Abstract
The biogeochemical cycling of nitrogen (N) in natural waterbodies, ranging from freshwaters to estuaries and seawater, is fundamental to the health of aquatic ecosystems. Anthropogenic pressures (agricultural runoff, atmospheric deposition, and wastewater discharge) have profound effects on these cycles, leading to widespread problems, such as eutrophication, harmful algal blooms, and contamination of drinking water sources. Monitoring of different N-species—ammonium (NH4+), nitrite (NO2−), nitrate (NO3−) ions, dissolved organic nitrogen (DON), and total nitrogen (TN)—is of crucial importance to protect and mitigate environmental harm. Traditional analytical methodologies, while providing accurate laboratory data, are hampered by logistical complexity, high cost, and the inability to capture transient environmental events in near-real time. In response to this demand, miniaturised microfluidic technologies offer the opportunity for rapid, on-site measurements with significantly reduced reagent/sample consumption and the development of portable sensors. Here, we review and critically evaluate the principles, state-of-the-art applications, inherent advantages, and ongoing challenges associated with the use of microfluidic colorimetry for N-species in a variety of environmental waterbodies. We explore adaptations of classical colorimetric chemistry to microfluidic-based formats, examine strategies to mitigate complex matrix interferences, and consider future trajectories with autonomous platforms and smart sensor networks for simultaneous multiplexed N-species determination.
1. Introduction
Nitrogen (N) is a fundamental nutrient in limiting primary productivity in many aquatic ecosystems []. Its natural biogeochemical cycle involves complex transformations between various inorganic and organic forms, including atmospheric nitrogen (N2) and ammonia (NH3), ammonium (NH4+), nitrite (NO2−), and nitrate (NO3−) ions, dissolved organic nitrogen (DON), and particulate organic nitrogen (PON) [,]. Key microbial processes, such as N fixation, nitrification, denitrification, and ammonification, govern the availability and fate of these species []. However, over the past century, human activities, primarily intensive agriculture (fertiliser use), combustion of fossil fuels (atmospheric N deposition), and discharge of untreated or inadequately treated wastewater (sewage) have drastically increased N loading levels into freshwater, estuarine, and coastal marine ecosystems globally [,] (Figure 1).
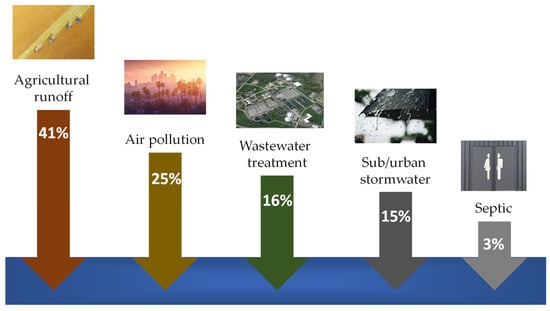
Figure 1.
An illustration of the sources of anthropogenic nitrogen pollution (adapted from []).
Anthropogenic N enrichment has severe ecological consequences []. Excessive inputs of bioavailable N, particularly NO3− and NH4+ ions, together with phosphorus (P) often trigger eutrophication, leading to excessive growth of algal species and aquatic plants [,]. In eutrophic lakes, streams, and marine waterbodies, the concentrations of N can exceed, 650, 1500, and 350 mg m3, respectively []. The subsequent decomposition of the organic matter by bacteria consumes dissolved oxygen (DO), creating hypoxic or anoxic conditions, commonly referred to as “dead zones,” which are detrimental to fish and other aquatic fauna [,]. Certain conditions of nutrient imbalance can also promote the proliferation of harmful algal blooms (HABs) that can produce toxins dangerous to wildlife and humans and cause significant economic losses [,]. Furthermore, elevated NO3− levels in groundwater and surface waters used for drinking can pose direct human health risks, most notably methemoglobinemia in infants []. Two cases of blue baby syndrome showed that infants became ill after being fed with water from private wells which contained 1.6–2.0 mM of NO3− []. Given these profound impacts, understanding the sources, transport, transformation, and fate of N-species in environmental waterbodies is crucial for effective environmental management, restoration efforts, and regulatory compliance (e.g., under the EU Water Framework Directive or the US Clean Water Act) [,]. This necessitates robust monitoring programs capable of providing accurate data on N-concentrations with sufficient sensitivity, precision, and spatiotemporal resolution to capture the dynamic nature of aquatic systems.
