Abstract
Temperature increase is one of the main effects of climate change occurring worldwide, with drastic impacts on both terrestrial and aquatic biota. Changes in the dominant macroalgal taxa in the Venice Lagoon have been analyzed in relation to the rise in air temperature recorded since 1973, highlighting the significant decline in cold-adapted species, which have been replaced by taxa more tolerant of higher temperatures. Cold-adapted species such as the native Fucus virsoides, Punctaria latifolia, Scytosiphon lomentaria, and many other Phaeophyceae are in decline, whereas thermophilic species such as the non-indigenous species (NIS) Gracilaria vermiculophylla, Agardhiella subulata, Solieria filiformis, Hypnea cervicornis, Caulacanthus okamurae, and many others have replaced the species that once dominated the lagoon. These changes have been associated with an average air temperature increase of approximately 2.5 °C. The highest increase has mostly been recorded for average minimum temperatures (+2.8 °C), compared to average maximum temperatures (+2.0 °C). As a result, Phaeophyceae have declined, while Rhodophyceae, especially recent NIS introductions, have colonized the lagoon bottoms. Changes in Chlorophyceae, on the other hand, appear to be more linked to the reduction of the lagoon’s trophic conditions, although the currently dominant species is Ulva australis, a NIS that has replaced the native Ulva rigida almost everywhere.
1. Introduction
Climate change, driven by a global rise in temperature, is having severe consequences on life on Earth, affecting both terrestrial and marine environments. These impacts include heat waves, catastrophic rainfall events, and rising sea levels due to ice melting. Global temperatures in 2024 were approximately 1.47 °C higher than the late 19th century preindustrial average (1850–1900), and the past ten years have been the warmest on record [1]. It is highly likely that the global mean sea surface temperature increased by 0.88 °C between 1850 and 1900 and 2011 and 2020, due to global warming, with most of this increase (0.60 °C) occurring between 1980 and 2020. However, the rise in water temperature is not uniform across regions, with some areas experiencing more significant changes. This is particularly evident in the Mediterranean Sea, where in 2023, water temperatures reached the highest levels recorded in modern history, with an average surface temperature increase of over 1 °C in just 25 years [2].
In choked and shallow environments such as the Venice Lagoon (Figure 1), temperature increase has been even more pronounced, affecting both sea levels and local biota. In fact, global mean sea level rose by 17 cm over the 20th century and keeps rising at an accelerating rate ([3,4,5]. Moreover, tide-gauge data, after accounting for subsidence, show that the sea level rise in the Venice Lagoon accelerated from 1.23 ± 0.13 mm y−1 between 1872 and 2019 to 2.76 ± 1.75 mm y−1 between 1993 and 2019 [6]. Beyond the evident consequences of rising sea levels, which by the end of the century could range from 32 to 62 cm, in the most optimistic scenario, to 58 to 110 cm, in the most extreme scenario, the increase in water temperature could have catastrophic effects on the lagoon’s biota. This different forecast depends on contributions from the melting of the Greenland and Antarctic ice sheets as well as water mass exchange through the Strait of Gibraltar.
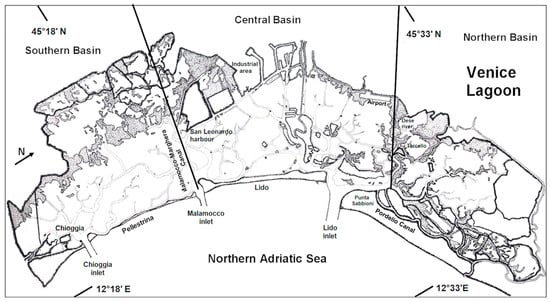
Figure 1.
Map of the Venice Lagoon.
Both plant and animal communities are already undergoing significant changes, with thermophilic species becoming more prevalent while cold-adapted species are declining or disappearing. Species change in response to global warming is further enhanced by the introduction of exotic species [7], which compete with native species and threaten biodiversity conservation. One particularly evident consequence in the Venice Lagoon is the sharp decline of species sensitive to rising minimum temperatures, which no longer allow the waters to freeze as regularly as they did until the mid-2010s. This is the case for the endemic species Fucus virsoides J. Agardh, which had already experienced a significant decline by 2015 and has nearly disappeared from the entire Adriatic Sea since the late 2000s [8]. A similar trend has been observed in many other Phaeophyceae, which are becoming increasingly rare. In contrast, thermophilic species, especially certain non-indigenous species (NIS), primarily introduced through aquaculture and wholesale fish markets [7,8,9], have found favorable conditions for their establishment and expansion in the lagoon.
The aim of this paper is to analyze the major changes in dominant macroalgal taxa in the Venice Lagoon, with a particular focus on the past twenty years. The study examines taxa that have significantly declined as well as those that have increased since the early 2010s, replacing previously dominant species, in relation to temperature changes recorded in the lagoon over the past 51 years.
In addition, a checklist of taxa recorded during this period is provided, highlighting the sharp decline of many cold-adapted Phaeophyceae, particularly the contraction of Fucus virsoides, which is disappearing from the entire lagoon and the increase in thermophilic Rhodophyceae, mainly NIS that tolerate higher temperatures and have colonized the lagoon bottoms, reducing the abundance of native taxa.
2. Materials and Methods
2.1. Study Area
The Venice Lagoon (Figure 1) is a large coastal basin covering approximately 549 km2, subdivided into three main sub-basins.
The Malamocco-Marghera Canal separates the South Basin from the Central Basin, while the North Basin is delineated by the Dese River and the Burano-Torcello tidal lands. These basins exhibit distinct morphological and ecological conditions, creating a diverse range of habitats that support high biodiversity [10,11,12]. Shallow, choked areas, characterized by limited water exchange, extreme salinity fluctuations (0–43), and temperature variations ranging from below 0 °C to 33 °C, with peaks up to 43 °C, alternate with deeper or shallow marine-influenced zones that are strongly affected by seawater inflows.
Water exchange occurs daily in areas near the three lagoon inlets (Lido, Malamocco, and Chioggia) and along the main canals, while in the most isolated areas, water renewal can take up to 40 days [13]. Currently, the lagoon has an average depth of approximately 1.2 m, whereas the main canals and lagoon inlets reach depths of 8–15 m, with some deeper pits (15–20 m) at canal intersections. The Malamocco inlet features a chasm exceeding 50 m in depth. The average tidal range in Venice is around 70–80 cm. However, the tidal range can vary significantly throughout the year, with high tide peaks exceeding 140 cm or even reaching much higher levels, while low tides can drop below 50 cm [14].
