Abstract
The dissipation pattern and mobility of applied pesticides in the soil represent a crucial process for pesticide safety and subsequent groundwater contamination. In this study, two distinct experiments were conducted to explore the environmental fate, dissipation, and mobility of two pesticides, phorate and boscalid, in greenhouse conditions and laboratory soil column studies, respectively. The role of organic matter and growing conditions was evaluated during dissipation and mobility studies. In the first study, commercial formulations of phorate (10 G) and boscalid (20% SC) were sprayed in the designated greenhouse for Korean cabbage following the recommended dosage. A sequential collection of plant samples (e.g., 0, 7, 14, 21 days) was performed. On the other hand, three sets of packing columns were prepared (control, biochar-amended, and H2O2 treated). The effect of organic matter addition or removal during the leaching of pesticides was explored. A 14-day interval after the last spray was suggested for safe spraying. After 30 days of leachate collection, no pesticide residue was detected in the leaching water, indicating the immobility of the studied pesticides. However, the metabolic transformation of phorate was evident during this column study, with slight mobility within soil columns. In particular, phorate sulfoxide and sulfone were mostly detected in the top soil layer (vadose zone) of the soil column. In summary, phorate and boscalid were considered immobile pesticides with moderate persistence in the soils. The safe pre-harvest interval should be maintained to reduce the health risk of pesticides.
1. Introduction
In the global context, regular monitoring for residual pesticides in farming lands is performed to evaluate the judicious application of pesticides and their impact on the surrounding environment [1,2,3]. In general, residual pesticide monitoring is evaluated either under open-field conditions or in controlled environments such as greenhouse investigations [4]. The fate of pesticides is initially controlled by pesticide distribution (e.g., residue in soil, water, air, plant, and animals), followed by biogeochemical transformation or redistribution [5]. The most important factor is pesticide persistence, which is often measured by dissipation half-life [1]. However, the half-life calculation is not directive for the final destination of the pesticide, as the transformation into metabolites and final mineralization need to be considered [6,7]. However, pesticide persistence and mobility are highly dependent on pesticide chemical properties, followed by soil and environmental factors. In particular, pesticide persistence and mobility are governed by microclimates, site conditions, and application methods [8]. Briefly, spatial and temporal variation is evident in pesticide fate and mobility studies due to the wide variation in climatic factors [4].
Diversified pesticides are studied for residual persistence and risk assessment in edible crops as routine studies across the globe [9,10]. In recent years, pesticide residues of endosulfan, cyazofamid, spirotetramat, and atrazine have been evaluated in various vegetables and fruits with a potential risk of transformed metabolites [3,9,11,12]. Those studies noticed that the transformation of toxic metabolites from parent pesticides represents a critical risk due to higher toxicity, and persistence in the fields. Thus, pesticides and their toxic metabolites should be considered for safety evaluation studies [13]. Phorate, an organophosphate pesticide with restricted use due to moderate persistence, is considered a vital agrochemical in farming lands and can be found in surface water [7]. Phorate can be easily transformed into oxidative toxic metabolites (e.g., sulfoxide, sulfone, and oxon) at applied sites, while the toxicity and persistence of phorate metabolites have been found to be higher than in parent pesticides [14,15]. In addition, boscalid, a carboximide fungicide, is widely used in horticultural crops [16] and has also been found in selected water bodies in the USA [17]. Further, soil organic matter, soil texture, and pH together affect the environmental fate of boscalid and phorate [15,18]. Due to the wide variation in their environmental fates, various triggering factors, and research gaps in previous studies, meticulous studies are necessary to explore the fate and mobility of phorate and boscalid in soils.
The dissipation and sorption of pesticides are governed by soil organic matter; in particular, the exogenous organic amendment (such as organic manure and biochar) have a positive correlation with the enhanced sorption and degradation of pesticides in soils [19,20,21]. For instance, pesticide degradation is enhanced by the amendment of biochar derived from diversified cheap feedstocks [11,21]. Pesticide degradation can be influenced by three major factors (soil chemical factors, such as organic matter, pH, cation exchange capacity (CEC), and catalytic factors; soil physical factors, including sunlight, temperature, moisture, and soil texture; and soil biological factors, such as microbes and functional enzymes) [22,23,24]. On the other hand, pesticide mobility is assessed through leaching potential, and the leaching index of pesticides is measured through Groundwater Ubiquity Score (GUS) values [8,25].
Dissipation studies on pesticides are performed either under open-field conditions [26,27], under controlled field conditions such as in a greenhouse [28], or under laboratory conditions [29]. As a result, a wide variation in dissipation patterns is evident when employing the same pesticide under various growing conditions. In contrast, the mobility of pesticides is evaluated under open-field conditions (e.g., with a field lysimeter) or in laboratory column experiments [8,30]. As a result, every experimental result is different due to diversified setups, even when employing the same pesticides. The leaching potential and mobility of phorate are dependent on the sorption capability of pesticides with soils, as the sorption of pesticide is evaluated by the distribution coefficient (Kd) and sorption coefficient (Koc) values [7]. Previous research has revealed the higher sorption of phorate and terbufos with soil particles, indicative of a possible lower tendency of leaching within the soil profile [15].
In previous studies, the dissipation of phorate and boscalid has been sparingly studied, while their mobility has not been meticulously concluded [15,31]. Thus, there is a gap in the information regarding the environmental fate and leaching index of phorate and boscalid. Considering this research gap, this study attempted to explore the dissipation behavior of phorate and boscalid through a greenhouse study and their mobility using laboratory-packed soil columns, respectively. The specific objectives of this study were to (i) study the effect of pesticide application methods on persistence and dissipation half-life evaluation in phorate and boscalid through greenhouse investigations, (ii) predict the safe pre-harvest interval (PHI) for phorate and boscalid application and perform risk assessments in greenhouses on Korean cabbage, (iii) compare the effect of organic matter during the leaching potential assessment of two pesticides using packing soil columns under laboratory conditions, and (iv) detect the transformation products (TPs) of phorate and their distribution in various depths in a soil column during leaching investigations.