Traditional analytical methods for N-species, primarily spectrophotometric/colorimetric techniques, are flexible [] and involve discrete sample collection, preservation, and transport to centralised testing laboratories. This often requires time-consuming manual or semi-automated analysis. While these methods have been developed in routine analysis because they are generally accurate and precise and respond to a wider range of analytes compared to some other traditional methods (e.g., electrochemistry, X-ray fluorescence, etc.), their logistical demands limit the frequency and spatial coverage of sampling, especially in remote or inaccessible locations []. This often results in an incomplete understanding of N-dynamics, as transient events (e.g., storm runoff, episodic discharges, and microbial activity) may be missed. Moreover, the cost per sample can be high, further constraining monitoring efforts. There is, therefore, a compelling need for innovative analytical tools that are reliable, rapid, accurate, precise, sensitive, cost-effective, portable, and capable of autonomous or semi-autonomous operation for in situ measurements in near-real time [,].
Microfluidic technologies have emerged as a powerful and versatile solution to address this analytical need [,]. By miniaturising analytical processes into µL volume, these lab-on-chip (LOC) systems offer the potential for rapid, high-throughput analysis with significantly reduced reagent consumption and waste generation, making them highly attractive for near-real-time continuous environmental monitoring applications []. In this review, we compare some of the most recent strategies in microfluidic field-deployable systems for colorimetric monitoring of N-species, commented on the dis/advantages, applications in environmental analysis, and future perspectives and trends.
2. Fundamentals of Microfluidic Platforms for Chemical Sensing
2.1. Non-Droplet Microfluidic Systems
Non-droplet microfluidics include continuous-flow microfluidic systems characterised by moving fluids through connected microchannels rather than handling them as discrete droplets. These systems have attractive features, such as simplicity, portability, disposability, and rapid analysis [,,,,,,,,,,,,]. The most significant issue in microfluidics is the method of moving fluid through the system channels. Flow-generation systems can be divided into two main groups: active and passive flow-generation systems. In active systems, valves, pumps, motors, micropumps, magnetic, and electric or thermal forces are typically included. Main types of passive systems include continuous-flow microchannels in which fluids are moved inside microchannels (e.g., Y-shaped or T-shaped channels) for controlled mixing of fluids. In capillary-driven microfluidics, the fluids are driven by capillary action without additional power supply (not by external pumps) such as in microfluidic paper-based device (µPAD) systems [,]. Detection on the µPAD can be carried out by comparing the colour intensity in the reaction zone, using cameras, scanners, or smartphones []. µPAD and a smartphone-based app allow for the simultaneous monitoring of some N-species []. The developed app can capture, process, and quantify the µPAD colorimetric outputs on-site. These devices have been recently used in environmental water monitoring with the development of digital communication technology [,,,,].
2.2. Droplet-Based Microfluidic Systems
Droplet-based microfluidics involves the precise generation and manipulation of discrete droplets as immiscible fluid segments within microfabricated channels [,]. Typically, an aqueous phase (containing the sample and reagent/s) is dispersed as droplets within a continuous, immiscible oil phase (Figure 2). Each droplet functions as an independent, isolated microreactor. As such, droplet-based systems have the advantage of long-duration deployment with high specificity, precision, and accuracy. Common passive droplet generation techniques, relying on interfacial tension and fluid dynamics, include T-junctions, in which the aqueous phase is injected perpendicularly into a main channel carrying the continuous oil phase (Figure 2) [,]. In flow-focusing formation, the aqueous phase is hydrodynamically controlled by two co-flowing streams of the oil phase through a narrow orifice, resulting in droplet formation due to Rayleigh–Plateau instability exacerbated by intra-fluid shear [,]. This method generally offers excellent control over droplet size and monodispersion. Co-flow (or co-axial flow) assumes that the aqueous phase is introduced via an inner capillary positioned coaxially within a larger channel carrying the oil phase. Droplets detach from the capillary tip []. The choice of geometry and flow rates dictates droplet size and generation frequency (Hz to kHz). Surfactants are typically added to the oil phase to stabilise droplet interfaces against coalescence and to control wetting properties at the channel walls [].