2.2. Macroalgal Changes
This study presents findings from 40 years of observations and research on macroalgal distribution, production and taxonomy ([15,16] and references therein) in the Venice Lagoon, with a focus on taxa that were once widespread and abundant but are now in decline, as well as newly introduced NIS that have thrived due to rising temperatures.
In this paper, we report the distribution of the cold-adapted species Fucus virsoides, an endemic relict species from the Tethys Ocean, during the early 2000s, when it was still abundant, and again in 2024, following its dramatic decline. Numerous studies conducted at lagoon soft and hard substrata in the whole lagoon on the framework of various projects [17,18,19,20,21,22,23,24] also reveal the near-total disappearance of other formerly common Phaeophyceae, such as Scytosiphon lomentaria and Punctaria latifolia.
At the same time, many Rhodophyceae, particularly NIS which tolerate or exhibit a preference for higher temperatures, allowing them to establish and spread throughout the lagoon [9], have successfully acclimated and expanded, replacing the previously dominant species.
2.3. Temperature Variation
In the Venice Lagoon, water temperatures are recorded through the SAMANET monitoring network (Venice Lagoon Environmental Monitoring Systems, Venice, Italy), which consists of 10 stations that continuously record water parameters (temperature, dissolved oxygen, salinity, pH, chlorophyll, turbidity) at half-hour intervals since 2002 [25]. This system was established to collect real-time environmental data, with the aim of monitoring and analyzing the ecological conditions of the lagoon. However, the datasets for many years are incomplete, and often there are gaps in days or months, so the annual values are not suitable for detecting long-term changes. Therefore, we used the daily air temperature dataset of Tessera, in Venice Airport at the edge of the central lagoon (Figure 1), recorded since 1973 [26]. For each year, we have calculated the average annual mean temperature (Tmean), the average annual minimum temperature (Tmin), and the average annual maximum temperature (Tmax). The results have been plotted in several graphs, highlighting the changes that have occurred over the last 51 years, during the cold (November–March) and warm (June–September) periods, as well as the number of days with temperatures exceeding 30 °C.
2.4. Statistical Analysis
Air temperatures have been analyzed determining the average annual mean temperature (Tmean), the average annual minimum temperature (Tmin) and the average annual maximum temperature (Tmax) recorded daily between 1973 and 2024. In addition, the average annual maximum temperatures recorded in March and in April during Fucus receptacle formation were calculated as well as the average maximum temperatures recorded in the warmest period June–September and the number of days with a temperature >30 °C. To verify whether air and water temperatures were similar or significantly different, temperature values recorded at 118 (2011) and 88 (2014, 2021, and 2023) stations equally distributed throughout the entire lagoon were analyzed. Sampling was carried out in late spring–early summer and repeated in autumn. The data were compared by calculating the mean, standard deviation (SD), minimum (min), and maximum (max) values, and by performing a one-way ANOVA (p < 0.05) using STATISTICA software, version 10 (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Species Changes
Since the middle of the second decade of the 2000s, a general decrease in many Phaeophyceae species has been observed in the Venice Lagoon and its marine littoral, accompanied by a progressive decline in both biomass and distribution. The most notable decline was that of the Phaeophycea Fucus virsoides, which, until 2015, colonized at least 70 linear kilometers of rocky substrates along the artificial Istrian stone panels, lagoon jetties, the docks of the historical center of Venice, and the shores of islands of all sizes (Figure 2).
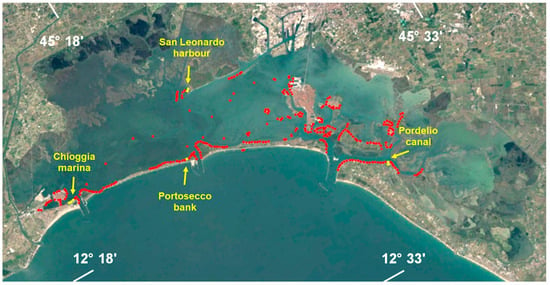
Figure 2.
Map of the Venice Lagoon. Red points indicate locations with Fucus virsoides presence until 2015. Yellow points represent locations where F. virsoides is currently present.
In 2024, this species has almost disappeared everywhere, surviving only in a few small areas (approximately 2–3 linear km2) where water exchange is higher, and temperature remains cooler. Similarly, Punctaria latifolia Greville and Scytosiphon lomentaria (Lyngbye) Link, two other taxa that previously formed dense belts in the mid-littoral zone and in shallow lagoon bottoms, are now very rare, with only occasional, isolated thalli observed. At the same time, some species that tolerate temperatures exceeding 30 °C for long periods without dying or decomposing, such as Gracilaria vermiculophylla, Agardhiella subulata, Solieria filiformis, and Hypnea cervicornis J. Agardh, have replaced the Ulvaceae, which were once dominant. A checklist of the taxa recorded since the mid-2000s with their abundance and temporal trends is reported in Table 1.

Table 1.
Species recorded since the early 2000s in Venice Lagoon. Abundance (+), trends of decline (↓), increase (↑), or stability (≈) across three ranges. NIS species are marked with an asterisk (*).
A list of 249 taxa was considered: 71 Chlorophyceae, 134 Rhodophyceae, 43 Phaeophyceae, and 1 Xanthophycea. Overall, 73 taxa showed a decrease (19 Chlorophyceae: −26.8%, 24 Rhodophyceae: −17.9%, 30 Phaeophyceae: −69.8%, and 1 Xanthophycea), while 30 taxa exhibited an increase in abundance (0 Chlorophyceae, 25 Rhodophyceae: +18.7%, and 5 Phaeophyceae: +11.6%). The other species remained almost unchanged with small variations depending on the year considered.
3.2. Temperature Changes
We recorded the mean, standard deviation, minimum and maximum values of air and water temperature in 118 (2011) and 88 (2014, 2021, and 2023) stations that are equally distributed in the lagoon (Table 2).

Table 2.
Mean, standard deviation (std), minimum (min), maximum (max) values and one-way ANOVA of air and water temperature recorded in the entire lagoon.
In 2011 and 2014 the difference between air and water temperature recorded in late spring-early summer and in autumn was 0.1 °C, whereas in 2021 and 2023 it ranged between 1.4 and 1.0 °C. The mean value of all data was 1.3 °C. The one-way ANOVA analysis shows that air and water temperature differences were not significant both in the single years and in all the data analyzed together.