2. Materials and Methods
2.1. Chemicals and Reagents
Two commercial formulations of pesticides (Phorate 10 G and Boscalid 20% SC) were purchased from Nonghyeop Co. Ltd. (Seoul, Republic of Korea). The analytical-standard (PESTANAL®) of phorate (purity ≥ 95.0%) and boscalid (purity ≥ 95.0%) were procured from Supelco and originated from Sigma-Aldrich International (Burlington, MA, USA). The vital physicochemical properties of the two pesticides are presented in Table 1. In addition, other related reagents (e.g., HPLC-grade water, acetonitrile, ammonium format, and formic acid) were obtained from Merck KGaA, Darmstadt, Germany. The other chemicals for minor uses including methanol, sodium hydroxide, and HCl were supplied by Fisher Scientific Ltd. (Seoul, Republic of Korea). The preparation of the stock solution and working solution of the chemicals studied were performed following the dilution method using appropriate organic solvents.

Table 1.
Physicochemical properties of studied pesticides.
2.2. Field Trial (Greenhouse Experiment)
A field trial was conducted in the greenhouse for dissipation studies on the two studied pesticides, phorate and boscalid, in Korean cabbage. A 120 m2 sized greenhouse cultivated with Korean cabbage located in the experimental field of Kyungpook National University, Republic of Korea, was used for the field investigation. The indoor temperature of the greenhouse was 11.2–21.4 °C, and the humidity was 15.4–63.2% throughout the experimental period. The detailed climatic observations data are available in Figure S1 (Supplementary File). The total greenhouse area was divided into 30 m2 four plots for four treatments (i.e., 0, 7, 14, and 21 PHIs). The plots were subdivided into 10-m2 (5 m × 2 m) subplots for the replications (n = 3) of treatments. Two commercial formulations of Phorate 10 G and Boscalid 20% SC were applied at the recommended rate. In contrast, Phorate was applied as a soil application followed by gentle irrigation for proper mixing. However, only water was sprayed on the control plot.
In this study, two methods of pesticide application were evaluated: foliar spray and soil applications. The dissipation pattern and persistence of the applied pesticides were varied due to the methods of pesticide application and types of formulation. After the specific period, the crops (Korean cabbage) were harvested, maintaining a PHI time of 21, 14, 7, and 0 days, respectively, after final spraying. The phorate persistence in soil and plant uptake at different days after application was also evaluated. Immediately after harvesting, the collected plant samples and soil samples were packed in iced boxes and transferred to the laboratory for further analysis.
2.3. Soil Column Experiment
A laboratory soil column experiment was conducted using a packed soil column (30 cm length and 5 cm diameter PVC column) under controlled laboratory conditions following the Organisation for Economic Co-operation and Development (OECD) guidelines [32]. The packing of the column using two different soils (sandy loam and loam) was prepared using a 2.00 mm sieve, and the physicochemical features of the soil are available in the Supplementary File (Table S1). The column packing was performed using a hammer, plank, and vibrator to avoid unexpected pores within the soil column layers. After filling the column with specified soils, the packed soil column was kept in water overnight to allow the capillary movement of water within the soil column. After the complete saturation of the packed soil column, the columns were moved to the plastic stands for the removal of excess water by the force of gravity. Briefly, three sets of columns were prepared for each type of soil: (i) the control column (only homogeneous soil packing), (ii) the biochar treated (the top 5 cm was amended with 5% biochar), and (iii) the H2O2-treated soil column. The salient features of the biochar used in this study are available in the Supplementary File (Table S2). The prime goal of the soil column test is to evaluate the effect of organic matter addition or removal during a leaching study on pesticides. Biochar is amended as an organic matter amendment, and H2O2-treated soil is used for organic matter removal purposes. A detailed description of biochar amendment and H2O2 treatment is available in the Supplementary File (Figure S2). As a result, a total of 12 soil columns were prepared using two types of soil (sandy loam and loam) with duplicate replications.
The designated pesticide with a specific concentration (200 µg/kg) was added to the top soil layer and kept for the drying of the solvent. After that, leaching was started, and a weekly leachate collection was performed over 30 days of soil column leaching investigation. The column set illustration is presented in Figure 1. In this study, two types of irrigation were assessed: (i) recommended irrigation (5 mL/day) and (ii) double the recommended irrigation (10 mL/day). After the leaching experiment, the soil column was kept in a freezer overnight, sectioned (each section was 10 cm in length), and air-dried until use in further analysis.
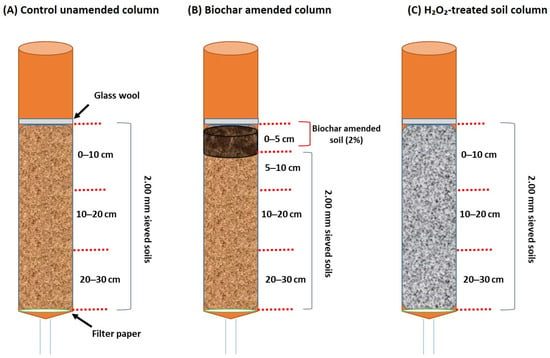
Figure 1.
Three sets of columns, including (A) control unamended column, (B) biochar amended column, and (C) H2O2-treated soil column, were designed for leaching and mobility assessment of two pesticides (phorate and boscalid).
2.4. Extraction of Soils and Water Sample Processing
2.4.1. Plant Sample Extraction
Uniform ground samples were made by grinding frozen plant samples under cryogenic conditions and stored at −20 °C until analysis. These samples remained viable for further analytical procedures for up to 30 days, as previously reported [3,12]. The modified QuEChERS technique was applied for extraction and clean-up. To extract residues, 10 g of ground plant material was combined with 20 mL of acetonitrile and 1% formic acid. The mixture was then agitated in a multitube shaker (Collomix shaker VIBA X.30; Gaimersheim, Germany) at 2500 rpm for 20 min. The extract was added with NaCl (1.0 g) and shaken for 1 min manually. The mixture was then centrifuged at 4000 rpm for 5 min. For the best possible recovery of metabolites produced from phorate, no additional commercial sorbents were utilized throughout the clean-up process. As a result, the complexity of the pigmented sample clean-up procedure was reduced to sequential centrifugation and filtration without any commercial sorbents. For LC-MS/MS analysis, the final vial was prepared by collecting 2 mL of the supernatant and filtering through a 0.2 μm PVDF syringe filter (Figure S3).
