taylor, M. P., & Mavoa, S. (2025). Arsenic in Soil: A Critical and Scoping Review of Exposure Pathways and Health Impacts. Environments, 12(5), 161. https://doi.org/10.3390/environments12050161