The trends of air temperature over the last 51 years (1973–2024) at Tessera, in the Venice Lagoon, are shown in Figure 3.
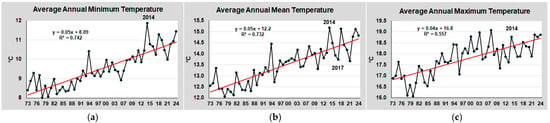
Figure 3.
Trend of air temperature at Tessera between 1973 and 2024: (a) average annual mean temperatures (Tmean); (b) average annual minimum temperatures (Tmin); (c) average annual maximum temperatures (Tmax) recorded daily over this period. The red lines are the linear trend lines of data. The equation and R2 of the linear lines are also reported in the graphs.
Tmean increased by approximately 2.5 °C (from 12.2 to 14.7 °C); Tmin by approximately 2.8 °C (from 9.1 to 10.9 °C), and Tmax by 2.0 °C (from 16.8 to 18.8 °C). The highest increase was recorded since 2014, when Tmin was 11.9 °C compared to 9.5 °C for the entire period. In this last decade, Tmin increased by 1.7 °C (from 9.2 to 10.9 °C). During the winter period, the lagoon waters have experienced only sporadic freezing events. Since 2020, freezing events have not occurred again, even in the more enclosed areas of the lagoon, where the water temperature rarely dropped below 3–4 °C. In previous years, significant freezing events were recorded in 1985 (air temperature −12 °C in January) and 2012 (−9 °C in February), when much of the lagoon was frozen. In the other years, freezing events occurred mainly in more enclosed areas.
The most significant temperature increases, recorded in March and April during the reproductive period of Fucus virsoides, are shown in Figure 4.
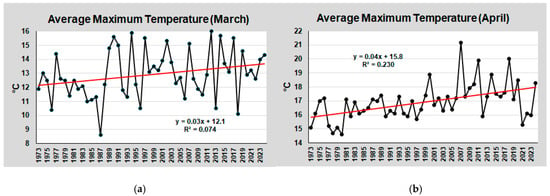
Figure 4.
Trend of air temperature at Tessera between 1973 and 2024: average annual maximum temperatures in March (a) and in April (b), recorded daily over this period. The red lines are the linear trend lines of data. The equation and R2 of the linear lines are also reported in the graphs.
Although annual values varied considerably, the average temperature in March increased by approximately 1.5 °C, and by more than 2.0 °C in April.
Similarly, the average maximum temperature during the warmest period (June–September) rose from approx. 28 °C to 30 °C, while the number of days with temperatures above 30 °C increased from 10 to 25 (Figure 5).
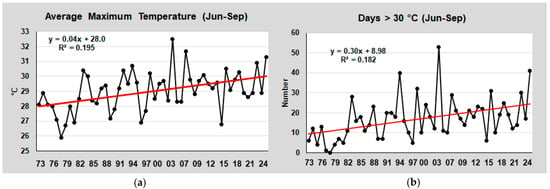
Figure 5.
(a) Trend of the average maximum air temperature in the period June–September at Tessera between 1973 and 2024; (b) Number of days with a temperature >30 °C in the period June–September. The red lines are the linear trend lines of data. The equation and R2 of the linear lines are also reported in the graphs.
4. Discussion
In the last forty years, the vegetation of the Venice Lagoon has undergone significant changes, both in terms of biomass and species dominance [16]. The decrease in biomass observed since the 1990s was primarily due to trophic changes, with a significant reduction in phosphorus and nitrogen species in both the water column and surface sediments ([27] and references therein). In contrast, changes in species dominance were driven by both the combined effects of nutrient reduction and increased temperatures. The reduction in trophic status favored the progressive decline in the abundance of many Chlorophyceae (−26.8%), particularly Ulvaceae, and the replacement of Ulva rigida C. Agardh by Ulva australis Areschoug, a NIS that thrives in lower nutrient concentrations [16]. Indeed, U. australis colonizes marine littorals and lagoon areas characterized by high water renewal and moderate nutrient concentrations, whereas U. rigida dominates the eutrophic waters around the urban centers of the lagoon. The abundance of Chlorophyceae species has not increased, and the lagoon is now predominantly dominated by Rhodophyceae, particularly Gracilariaceae, Solieriaceae, and Cystocloniaceae.
This change is also clearly visible when observing the banks of the historical center of Venice and the islands of the lagoon. In the past, during low tide, the hard substrata and island shores were covered by dense belts of filamentous/laminar Chlorophyceae, mostly belonging to the genera Blidingia, Ulva, Chaetomorpha, and Cladophora. Currently, the green algae have almost disappeared everywhere. Therefore, the banks of the islands and the shores of the lagoon are now characterized by a dense stratification of Rhodophyceae, especially turf-forming algae such as Caulacanthus okamurae Yamada, another intertidal NIS of tropical origin (China, Korea, Taiwan) that has recently also colonized the Mar Piccolo of Taranto [28].
Climatic changes, with a general increase in temperature, have already favored the loss of many Fucales around the world [29,30,31]. The same negative trend was recorded in the Venice Lagoon with the decline of many Phaeophyceae, especially mid-littoral species such as Fucus virsoides, Scytosiphon lomentaria, and Punctaria latifolia, which, at low tides, are exposed to air, intense sunlight, and desiccation. Conversely, their decrease or disappearance favored the spread of taxa that are very resistant to high temperatures, many of which are NIS, such as G. vermiculophylla, A. subulata, S. filiformis, and H. cervicornis [9]. Generally, marine algae inhabiting the intertidal zone are tolerant to freezing and desiccation, showing higher photosynthetic rates during periods of air exposure. However, these rates drop dramatically when the algae are exposed to temperatures >30 °C and rapidly lose their water content over time, as reported for Fucus virsoides by [32] and Fucus vesiculosus Linnaeus [33], while changes in nutrient concentrations appear less important [9]. Similar results were obtained for other Phaeophyceae living in the intertidal zone, as found by [34] for Hesperophycus harveyanus (Decaisne) Setchell and N.L. Gardner (now recorded as Fucus ceranoides Linnaeus), and Pelvetia fastigiata f. gracilis (now recorded as Silvetia compressa (J. Agardh) E. Serrão, T.O. Cho, S.M. Boo and Brawley), or by [35] for Mastocarpus papillatus (C. Agardh) Kützing, and by [36] for Fucus spiralis Linnaeus.