2.4.2. Extraction of Field Soil and Column Soils
The leachate collected at a specified period was filtered immediately after collection, and the leachate was analyzed through LC-MS/MS. It is noted that during leachate filtration, no extraction and purification was performed. However, after the 30-day leaching experiment, the soil columns were kept freezing (−20 °C) overnight (24 h). Additionally, the frozen columns were divided into segments, each with a soil depth of 10 cm, and they were air-dried for a whole day. A QuEChERS extraction (EN kit) of the column soils (separated at various depths) was performed for the extraction of residual pesticides and associated metabolites. In short, a conical tube was filled with a 10 g soil sample. After adding 10 milliliters of distilled water and 10 milliliters of acetonitrile, the mixture was shaken for ten minutes. A QuEChERS extraction pouch was added to separate the extracted pesticide from the water, and a dSPE kit was used for cleaning up the centrifuged samples. It should be noted that the possibility of losing transformed metabolites during this leaching experiment was minimized by the avoidance of using clean-up materials (e.g., C18, graphitized carbon black-GCB, or florisil), keeping only MgSO4 and PSA.
2.5. Instrumental Analysis and Quality Control
Agilent liquid chromatography (LC) derived from the Agilent Technologies 1200 series coupled with a 5500 AB Sciex Q TRAP tandem mass spectrometer (MS/MS) was used for the simultaneous analysis of two pesticides, phorate and boscalid, and five oxidative metabolites of phorate (sulfoxide, sulfone, oxon, oxon sulfoxide, and oxon sulfone). A C18 column (Kinetex 100 × 4.6 mm, 3 µm) supplied by Phenomenex was employed as the stationary phase to separate the studied chemicals at a specific retention time (RT). Mobile phase A (methanol with 0.1% formic acid and 5 mM ammonium formate) and B (distilled water with 0.1% formic acid and 5 mM ammonium formate) were used in a gradient mode throughout the analysis period. In addition, MS/MS was performed under positive electrospray (ESI+) through MRM (multiple reaction monitoring) parameters. The detailed analytical explanation and MRM are available in a Supplementary File (Figure S4 and Table S3).
The specificity, linearity, recovery, limit of quantification (LOQ), and limit of detection (LOD) were assessed as to whether they satisfied the quality control or validation of the analytical method. Briefly, the matrix-matched calibration curve was evaluated to study the linearity, while the specificity was assessed by comparing standard and blank chromatogram observations. In short, LOD is the lowest concentration of the analytes that can be detected during precision analysis using LC-MS/MS. On the other hand, the limit of quantification (LOQ) is the lowest concentration that can be measured with an acceptable level of accuracy and precision, whereas LOD indicates the ability to detect an analyte.
2.6. Calculation of Half-Life and Leaching Index
The dissipation pattern of the studied pesticides followed first-order kinetics and can be expressed as Equation (1), as follows [3]:
where Ct represents the concentration (mg/kg) after time t (days), C0 is the initial concentration (mg/kg), and k is the rate constant.
Ct = C0 e−kt
Similarly, the half-life for each pesticide was calculated using Equation (2):
where t1/2 is the half-life (days), and K is the rate constant.
t1/2 = ln2/k
The maximum residue limit (MRL) of specific pesticides is derived from the Ministry of Food and Drug Safety (MFDS) MRL database [33].
On the other hand, the leaching potential is calculated by the Groundwater Ubiquity Score (GUS) Equation (3), and Leachability Index (LIX) Equation (4).
(GUS > 2.8: Mobile; 1.8 > GUS < 2.8: Transition; GUS < 1.8: Immobile)
(LIX = 1: Very mobile; 0.1 > LIX < 1: Mobile; 0 > LIX < 0.1: Transition; LIX = 0: Immobile), where k = first-order rate constant, t½ = half-life (days) of pesticide, KOC = coefficient of adsorption to organic carbon.
GUS = logt½ · (4 − logKOC)
LIX = exp (–k · KOC)
2.7. Statistical Analysis
The statistical analysis of this study was performed by Microsoft Excel with Solver Add-in. The prepared graphs are rectified by SigmaPlot 14.5. Unless specified otherwise, the mean values of three replicates are presented in the tables.
3. Results and Discussion
3.1. Method Validation
The method validation was performed through the specificity, linearity, recovery, LOD, and LOQ of the analysis. In this study, the overall quality control of the analytical method was performed. In a nutshell, the linearity of this established method was proven by a calibration curve prepared by 5 µg/L to 100 µg/L. The coefficient of determination (r2) ≥ 0.99 indicates acceptable linearity. The set LOQ is 10 µg/kg, and LOD is 2 µg/kg for the analysis of the two studied pesticides. This setting of LOQ and LOD is based on the signal–noise ratio (S/N ratio) of the LC-MS/MS instrument. In addition, the accuracy of this method is established through recovery (%) assessment. The calculated average recovery (%) was within the range of 85.3–104.8%, and the %RSD (relative standard deviation) was within 1.2–4.1%. As a result, this proposed method for the simultaneous analysis of boscalid, phorate, and five metabolites of phorate was established with acceptable analytical validation criteria (Table 2).

Table 2.
Recovery (%), relative standard deviation (RSD %), LOD, and LOQ of the analytes during the simultaneous analysis of boscalid and phorate, including their five metabolites.
The validation of the analytical method is a routine task for the quality control of any suggested or proposed analytical method. The acceptable quality control criteria in terms of linearity, specificity, recovery, LOQ, and LOD are evident in earlier studies [20,27,34]. For instance, a recent study established an efficient method for multi-residue pesticide analysis including organophosphate and pyrethroid, while average recoveries varied from 105.54% to 120% with an RSD value from 0.7% to 4.8% [35]. In addition, the proposed method of flumioxazin analysis in four Chinese soils was validated by established LOQ (0.01 mgKg−1), acceptable recovery (78.8% to 102.8%), and linearity (r2 ≥ 99) [36]. Our proposed method is highly precise and falls within the quality criteria for the simultaneous analysis of phorate and boscalid using LC-MS/MS.