Fucus virsoides is a glacial relict species endemic to the Adriatic Sea, growing on rocky substrata from Ancona to Venice and Trieste on the western coasts of Italy, and from the eastern coasts of Slovenia and Croatia up to Dürres in Albania [8,37,38,39,40,41,42,43]. The species was first reported in the Venice Lagoon by [44] and subsequently by all researchers who studied macroalgae in this environment [15].
In the Venice Lagoon, F. virsoides was present both inside the lagoon and along the marine shores of Lido and Pellestrina until the early 2000s. Subsequently, it disappeared from the seashores (panels and lagoon jetties) but remained very common inside the lagoon. Here, it colonized large areas with dense populations, particularly along the shores of Punta Sabbioni, Lido, Pellestrina, Chioggia, and the small islands scattered throughout the lagoon (Figure 2). It was even found in some embankments and inner canals of Venice’s historical center (e.g., S. Barnaba Canal, Rio Novo), where high pollution levels made its presence unexpected.
The decline of Fucus populations in the lagoon was first observed in 2015–2016, when they rapidly disappeared from almost all areas. At present, only a few residual populations persist inside the harbor of San Leonardo in the central lagoon, in the Pordelio Canal at Punta Sabbioni in the northern lagoon (Figure 2), and along the shores of a marina in Chioggia, but every year they decrease more and more. These areas are protected from direct wave action and benefit from high seawater renewal, which mitigates extreme temperature fluctuations. Some thalli or very small populations can still be found in areas where F. virsoides was once abundant, but their presence is now only occasional.
Because systematic data on water temperatures in the lagoon or the adjacent sea littoral are not available, we considered the daily changes in air temperature recorded since 1973 at a weather station at Venice Airport (Tessera, [26]) located on the edge of the central lagoon basin. This choice was corroborated by an analysis of air and water temperatures recorded in 88–118 stations spread in the entire lagoon in 2011, and 2014 before the highest decline in Phaeophyceae, and subsequently in 2021 and 2023. Results show that air and water temperatures did not differ significantly (Table 1). Indeed, the lagoon’s average depth is only 1.2 m, with extensive shallow areas around 0.5 m, and a mean tidal excursion of 70–80 cm. Consequently, the temperatures of both air and water quickly reach similar values. In addition, the macroalgal species showing the highest changes are mainly mid-littoral species that are exposed to air temperatures twice a day. Intense sunlight, desiccation, or freezing events can severely affect their presence or absence. Therefore, air temperature can play a major role in regulating the distribution of these species, providing information on their possible decline or spread.
The analysis of daily air temperatures over the past 51 years shows a significant increase, particularly in the average annual minimum values (approximately +2.8 °C). In the past, winter water temperatures were much colder, and numerous freezing events were recorded. This progressive increase in temperature suggests that, in the coming years, many other Phaeophyceae will also disappear, especially those in the mid-littoral zone, which are continuously exposed to the air during low tide. The species most at risk are those that grow in winter and early spring, such as the taxa belonging to the genera Asperococcum, Petalonia, Stictyosiphon, and Striaria, and disappear in May–June as temperatures rise.
Some authors [45] reconstructed freezing events in the Venice Lagoon over the past 1400 years. The first documented occurrence dates back to 604 AD, when the monk Paulus Diaconus (720–799), who lived in Aquileia (approximately 100 km northeast of Venice), reported that the winter was so harsh that it killed vineyards almost everywhere and even caused the lagoon to freeze over. He went on to document 57 additional freezing events. The most recent ones occurred in 1929, 1956, 1985, and 2012.
Between January and February 1929, air temperatures ranged from −8.5 °C to −13 °C, forming ice slabs 15–20 cm thick, allowing people to walk from one island to another. In January–February 1956 and 1985, arctic air masses and strong Bora winds caused temperatures to drop to −8 °C and −12 °C, respectively, freezing the entire lagoon once again.
In the following years, despite many canals regularly freezing (Figure 3), the last widespread freezing event affecting a large part of the lagoon occurred in 2012 when air temperatures dropped to −9 °C. Although this event was less severe than previous ones, the lagoon was partially frozen, and floating ice slabs invaded the canals of the city and surrounding islands. Between 2012 and 2015, occasional freezing events were recorded only in choked areas, salt marshes, and some canals. In the years since, until today, water temperatures in January–February have rarely dropped below 3–4 °C. At the same time, Fucus virsoides and other Phaeophyceae species, such as Punctaria latifolia and Scytosiphon lomentaria, which were abundant 10–20 years ago, have undergone a rapid decline and are currently disappearing.
Scytosiphon lomentaria is a species that presents tubular, filamentous gametophytic macrothalli in late winter to early spring and crustose, sporophytic microthalli in summer. In the past, in early spring, the macrothalli formed dense belts of tubular filaments, 40–60 cm long, in the mid-littoral zone of all hard substrata along marine and lagoon embankments, with a biomass of several kilograms of fresh weight per square meter. Currently, only isolated, occasional tubular filamentous macrothalli are present, accounting for only a few grams per square meter. In Denmark, the formation of plurilocular sporangia on macrothalli occurred at temperatures between 11 °C and 18 °C. Above 18 °C, macrothalli disappeared both in nature and in culture [46]. The sporophytic crusts produced unilocular sporangia at temperatures between 16 °C and 23.8 °C, thus functioning as the reproductive system during the summer. Higher temperatures inhibited the growth of both macrothalli and crusts, reducing the presence of this species, as also observed along Scotland’s coast [47], except in the far northwest, where waters were colder.
Similarly, P. latifolia, was very abundant in the past, and in 1984 covered the lagoon bottoms around the historical center of Venice with luxuriant populations (2–3 kg FW m−2). However, at present, this species has almost disappeared.
All these species have particularly suffered from global warming because they live in the mid-littoral zone and are exposed to the air during low tides. In particular, Fucus virsoides reproduces in late winter to early spring, and if temperatures are higher than 10–15 °C, reproduction is inhibited (Figure 4). A relevant study on the spawning conditions of six species of Fucus, carried out at the University of Groningen (The Netherlands) by [48], showed that reproduction depended on different conditions of photoperiod and temperature. The receptacles were not produced under short-day or long-day conditions. Generally, plants in spring initiate receptacle formation under a 12:12 h photoperiod [49]. Therefore, if temperatures are too high during this period, receptacle production is inhibited.