3.2. Dissipation Pattern and Half-Life
Dissipation behavior and half-life (t1/2) calculations were calculated using first-order kinetic equations for two distinct studies including (i) field observations and (ii) laboratory soil column investigations. In the field condition (first study), a commercial formulation of boscalid was sprayed over standing crops, while phorate was mixed with soils. Thus, the half-life of boscalid, 20% SC (Soluble Concentrate) was calculated for Korean cabbage and soils separately. In particular, the half-life was calculated at various pre-harvest intervals (PHIs) (Table 3). On the other hand, phorate is a soil-applied pesticide; thus, the half-life was calculated in the soil at a specific PHI (21 days). In general, foliar-sprayed pesticides are initially adsorbed or deposited in the foliage of standing crops, followed by soil deposition. As a result, the half-life of boscalid was calculated both for Korean cabbage and for soils. According to Table 3, the dissipation half-life of the applied boscalid varied with different PHIs in greenhouse Korean cabbage. In addition, a longer persistence of boscalid was evident in soils compared to Korean cabbage foliage.

Table 3.
Half-life of phorate and boscalid at different PHIs (pre-harvest intervals) both in field investigations and laboratory column studies.
On the other hand, in the second study using laboratory soil columns, the dissipation half-lives of boscalid and phorate were compared by calculating the total residue in the soils of columns. This study focuses on the influence of organic matter addition (e.g., biochar amendment) or organic matter removal (e.g., H2O2 treated soils) during this half-life investigation. The addition of biochar as an organic amendment accelerates the degradation speed of the studied pesticides and shows a short half-life value compared to H2O2-treated columns. The partial removal of organic matter through H2O2 treatment causes the longer persistence of phorate and boscalid as compared to the control and biochar-treated columns. The calculation of half-life is crucial to the establishment of safety guidelines for pesticide applications, including maximum residue limit (MRL) and safe pre-harvest interval (PHI) evaluations [1,2,10]. Soil organic matter is considered a key factor for controlling the sorption, degradation, and persistence of pesticides applied in soils [11,19,31]. The addition of exogenous organic matter causes the accelerated degradation of pesticides due to higher soil activity including microbial stimulations and activated catalytic properties [19,37]. In the presence of amended organic matter, soil microbial activity is triggered and the functional enzymes become reactive for the efficient removal of pesticides and their toxic metabolites in soils [15,37]. In addition, organic amendment can enhance the adsorption capacity of soils for various organic pollutants. In addition, organic matter removal by H2O2 treatment can resist pesticide dissipation [38]. On the other hand, the pesticide dissipation pattern in plant foliage is governed by the leaf orientation, leaf area, and stomatal penetration of pesticides [3,6,28]. Thus, separate studies are needed to explore pesticide dissipation under different soil and plant foliage conditions to more accurately predict pesticide fate and environmental persistence.
The specific dissipation pattern is illustrated in Figure 2. According to Figure 2, the dissipation pattern of boscalid exponentially increased over time. The lowest residual boscalid (0.09 mg/kg) was detected 21 days after the final spray. In contrast, the dissipation behavior of pesticides in soil exhibited the opposite scenario. In general, a soil-applied pesticide (e.g., phorate) is initially deposited in soil, and subsequent uptake by plant roots is evident. In contrast, foliar-applied pesticides (e.g., boscalid) are initially deposited in plant foliage, followed by soil deposits. In Korean cabbage, the maximum residue limit (MRL) set by the Korean government (MFDS) for boscalid is 3.0 mg/kg, and for phorate, it is 0.05 mg/kg [33]. Thus, 14 days is considered a safe PHI compared to the MRL of the studied pesticides.
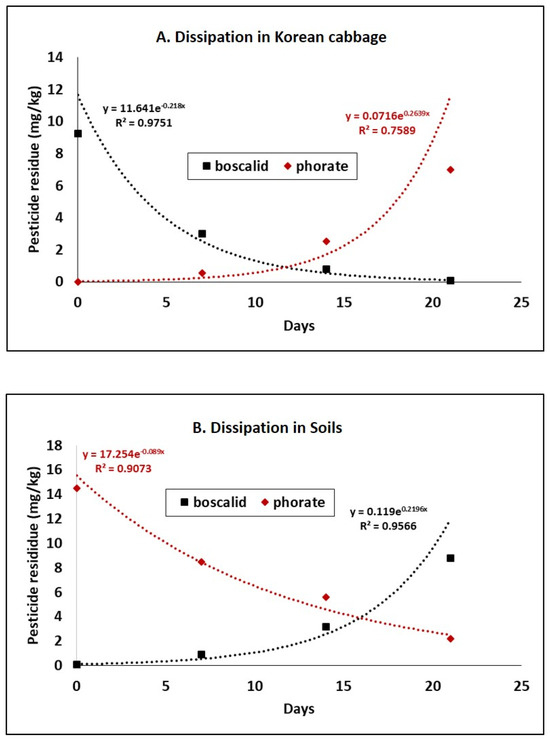
Figure 2.
Dissipation patterns of boscalid and phorate in Korean cabbage (A) and soils (B), respectively. The dissipation behavior at different PHIs is exponentially illustrated to explore the dissipation rate and associated factors.