This seems to be the case for F. virsoides in the Venice Lagoon, which has almost disappeared in the last ten years. Figure 4 shows the average maximum temperatures recorded in March and April since 1973. In March, water temperatures fall within the range suitable for receptacle formation, but the photoperiod is too short. In contrast, in April, the photoperiod is optimal, but the temperature is too high, almost always exceeding 15 °C, with an increasing trend in recent years. These considerations can explain the decline of this species over the past decade, even though F. virsoides may have slightly different photoperiod and temperature thresholds.
If we consider the optimal growth temperature for Fucus vesiculosus in the North Sea, it ranges between 10 and 20 °C, with strongly reduced growth above 20 °C and an upper lethal limit of 28 °C after one week of exposure [50,51]. These results were confirmed by [33], who, in three-week exposure experiments, found that F. vesiculosus was able to grow and survive within a temperature range of 5 to 26 °C without any injury or visible damage to the apical growing meristem. However, at higher water temperatures (≥27 °C), growth rapidly decreased from the third day onwards, and progressive necrosis was observed at 28 and 29 °C.
Since the average maximum temperature of the lagoon has also increased by approximately 2.0 °C, we cannot exclude the possibility that the decline of F. virsoides has been further exacerbated by prolonged periods with temperatures exceeding 28 °C (Figure 5) or, at the very least, that it has contributed synergistically to the species’ decline.
Indeed, temperatures above 30 °C, heatwaves, and severe storm events are pushing environmental conditions beyond the species’ tolerance limits, increasing thallus desiccation and damage while reducing photochemical activity [52,53].
In 1973, the average maximum temperature during the warm period (June–September) was approx. 28 °C. Over the following years, it progressively increased, reaching approx. 30 °C in 2024. Similarly, the average number of days with temperatures exceeding 30 °C was 9 in 1973, rising to 25 by 2024. However, studies on the optimal reproductive periods for this species are still necessary to confirm or refute these conclusions.
The most widespread species resistant to high water temperatures are NIS recorded in the lagoon since the early 2000s: Agardhiella subulata and Solieria filiformis in 2003; Gracilaria vermiculophylla in 2008; and Hypnea cervicornis in 2009. By 2019, these species accounted for 92.3% of the total NIS biomass in the lagoon (G. vermiculophylla: 45.3%, A. subulata: 25.1%, H. cervicornis: 19.3%, and S. filiformis: 2.57%).
Gracilaria vermiculophylla is a habitat-forming species that colonizes muddy substrata in the choked areas of the central and southern lagoon, particularly in more turbid regions where it tends to dominate. Owing to its resistance to high temperatures and high concentrations of phycoerythrin and phycocyanin, it has successfully colonized bare sediments where other species cannot survive. These habitats are particularly suitable for juvenile fish (nursery areas), shrimps, and numerous benthic macrofaunal species [54,55]. It is often found alongside other Gracilariaceae and Solieriaceae, especially the native species Gracilariopsis longissima (S.G. Gmelin) Steentoft, L.M. Irvine and Farnham, Gracilaria gracilis (Stackhouse) Steentoft, L.M. Irvine and Farnham, as well as the NIS A. subulata and S. filiformis. These species coexist across a range of habitats. G. vermiculophylla and S. filiformis occupy the most turbid and degraded areas, where even native species are unable to survive. In contrast, H. cervicornis, a NIS first recorded in 2009, prefers clearer waters. It forms aggregates with various other macroalgae, especially the native Chondria capillaris (Hudson) M.J. Wynne, Spyridia filamentosa (Wulfen) Harvey, and Centroceras gasparrinii subsp. minus M.A. Wolf, Buosi, Juhmani, and Sfriso, or inhabits aquatic angiosperm meadows in other shallow lagoon areas.
To these species, we must also add Caulacanthus okamurae, a NIS only recently distinguished through molecular analyses [28], previously confused with C. ustulatus (Turner) Kützing. This NIS has almost completely replaced Gelidium spp. and Gymnogongrus griffithsiae (Turner) Martius on intertidal hard substrata.
An exceptional case is Grateloupia yinggehaiensis H.W. Wang and R.X. Luan, first recorded in 2008. This species thrives in particularly warm waters and grows exclusively near the cooling water discharges (35 m3 s−1) of the Fusina thermoelectric power plant. These discharges are 10–14 °C warmer than natural waters, raising the temperature of the surrounding areas by 4–5 °C, with the greatest difference observed in winter [56]. This species is native to tropical regions such as Hainan Province (China), which experiences a tropical moist monsoonal climate with winter temperatures ranging from 16 to 21 °C [57]. In Venice, winter temperatures until the second half of the 2010s were generally close to 0 °C, with frequent freezing events. However, the thermal power plant has ensured a minimum temperature of 6–8 °C, allowing reproductive cells or basal crusts to survive even during the coldest periods.
5. Conclusions
Climate change, characterized by a progressive rise in air temperatures and significant impacts, particularly on minimum water temperatures, has had a major effect on lagoon vegetation over the past two decades. Freezing events are now rare, even in the most choked areas of the lagoon. These changes have severely affected native cold-water taxa, which are in sharp decline. At the same time, they have facilitated the establishment and spread of many NIS that are better adapted to elevated temperatures. As a result, almost all Phaeophyceae are declining, and some intertidal taxa are now very rare or even nearing extinction. In contrast, several thermophilic taxa, particularly exotic Rhodophyceae, have colonized both hard and muddy substrata, altering the dominant vegetation, which was previously composed largely of Ulvaceae.
However, the general decline of Chlorophyceae is also attributable to a significant reduction in nutrients in both the water column and surface sediments, as reported by ([15,16] and references therein). Thus, the current composition of lagoon vegetation is the result of a synergistic interaction between climate change and reduced anthropogenic pressures.
Similar results have been recorded in the lagoons of the Po Delta (Caleri, Goro, Barbamarco) and at Fattibello in the Valli di Comacchio, where Rhodophyceae have almost completely replaced Chlorophyceae. Indeed, recent sampling showed that the dominant taxa in these lagoons are currently the non-indigenous species Gracilaria vermiculophylla, Solieria filiformis, and Ulva australis. In addition, among the ten most frequently observed taxa, six are NIS species [58].
In the coming years, the ongoing decrease in nutrients [27] and rising temperatures [1,2,26] are expected to further transform lagoon vegetation. Species sensitive to elevated temperatures may decline or disappear, while the introduction of new NIS will continue to partially replace native species. This process has not led to a reduction in biodiversity, but rather a decline in the abundance of native species. Unlike other NIS, such as the ctenophore Mnemiopsis leidyi Agassiz [59,60,61] or the blue crab Callinectes sapidus Rathbun [62,63], which have had dramatic impacts on native species and the fisheries economy, allochthonous macroalgae have so far contributed only to an enrichment in the number of taxa present in the lagoon [9,54].
Author Contributions
Conceptualization, A.S.; methodology, A.S. and A.A.S.; validation, A.S., Y.T., and A.A.S.; formal analysis, A.S.; investigation, A.S., Y.T., and A.A.S.; resources, A.S.; data curation, A.S., Y.T., and A.A.S.; writing—original draft preparation, A.S.; writing—review and editing, A.A.S. and Y.T.; supervision, A.S., Y.T., and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this paper received no external funding. However, most of the results presented in this paper are based on observations collected over the last 15–20 years during the implementation of numerous projects. The main ones were those of the Veneto Region (references [17,18,19,20,21]), of CORILA (reference [22]), and the LIFE SERESTO and LIFE LAGOON REFRESH projects (references [23,24]).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NIS | Non-indigenous species |
| Tmin | Average minimum temperature |
| Tmean | Average mean temperature |
| Tmax | Average maximum temperature. |
References
- NASA (National Aeronautics and Space Administration). Global Climate Change. Available online: https://climate.nasa.gov/vital-signs/global-temperature/?intent=121 (accessed on 25 February 2025).
- ENEA (Agenzia Nazionale per le Nuove Tecnologie, l’Energia e lo Sviluppo Economico Sostenibile) and INGV (Istituto Nazionale di Geofisica e Vulcanologia). Environment: Mediterranean Temperature, 1 °C Warmer in Last 25 Years. 2025. Available online: https://www.ingv.it/en/press-and-urp/Press/Press-releases/5650-Mediterranean-environment-temperature-over-1-c-more-in-the-last-25-years (accessed on 20 February 2025).
- Dangendorf, S.; Hay, C.; Calafat, F.M.; Marcos, M.; Piecuch, C.G.; Berk, K.; Jensen, J. Persistent acceleration in global sea-level rise since the 1960s. Nat. Clim. Chang. 2019, 9, 705–710. [Google Scholar] [CrossRef]
- IPCC. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2022: Impacts, Adaptation, and Vulnerability; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar] [CrossRef]
- Giupponi, C.; Bidoia, M.; Breil, M.; Di Corato, L.; Kumar Gain, A.; Leoni, V.; Fard, B.-M.; Pesenti, R.; Umgiesser, G. Boon and burden: Economic performance and future perspectives of the Venice flood protection system. Reg. Environ. Change 2024, 24, 44. [Google Scholar] [CrossRef]
- Zanchettin, D.; Bruni, S.; Raicich, F.; Lionello, P.; Adloff, F.; Androsov, A.; Antonioli, F.; Artale, V.; Carminati, E.; Ferrarin, C.; et al. Sea-level rise in Venice: Historic and future trends. Nat. Hazards Earth Syst. Sci. 2021, 21, 2643–2678. [Google Scholar] [CrossRef]
- Van der Loos, L.M.; Bafort, Q.; Samuel, S.; Ballesteros, E.; Bárbara, I.; Bercibar, E.; Blanfuné, A.; Bogaert, K.; Bouckenooghe, S.; Boudouresque, C.-F.; et al. Non-indigenous seaweeds in the Northeast Atlantic Ocean, the Mediterranean Sea, and Macaronesia: A critical synthesis of diversity, spatial and temporal patterns. Eur. J. Phycol. 2024, 59, 127–156. [Google Scholar] [CrossRef]
- Descourvières, E.; Bandelj, V.; Sfriso, A.; Orlando-Bonaca, B.; Mačić, V.; Iveša, L.; Kipson, S.; Gljušćić, E.; Battelli, C.; Moro, I.; et al. Toward the first documented extinction of a marine macroalga in the Mediterranean Sea? Reg. Environ. Change 2024, 24, 132. [Google Scholar] [CrossRef]
- Sfriso, A.; Buosi, A.; Wolf, M.A.; Sfriso, A.A. Invasion of alien macroalgae in the Venice Lagoon, a pest or a resource? Aquat. Invasions 2020, 15, 245–270. [Google Scholar] [CrossRef]
- Gagič, M.; Solidoro, C. Lagoon of Venice. Circulation, water exchange and ecosystem functioning. J. Mar. Syst. 2004, 51, 1–4. [Google Scholar] [CrossRef]
- Tagliapietra, D.; Zanon, V.; Frangipane, G. Modello di Zonazione Gerarchica dei Bassofondali Della Laguna di Venezia, WP1, Tipologie Ambientali Lagunari (Habitat Acquatici Lagunari). Relazione Finale del Programma di Ricerca CORILA, 2004-2006, Linea 3.11: Indicatori ed Indici di Qualità Ambientale per la Laguna di Venezia; CORILA: Venice, Italy, 2006; pp. 1–8. [Google Scholar]
- Tagliapietra, D.; Volpi Ghirardini, A. Notes on coastal lagoon typology in the light of the EU Water Framework Directive: Italy as a case study. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2006, 16, 457–467. [Google Scholar] [CrossRef]
- Cucco, A.; Umgiesser, G. Modelling the Venice Lagoon residence time. Ecol. Modell. 2006, 193, 34–51. [Google Scholar] [CrossRef]
- Variazioni del Livello Medio del Mare. Available online: https://www.comune.venezia.it/it/content/variazioni-livello-medio-mare (accessed on 20 February 2025).
- Sfriso, A.; Curiel, D.; Rismondo, A. The Venice Lagoon. In Flora and Vegetation of the Italian Transitional Water Systems; Cecere, E., Petrocelli, A., Izzo, G., Sfriso, A., Eds.; CoRiLa: Venice, Italy, 2009; pp. 17–80. [Google Scholar]
- Sfriso, A.; Buosi, A.; Tomio, Y.; Wolf, M.; Sciuto, K.; Sfriso, A.A. Macrophyte changes in transitional water systems: Role of water and sediment parameters, the Venice Lagoon as study case. Ecol. Indic. 2024, 159, 111623. [Google Scholar] [CrossRef]
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). Line 2: EQB: Macrophytes, Mo.V.Eco. I, Final Report, 2012; pp. 23 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=294424&highlight=true (accessed on 20 February 2025).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes, Mo.V.Eco. II, Final Report, 2015; pp. 27 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=379241&highlight=true (accessed on 20 February 2025).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes, Mo.V.Eco. III, Final Report, 2019; pp. 26 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=367174 (accessed on 20 February 2025).