3.3. Distribution of Pesticides in the Soil Column
In the soil column study, the distribution of pesticides (e.g., boscalid and phorate) was measured at different soil layer depths. The packed soil column revealed that most of the detected phorate was within the top soil column layer (10 cm) and no phorate was found in the deeper soil layer (e.g., at 20 and 30 cm depth in the soil column) irrespective of the soil texture (e.g., sandy loam and loam soil) (Table 4). Thus, phorate was considered a less mobile or immobile pesticide. On the other hand, boscalid residue was distributed both in the 10 cm layers and 20 cm layers of soil columns, indicating that boscalid has a more moderate mobility than phorate. However, the biochar-amended columns showed a more rapid disappearance of parent pesticides than the H2O2-treated soil columns. This may happen due to the biochar-amended rapid transformation of studied boscalid and phorate in the soil column investigation through augmented organic carbon. In a previous study, the faster degradation of pesticides was evident [15]. The slow movement of pesticides is also documented due to biochar amendment in the soil column due to higher adsorption by the biochar-amended soil column [19]. Further studies are recommended for elucidating the exact mechanism behind the faster degradation of pesticides in biochar-amended soil treatments.

Table 4.
Distribution of phorate and boscalid residue in different layers of soil column after 30-day soil column investigations.
The total distribution of phorate and boscalid is illustrated in Figure 3. According to Figure 3, the top layer of the soil column (0–10 cm) is considered an “active zone” for the distribution of both parent pesticides and metabolic transformation. Most of the residual phorate and its metabolites were found in the vadose zone (0–10 cm top soil layer) of the soil column. On the other hand, boscalid is moderately distributed in the sub-soil region of the column (e.g., the 10–20 cm soil layer). In general, the strong adsorption potential of pesticides tends to bind with soil particles and have less tendency toward leaching loss. The studied pesticides (i.e., phorate and boscalid) are hydrophobic and exhibited a strong sorption potential with soil surfaces, as reported by previous studies [15,20]. As a result, the distribution of parent pesticides, including their metabolic transformation, was found to be limited within the surface layer of soil columns. In contrast, the herbicides with high water solubility were found to be distributed in the deep soil layer and may have caused groundwater contamination through leaching [8,25]. Thus, comparative studies should be conducted with high hydrophobic and hydrophilic pesticides in soil column investigations.
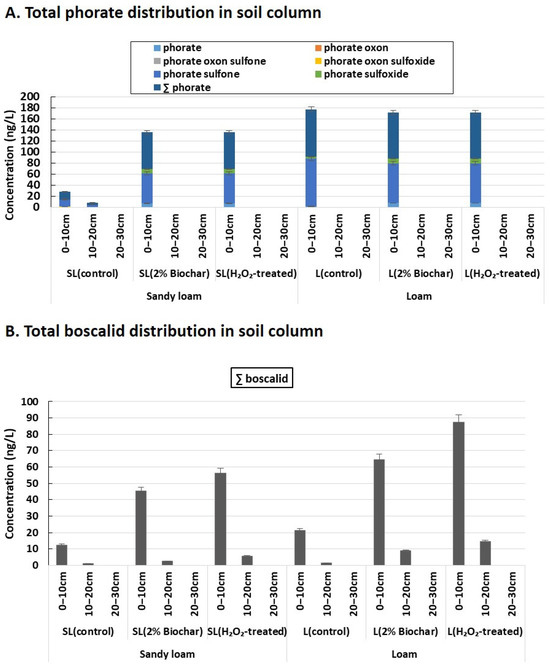
Figure 3.
Total distribution of phorate and boscalid into the soil columns. The distribution of phorate, including metabolites (A), and boscalid in three sets of columns using sandy loam (SL) and loam (L) soils (B).
3.4. Distribution of Toxic Metabolites of Phorate in the Soil Column
As the previous distribution study noticed, the phorate distribution was limited to the top soil layer (10 cm vadose zone) (Table 4). Thus, further detailed studies were conducted to identify the phorate metabolites in the layers of soil columns. In general, phorate can be transformed into five oxidative and toxic metabolites in soils. Thus, in this study, the transformation of parent phorate into metabolites was meticulously investigated in a soil column assessment. The metabolite transformation and distribution were positively mediated by biochar amendment and negatively correlated by H2O2 treatment (Figure 4). It should be noted that the irrigation rate (e.g., 5 mL/day or 10 mL/day) had no significant effects on pesticide distribution and metabolic transformation. As the metabolite transformation was mediated by soil organic matter and microbes, including their functional enzymes, the amendment of biochar enhanced the metabolic transformation. In contrast, the removal of soil organic matter by H2O2 treatment caused slow metabolic transformations. Interestingly, the biochar amendment enhanced pesticide degradation, as well as increasing the risk of the emergence of potentially toxic metabolites. This metabolic transformation, mediated by organic amendment, is considered a hidden threat to pesticide safety management [19,21]. Our study indicated that the distribution of parent pesticides and transformed metabolites was mostly limited to the top soil layer (0–10 cm vadose zone), irrespective of irrigation rates or organic matter treatments. In a previous study, the rhizosphere (top 10 cm root zone) was considered a vital zone for soil chemistry and the interaction of soil microbes due to root exudates and microbial biodiversity [39]. Due to the multifaceted feature of the topsoil zone, most of the pesticide adsorption, degradation, and metabolic transformation occurred in this soil zone. In contrast, the microbial activity decreased due to a shortage of nutrients and oxidative pressure in the deeper layer of soils.
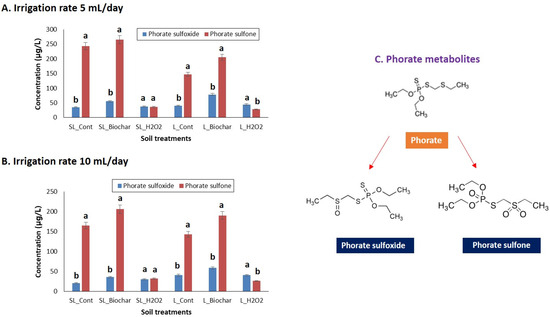
Figure 4.
Role of organic matter addition or removal in metabolic transformation of phorate in soil columns; (A) metabolic transformation at 5 mL/day irrigation rate, (B) metabolic transformation at 10 mL/day irrigation rate, and (C) the detected metabolites of phorate. The data shown in the bar graphs are statistically significant (p < 0.05). The error bar in the graph shows the standard deviation (SD) of means (n = 3). The similar letters a indicating the non-significance and the dissimilar letters (e.g., ‘a’ and ‘b’) indicating the significance at p < 0.05.
3.5. Role of Organic Matter in Metabolic Transformation
The role of organic matter is closely evaluated in phorate transformation into toxic metabolites, and the comparative metabolic transformation is illustrated in Figure 4. In general, organic amendment is used for soil conditioning including organic carbon augmentation, ultimately improving the overall fertility status of soils. In this soil column study, the top soil layer (0–5 cm) was amended with organic amendment (2% biochar) to evaluate the effect of biochar during leaching and metabolic transformation within the soil column. Similarly, H2O2-treated soils were used for representing the partially organic matter-removed soil. The H2O2-treated soil columns are expected to cause zero metabolic transformation due to a lack of organic matter. However, the functional enzymes of soils that remain in the H2O2-treated soils become reactivated after the hydration of soil columns and perform the slow degradation and metabolic transformation of phorate in soil columns. In general, H2O2-treated soil columns lack organic carbon, resulting in the slow degradation of pesticides and sluggish metabolic transformation. As a result, the organic amendment acted as the positive factor for enhanced pesticide adsorption and degradation, while H2O2 treatment (soil organic matter removal) was treated with negative factors. The role of organic amendment during pesticide degradation and leaching studies is documented in previously published papers [40,41]. The addition of organic matter will increase the amount of dissolved organic matter (DOM) in soils. This DOM can improve the soil adsorption capacity. The quality and concentration of DOM (derived from organic amendment) are controlled by the types of feedstock of organic amendment and the processing factors [19]. Several earlier studies demonstrated that the addition of organic amendments such as biochar derived from diversified feedstock had a positive correlation with the enhanced sorption and degradation of pesticides in soils [42]. On the contrary, H2O2-treated soil has negligible organic carbon and retards the degradation and metabolic transformation of pesticides in soils [38]. However, no studies have documented a comparative assessment of organic matter addition and organic matter removal during the degradation and leaching of pesticides. Thus, the current study will be the first study to compare the influence of organic matter addition and removal during pesticide degradation and leaching studies. In particular, the comparative study for exploring the effect of organic amendment with biochar and organic matter removal technology (H2O2 washing and boiling) during phorate and boscalid degradation and mobility in soils is a pioneering study.
3.6. Mobility and Leaching Index
Among the various technologies for the leaching test of pesticides in soils, we employed the packed soil column investigation under laboratory conditions following OECD guidelines [32]. The mobility and leaching of applied pesticides into the soil layer are dependent on the pesticide adsorption capability, solubility, degradation pattern, and metabolic transformation of the specific pesticide [40]. To understand the potential risk of pesticides to groundwater and surface water, determining the leaching index and mobility potential of specific pesticides is necessary. For example, the diversified leaching behavior of five pesticides was documented using frozen and unfrozen soil columns [8]. The leaching index values include GUS, and LIX (the values of GUS and LIX can be derived from eq. iii and eq. iv from the Methodology Section). According to the GUS and LIX values, both pesticides were considered “immobile”, and no residual pesticides were detected in the leachate collected either from 5 mL/day or 10 mL/day irrigation rates. The details of GUS, LIX, and leaching index in this study and adopted from previous study are presented in Table S4 [41]. According to the data in Table S4, there is no risk of groundwater contamination by parent pesticides such as phorate and boscalid. Interestingly, a trace amount of phorate sulfoxide (0.9 mg/kg) was detected in the leachate collected from the fourth week (10 mL/day). This is indicative of the slight mobility of transformed metabolites into groundwater, posing the risk of groundwater contamination as compared to parent pesticides. Previously published research has demonstrated that biochar amendment can modulate the leaching behavior of pesticides in soil column studies [43,44]. In particular, biochar prepared from high-temperature pyrolysis can reduce pesticide mobility and affect the sorption and degradation magnitude [43]. In general, the higher sorption mediated by biochar amendment can cause decreased leaching. In this study, regardless of biochar treatments, the studied pesticides showed immobility in the column test. However, more detailed studies can be performed for aging column experiments and real field studies for exploring both the leaching and surface runoff potential of phorate and boscalid.
3.7. Limitations of the Study
In this study, two separate experiments including field observation for the dissipation of studied pesticides and packed soil column investigations for exploring the leaching and mobility of pesticides in soils were developed. These experimental conditions were limited to the protocol and guidelines provided by regulatory authorities. Thus, several research pitfalls, described below, were identified during those studies and can be explored by further studies to minimize these research shortcomings.
- (a)
- This study was conducted under greenhouse conditions; thus, the safe PHI derived from this study cannot be used as a reference value if a crop is grown under open-field conditions. As a result, the climatic conditions should be considered during open-field dissipation investigations.
- (b)
- The half-life calculation followed first-order kinetics, but many recent studies indicate that pesticide dissipation does not always follow the first-order rate decline [1,45]. Thus, second-order kinetics or dual kinetics should be considered and corroborated to reduce data biases.
- (c)
- The column study followed the OECD guidelines for packed soil column experiments; however, other guidelines can be compared for more specific investigations. Moreover, a field lysimeter can be installed and the leaching behavior can be assessed under laboratory soil column and field conditions to minimize the climatic factors mediating data variations.
- (d)
- In this study, biochar derived from rice straw was used as an organic amendment. Several organic amendments derived from wastewater are gaining popularity for this purpose as a new strategy for sustainable waste management. Future studies can be designed to explore more organic amendments to enhance the sustainable management of waste and organic pollutants.