- Regione del Veneto. Monitoring Plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes- Mo.V.Eco. IV, Final Report, 2022; pp. 19 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=386914 (accessed on 20 February 2025).
- Regione del Veneto. Monitoring plan for the Venice Lagoon Pursuant to Directive 2000/60/EC Aimed at Defining the Ecological Status (Legislative Decree N. 152/2006 s.m.i). EQB: Macrophytes- Mo.V.Eco. V, Final Report, 2024; pp. 26 + Tables and Figures. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDecreto.aspx?id=468004 (accessed on 20 February 2025).
- CORILA (Consorzio per il coordinamento delle ricerche inerenti al sistema lagunare di Venezia). Venezia2021. Programma di Ricerca Scientifica per Una Laguna “Regolata. Linea 3.3”. Produzione Primaria, Comunità Microbica, Bentonica, Planctonica e Nectonica Lagunare WP3.3.1. Aggiornamento Della Trofia, Della Speciazione e Produzione Primaria Delle Macrofite, Impatto dei Tassi di Sedimentazione e dei Processi di Erosione/Sedimentazione in Laguna di Venezia. 2022. Available online: https://www.corila.it/portfolio/venezia-2021-2/?doing_wp_cron=1743436511.5847239494323730468750 (accessed on 20 February 2025).
- Life SERESTO. Habitat 1150* (Coastal Lagoon) Recovery by SEagrass RESTOration. A New Strategic Approach to Meet HD & WFD Objectives. LIFE12-NAT-IT-000331. Available online: https://una.city/nbs/venezia/life-seagrass-restoration (accessed on 20 February 2025).
- Life LAGOON REFRESH. Coastal Lagoon Habitat (1150*) and Species Recovery by Restoring the Salt Gradient Increasing Fresh Water Input. LIFE16-NAT-IT-000663. Available online: www.lifelagoonrefresh.eu (accessed on 20 February 2025).
- Ferrari, G.; Badetti, C.; Ciavatta, S. Real-Time Monitoring of the Venice Lagoon. The Magistrato alle Acque Develops a Monitoring Network to Study the Evolution of the Venice Lagoon Ecosystem. Sea Technol. 2004, 45, 22–24. [Google Scholar]
- Archivio Meteo Storico. Meteo.it. Available online: https://www.ilmeteo.it/portale/archivio-meteo (accessed on 20 February 2025).
- Sfriso, A.; Buosi, A.; Mistri, M.; Munari, C.; Franzoi, P.; Sfriso, A.A. Long-term changes of the trophic status in transitional ecosystems of the northern Adriatic Sea, key parameters and future expectations: The lagoon of Venice as a study case. Nat. Conserv. 2019, 34, 193–215. [Google Scholar] [CrossRef]
- Petrocelli, A.; Wolf, A.M.; Cecere, E.; Sciuto, K.; Sfriso, A. Settlement and spreading of the introduced seaweed Caulacanthus okamurae (Rhodophyta) in the Mediterranean Sea. Diversity 2020, 12, 129. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Orlando-Bonaca, M.; Mannoni, P.-A.; Poloniato, D.; Falace, A. Assessment of Fucus virsoides distribution in the Gulf of Trieste (Adriatic Sea) and its relation to environmental variables. Bot. Mar. 2013, 56, 451–459. [Google Scholar] [CrossRef]
- Iveša, L.; Djakovac, T.; Devescovi, M. Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 2016, 106, 162–173. [Google Scholar] [CrossRef]
- Gessner, F.; Hammer, L. Physiological Investigations on the Tolerance of Fucus virsoides (don) J. Ag. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1971, 56, 581–597. [Google Scholar] [CrossRef]
- Graiff, A.; Liesner, D.; Karsten, U.; Bartsch, I. Temperature tolerance of western Baltic Sea Fucus vesiculosus—Growth, photosynthesis and survival. J. Exp. Mar. Bio. Ecol. 2015, 471, 8–16. [Google Scholar] [CrossRef]
- Oates, B.R.; Murray, S.N. Photosynthesis, dark respiration and desiccation resistance of the intertidal seaweeds Hesperophycus harveyanus and Pelvetia fastigiata f. gracilis. J. Phycol. 1983, 19, 371–380. [Google Scholar] [CrossRef]
- Bell, E.C. Photosynthetic response to temperature and desiccation of the intertidal alga Mastocarpus papillatus. Mar. Biol. 1993, 117, 337–346. [Google Scholar] [CrossRef]
- Madsen, T.V.; Maberly, S.C. A Comparison of air and water as environments for photosynthesis by the intertidal alga Fucus spiralis (Phaeophyta). J. Phycol. 1990, 26, 24–30. [Google Scholar] [CrossRef]
- Linardić, J. Studije o jadranskom fukusu (Fucus virsoides). Acta Bot. Croat. 1949, 12, 7–131. [Google Scholar]
- Munda, I.M. Seasonal and ecologically conditioned variations in the Fucus virsoides association from the Istrian coast (northern Adriatic). Dissertations. SAZU 1972, 15, 1–33. [Google Scholar]
- Munda, I.M. Benthic marine algae as reflection of environment changes in the Northern Adriatic Sea. In Climate warming and related changes in Mediterranean marine biota. Helgoland, CIESM. In Proceedings of the CIESM Workshop Monographs, Menton, France, 1–4 October 2008; Volume 35, pp. 65–72. [Google Scholar]
- Zavodnik, N.; Iveša, L.; Travizi, A. Note on recolonisation by fucoid algae Cystoseira spp. and Fucus virsoides in the North Adriatic Sea. Acta Adriat. 2002, 43, 25–32. [Google Scholar]
- Rindi, F.; Battelli, C. Spatio-temporal variability of intertidal algal assemblages of the Slovenian coast (Gulf of Trieste, northern Adriatic Sea). Bot. Mar. 2005, 48, 96–105. [Google Scholar] [CrossRef]
- Rindi, F.; Gavio, B.; Díaz-Tapia, P.; Di Camillo, C.G.; Romagnoli, T. Long-term changes in the benthic macroalgal flora of a coastal area affected by urban impacts (Conero Riviera, Mediterranean Sea). Biodivers. Conserv. 2020, 29, 2275–2295. [Google Scholar] [CrossRef]
- Mačič, V. Distribution of seaweed Fucus virsoides J. Agardh in Boka Kotorska Bay (South Adriatic Sea). Ann. Ser. Hist. Nat. 2006, 16, 1–4. [Google Scholar]
- Zanardini, G. Synopsis algarum in Mari Adriatico hucusque Collectarum cui accedunt monographia siphonearum nec non generales de algarum vita et structura disquisitiones cum tabulis auctoris manu ad vivum depictis. Mem. Reale Accad. Sci. Torino. Ser. II Tomo IV 1841, 4, 105–255. [Google Scholar]
- Camuffo, D.; Bertolin, C.; Craievich, A.; Granziero, R.; Enzi, S. When the Lagoon was frozen over in Venice from A.D. 604 to 2012: Evidence from written documentary sources, visual arts and instrumental readings. Méditerranée. 2017. [Online], Varia, Online since 07 February 2017. Available online: http://mediterranee.revues.org/7983 (accessed on 1 January 2025).