4. Conclusions
In summary, phorate and boscalid exhibited diversified dissipation patterns under different growing conditions and application methods. In particular, the half-life of boscalid in Korean cabbage varied with different pre-harvest intervals (PHIs). The dissipation rate of applied pesticide increased over time (i.e., the PHI) due to stomatal penetration and subsequent metabolic transformation. The dissipation behavior of the two pesticides varied in soils according to the solubility, formulation, and application method in the fields. Boscalid is applied as a foliar spray, while phorate is mixed as a soil pesticide. Thus, the application method of pesticides triggers the dissipation pattern of specific pesticides. The dissipation of pesticides is critically governed by the method of application in the field. On the other hand, a soil column study demonstrated that both boscalid and phorate were non-leachers (i.e., no residual pesticide was detected in leachate) irrespective of irrigation rates and soil types. A slight mobility was evident for phorate sulfoxide in the leachate at 30 days after column investigations. The dissipation half-life was also evaluated through column tests to compare the effect of organic matter addition (e.g., biochar amendment) and organic matter removal (e.g., H2O2 treatment). The addition of biochar as an organic amendment exhibited a positive correlation with pesticide degradation and reduced the leaching movement of pesticides into the soil column layers. However, H2O2 treatment is considered a negative factor for enhanced pesticide degradation. Further studies should be attempted to explore the climatic factors during pesticide dissipation and mobility assessments. In particular, real field research, the application of different organic matters, and diversified methods should be adopted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12040123/s1, Figure S1: Indoor weather conditions (Temperature and Humidity) during the test period for monitoring dissipation of phorate and boscalid under Greenhouse conditions, Figure S2: The flowchart shows the soil sample preparation including sieving and autoclaving during adsorption studies using biochar derived from rice husk as an organic amendment, Figure S3: A simplified flow diagram showing the EN QuEChERS method for soil column extraction (A), and extraction of Korean cabbage (B), for two studied pesticides, following the OECD guidelines. Figure S4: Instrumental parameter and technical feature of LC-MS/MS during the simultaneous analysis of Terbufos and Phorate in investigated Korean cabbage and soil samples.; Table S1: Chemical features (pH, organic carbon-OC %) of studied soils and soil treatments, Table S2: Physicochemical feature of rice husk biochar (pristine form fresh biochar) (supplied by commercial biochar supplier Korea Biochar Agricultural Corporation Co. Ltd.), Table S3: MRM parameters for determination of pesticides. Table S4: Leaching index including Groundwater Ubiquity Score (GUS), Leachability Index (LIX) of phorate and boscalid using soil column investigation.
Author Contributions
Conceptualization, A.S. and R.N.; methodology, R.N.; investigation, R.N.; data curation, M.M.R.; writing—original draft preparation, A.S. and R.N.; writing—review and editing, M.M.R. and A.K.H.; supervision, A.S. and A.K.H.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2023 MFDS (Ministry of Food and Drug Safety) research fund.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors are grateful to the laboratory staff and technicians for their kind support and technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Nandi, R.; Kwak, S.Y.; Lee, S.H.; Sarker, A.; Kim, H.J.; Lee, D.J.; Heo, Y.J.; Kyung, K.S.; Kim, J.E. Dissipation Characteristics of Spirotetramat and Its Metabolites in Two Phenotypically Different Korean Vegetables under Greenhouse Conditions. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 964–976. [Google Scholar] [CrossRef]
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Islam, T.; Rahman, S.; Nandi, R.; Kim, J.E. Uncertainty of Pesticides in Foodstuffs, Associated Environmental and Health Risks to Humans—A Critical Case of Bangladesh with Respect to Global Food Policy. Environ. Sci. Pollut. Res. 2021, 28, 54448–54465. [Google Scholar] [CrossRef]
- Dar, M.A.; Baba, Z.A.; Kaushik, G. A review on phorate persistence, toxicity and remediation by bacterial communities. Pedosphere 2022, 32, 171–183. [Google Scholar] [CrossRef]
- Holten, R.; Larsbo, M.; Jarvis, N.; Stenrød, M.; Almvik, M.; Eklo, O.M. Leaching of Five Pesticides of Contrasting Mobility through Frozen and Unfrozen Soil. Vadose Zone J. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A Worldwide Review of Currently Used Pesticides’ Monitoring in Agricultural Soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade Impact of Maximum Residue Limits in Fresh Fruits and Vegetables. Food Policy 2022, 106, 102203. [Google Scholar] [CrossRef]
- Deng, H.; Feng, D.; He, J.X.; Li, F.Z.; Yu, H.M.; Ge, C.J. Influence of biochar amendments to soil on the mobility of atrazine using sorption-desorption and soil thin-layer chromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Sarker, A.; Lee, S.H.; Kwak, S.Y.; Nam, A.J.; Kim, H.J.; Kim, J.E. Residue Monitoring and Risk Assessment of Cyazofamid and Its Metabolite in Korean Cabbage Under Greenhouse Conditions. Bull. Environ. Contam. Toxicol. 2020, 105, 595–601. [Google Scholar] [CrossRef]
- Medina-Pastor, P.; Triacchini, G. The 2018 European Union report on pesticide residues in food. EFSA J. 2020, 18, e06057. [Google Scholar] [CrossRef]
- Chen, J.P.; Pehkonen, S.O.; Lau, C.C. Phorate and Terbufos adsorption onto four tropical soils. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 55–61. [Google Scholar] [CrossRef]
- Sarker, A.; Kim, T.K.; Kim, S.I.; Jeong, W.T. Influence of Soil Types and Organic Amendment During Persistence, Mobility, and Distribution of Phorate and Terbufos in Soils. Korean J. Pestic. Sci. [CrossRef]