- Kristiansen, A.; Møller Pedersen, P.; Moseholm, L. Growth and reproduction of Scytosiphon lomentaria (Fucophyceae) in relation to temperature in two populations from Denmark. Nord. J. Bot. 1991, 11, 375–383. [Google Scholar] [CrossRef]
- Scottish Association for Marine Science (SAMS). Significant Changes in Scotland’s Rocky Intertidal Zones. 2025. Available online: https://www.cmscoms.com/?p=42868 (accessed on 20 February 2025).
- Feis, M.E. Reproduction of the Genus Fucus. Bachelor’s Thesis, Department of Marine Benthic Ecology and Evolution, Biological Centre, University of Groningen, Groningen, The Netherlands, 2010. [Google Scholar]
- Berger, R.; Malm, T.; Kautsky, L. Two reproductive strategies in Baltic Fucus vesiculosus (Phaeophyceae). Eur. J. Phycol. 2001, 36, 265–273. [Google Scholar] [CrossRef]
- Fortes, M.D.; Lüning, K. Growth Rates of North Sea Macroalgae in Relation to Temperature, Irradiance and Photoperiod. Helgoländer Meeresunters. 1980, 34, 15–29. [Google Scholar] [CrossRef]
- Lüning, K. Temperature tolerance and biogeography of seaweeds: The marine algal flora of Helgoland (North Sea) as an example. Helgoländer Meeresunters. 1984, 38, 305–317. [Google Scholar] [CrossRef]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine heatwaves under global warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Rohat, G.; Perroud, M.; Goyette, S.; Kasparian, J. Shifting velocity of temperature extremes under climate change. Environ. Res. Lett. 2020, 15, 34027. [Google Scholar] [CrossRef]
- Sfriso, A.; Wolf, M.A.; Maistro, S.; Sciuto, K.; Moro, I. Spreading and autoecology of the invasive species Gracilaria vermiculophylla (Gracilariales, Rhodophyta) in the lagoons of the north-western Adriatic Sea (Mediterranean Sea, Italy). Estuar. Coast. Shelf Sci. 2012, 114, 192–198. [Google Scholar] [CrossRef]
- Ramus, A.P.; Silliman, R.B.; Thomsen, M.S.; Long, Z.T. An invasive foundation species enhances multifunctionality in coastal systems. Proc. Natl. Acad. Sci. USA 2017, 114, 8580–8585. [Google Scholar] [CrossRef]
- Wolf, M.A.; Sfriso, A.; Moro, I. Thermal pollution and settlement of new tropical alien species: The case of Grateloupia yinggehaiensis (Rhodophyta) in the Venice Lagoon. Estuar. Coast. Shelf Sci. 2014, 147, 11–16. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, H.; Sheng, Y.; Lü, J.; Luan, R. Morphological observation and rbcL gene sequences studies of two new species, Grateloupia dalianensis H.W. Wang et D. Zhao. Sp. Nov. and G. yinggehaiensis H.W. Wang et R.X. Luan, sp. nov. (Halymeniaceae, Rhodophyta) from China. Acta Oceanol. Sin. 2012, 31, 109–120. [Google Scholar] [CrossRef]
- Life TRANSFER. Seagrass Transplantation for Transitional Ecosystem Recovery. LIFE19-NAT-IT-000264. Available online: www.lifetransfer.eu (accessed on 22 April 2025).
- Haslob, H.; Clemmensen, C.; Schaber, M.; Hinrichsen, H.-H.; Schmidth, J.O.; Voss, R.; Kraus, G.; Köst, F.W. Invading Mnemiopsis leidyi as a potential threat to Baltic fish. Mar. Ecol. Prog. Ser. 2007, 349, 303. [Google Scholar] [CrossRef]
- Roohi, A.; Fazli, H.; Rowshantabari, M.; Rahmati, R.; Khodaparast, N. Effect of an invasive species Mnemiopsis leidyi on the zooplankton community structure in the Caspian Sea. J. Great Lakes Res. 2024, 50, 102420. [Google Scholar] [CrossRef]
- Piccardi, F.; Poli, F.; Sguotti, C.; Tirelli, V.; Borme, D.; Mazzoldi, C.; Barausse, A. Assessing the impact of the invasive ctenophore Mnemiopsis leidyi on artisanal fisheries in the Venice Lagoon: An interdisciplinary approach. Hydrobiologia 2025, 852, 2387–2405. [Google Scholar] [CrossRef]
- Gavioli, A.; Mancinelli, M.; Turolla, E.; Lanzoni, M.; Paesanti, V.; Soana, E.; Eggleston, D.B.; Christian, R.R.; Castaldelli, G. Impacts of the invasive blue crab Callinectes sapidus on small-scale fisheries in a Mediterranean lagoon using fishery landing data. Sci. Tot. Environ. 2025, 974, 179236. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, F.; Fucilli, V.; Pinto, H.; Elston, J.N.; Carignani, A.; Petrontino, A.; Bozzo, F.; Frem, M. Socio-economic impacts of the recent bio-invasion of Callinectus sapidus on small-scale artisanal fishing in southern Italy and Portugal. Front. Mar. Sci. 2024, 11, 2024. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).