- Smith, D.L.; Garrison, M.C.; Hollowell, J.E.; Isleib, T.G.; Shew, B.B. Evaluation of Application Timing and Efficacy of the Fungicides Fluazinam and Boscalid for Control of Sclerotinia Blight of Peanut. Crop Prot. 2008, 27, 823–833. [Google Scholar] [CrossRef]
- Reilly, T.J.; Smalling, K.L.; Orlando, J.L.; Kuivila, K.M. Occurrence of Boscalid and Other Selected Fungicides in Surface Water and Groundwater in Three Targeted Use Areas in the United States. Chemosphere 2012, 89, 228–234. [Google Scholar] [CrossRef]
- Bhatt, D.; Srivastava, A.; Srivastava, P.C. An Insight into the Sorption Kinetics of Boscalid onto Soils: Effect of General Soil Properties. Chemosphere 2023, 325, 138274. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukhopadhyay, A.; Mukherjee, I. A Laboratory Study on Adsorption–Desorption Behavior of Flupyradifurone in Two Indian Soils: Effect of Soil Properties and Organic Amendment. J. Soils Sediments 2022, 22, 2022–2035. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Crépet, A.; Luong, T.M.; Baines, J.; Boon, P.E.; Ennis, J.; Kennedy, M.; Massarelli, I.; Miller, D.; Nako, S.; Reuss, R.; et al. An International Probabilistic Risk Assessment of Acute Dietary Exposure to Pesticide Residues in Relation to Codex Maximum Residue Limits for Pesticides in Food. Food Control 2021, 121, 107563. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, Distribution Pathways and Effects on Human Health—A Review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Živančev, N.V.; Kovačević, S.R.; Radović, T.T.; Radišić, M.M.; Dimkić, M.A. Mobility and sorption assessment of selected pesticides in alluvial aquifer. Environ. Sci. Pollut. Res. 2019, 26, 28725–28736. [Google Scholar] [CrossRef]
- Karlsson, A.S.; Weihermüller, L.; Tappe, W.; Mukherjee, S.; Spielvogel, S. Field Scale Boscalid Residues and Dissipation Half-Life Estimation in a Sandy Soil. Chemosphere 2016, 145, 163–173. [Google Scholar] [CrossRef]
- He, Y.; Meng, M.; Yohannes, W.K.; Khan, M.; Wang, M.; Abd EI-Aty, A.M.; Hacımüftüoğlu, F.; He, Y.; Gao, L.; She, Y. Dissipation Pattern and Residual Levels of Boscalid in Cucumber and Soil Using Liquid Chromatography-Tandem Mass Spectrometry. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Jeong, W.T.; Kyung, K.S.; Lee, H.D.; Kim, D.; Song, H.S.; Kang, Y.; Noh, H.H. Dissipation and Distribution of Picarbutrazox Residue Following Spraying with an Unmanned Aerial Vehicle on Chinese Cabbage (Brassica campestris var. pekinensis). Molecules 2021, 26, 5671. [Google Scholar] [CrossRef]
- Lv, L.; Su, Y.; Dong, B.; Lu, W.; Hu, J.; Liu, X. Dissipation Residue Behaviors and Dietary Risk Assessment of Boscalid and Pyraclostrobin in Watermelon by HPLC-MS/MS. Molecules 2022, 27, 4410. [Google Scholar] [CrossRef]
- Negi, S.; Srivastava, A.; Pachauri, S.P.; Pathak, A.; Srivastava, P.C. Modulating Leaching Behavior of Boscalid Fungicide in Soil Through Organic Amendments. Soil Sediment Contam. 2024, 34, 71–84. [Google Scholar] [CrossRef]
- Loffredo, E.; Carnimeo, C.; D’Orazio, V.; Colatorti, N. Sorption and Release of the Pesticides Oxyfluorfen and Boscalid in Digestate from Olive Pomace and in Digestate-Amended Soil. J. Soils Sediments 2024, 24, 1489–1506. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Test No. 312: Soil Leaching Test; OECD: Paris, France, 2004. [Google Scholar]
- MFDS PRL Ministry of Food and Drug Safety—Pesticide Residue Limit 2021. Pesticide Residue Limit (Pesticide MRLs). 2024. Available online: https://www.foodsafetykorea.go.kr/foodcode/02_01.jsp (accessed on 1 July 2024).
- Brandi, J.; Siragusa, G.; Robotti, E.; Marengo, E.; Cecconi, D. Analysis of Veterinary Drugs and Pesticides in Food Using Liquid Chromatography-Mass Spectrometry. TrAC-Trends Anal. Chem. 2024, 179, 117888. [Google Scholar] [CrossRef]
- Hua, L.; Dang, F.; Yu, L.; Zhao, H.; Wei, T.; An, F. Soil Residues and Crop Accumulation of Organophosphorus and Pyrethroid Pesticides in Agricultural Fields in Shaanxi, China. J. Soils Sediments 2024, 24, 2713–2723. [Google Scholar] [CrossRef]
- Chen, Y.; Han, J.; Chen, D.; Liu, Z.; Zhang, K.; Hu, D. Persistence, Mobility, and Leaching Risk of Flumioxazin in Four Chinese Soils. J. Soils Sediments 2021, 21, 1743–1754. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S.; Singh, U.B. Pesticide-Tolerant Microbial Consortia: Potential Candidates for Remediation/Clean-up of Pesticide-Contaminated Agricultural Soil. Environ. Res. 2023, 236, 116724. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Vicente, F.; Rodriguez, S.; Lafuente, A.L. In Situ Oxidation Remediation Technologies: Kinetic of Hydrogen Peroxide Decomposition on Soil Organic Matter. J. Hazard. Mater. 2009, 170, 627–632. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, F.; Wahid, S.A.; Jalili, B. Sorption, Degradation and Leaching of Pesticides in Soils Amended with Organic Matter: A Review. Adv. Environ. Technol. 2017, 3, 119–132. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; El Aatik, A.; Vela, N.; Fenoll, J.; Navarro, S. Exogenous Organic Matter as Strategy to Reduce Pesticide Leaching through the Soil. Arch. Agron. Soil Sci. 2021, 67, 934–945. [Google Scholar] [CrossRef]
- Sarker, A.; Shin, W.S.; Al Masud, M.A.; Nandi, R.; Islam, T. A Critical Review of Sustainable Pesticide Remediation in Contaminated Sites: Research Challenges and Mechanistic Insights. Environ. Pollut. 2024, 341, 122940. [Google Scholar] [CrossRef]
- Manna, S.; Singh, N. Biochars Mediated Degradation, Leaching and Bioavailability of Pyrazosulfuron-Ethyl in a Sandy Loam Soil. Geoderma 2019, 334, 63–71. [Google Scholar] [CrossRef]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow Bone Char as a Sorbent to Increase Sorption and Decrease Mobility of Hexazinone, Metribuzin, and Quinclorac in Soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Sarker, A.; Kim, D.; Jeong, W.T. Environmental Fate and Sustainable Management of Pesticides in Soils: A Critical Review Focusing on Sustainable Agriculture. Sustainability 2024, 16, 10741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).