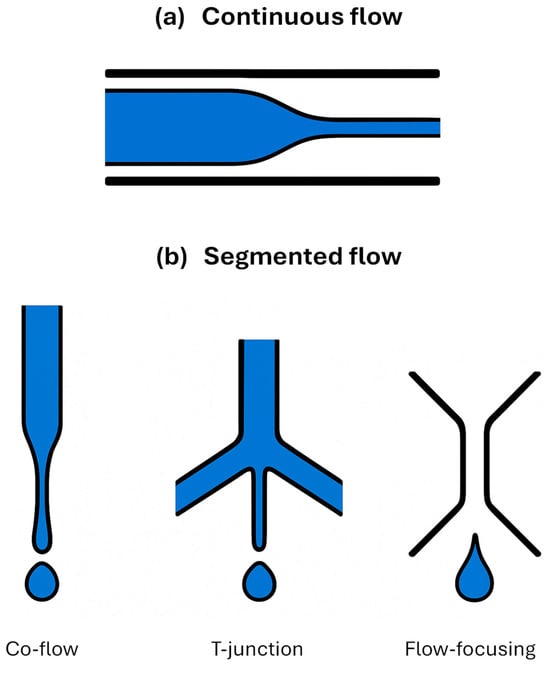
Figure 2.
Schematic diagrams of (a) the continuous vs. segmented flow in microfluidic systems; (b) droplet generation strategies: capillary, T-junction, and flow-focusing formation.
Alongside these traditional droplet formation techniques, the use of peristaltic pumps has enabled phased micro-pumping, in which the sample, reagents, and carrier oil are pumped in an anti-phase approach []. This technique allows for very precise control of the droplet dynamics, with the droplet size and composition being governed by the size of the grooves in the peristaltic pump roller. This process allows for much more robust droplet generation, as the dynamics are independent of the overall flow rate, unlike in co-flow regimes. In our recently developed microfluidic systems suitable for environmental analyses [,], this approach has been applied and demonstrates stable droplet generation over long deployments.
2.3. On-Chip Droplet Operations
Once formed, droplets can be subjected to a variety of manipulations to perform complex analytical protocols. Droplets containing different reagents (e.g., salicylate and nitroprusside reagents for NH4+) or water samples can be merged to initiate reactions, passively (e.g., at channel expansions) or actively (e.g., using electro-coalescence or acoustic forces) [,]. Complete homogeneous mixing of reagents with the sample is crucial to the precision and accuracy of the analyses. Single droplets can be divided into multiple smaller droplets for parallel processing to measure precision []. Rapid mixing within droplets is achieved via internal chaotic advection generated by turbulent shear at the droplet interface as it moves through curved or serpentine channels, significantly accelerating reaction rates and reducing the time to reach chemical equilibrium [,]. Delay lines (e.g., extended, long, serpentine channels) provide controlled residence times for reactions to proceed to completion []. If required, the droplets can be selectively routed based on their contents (e.g., after an optical measurement) using active sorting mechanisms like dielectrophoresis (DEP) or surface acoustic waves (SAW) [,].
2.4. Intrinsic Advantages of Microfluidic Droplet-Based Systems
Similar to non-droplet microfluidics, the droplet-based formats offer several key advantages for chemical analysis, including miniaturisation and reduced reagent/sample consumption (typically 103 to 106-times less than conventional methods), lower costs, and minimised chemical waste, which is a significant benefit for field deployments where reagent transport and waste disposal can be problematic [,]. Enhanced mixing and short diffusion distances within droplets accelerate reaction kinetics, often reducing analysis times from h to min or even sec [,].
High throughput and temporal resolution enable the ability to generate and analyse droplets at high frequencies, which then allows for rapid sequential measurements enabling high-temporal-resolution monitoring of dynamic processes []. Each droplet acts as an isolated microreactor; their separation prevents cross-contamination between sequential samples and eliminates axial dispersion (i.e., Taylor dispersion), which is common in continuous-flow systems, leading to sharper signals []. In continuous flow, due to in-tube mixing and diffusion, signal smearing occurs and therefore sensitivity is low, as is temporal resolution. In droplet-based flow, there is no mixing or diffusion, nor smearing, and better resolution and temporal discretisation are obtained. The small scale of microfluidic devices facilitates the development of compact, automated, and portable analytical systems suitable for on-site and field deployments [,].
3. Colorimetric Detection in Microfluidic Systems
3.1. Principles of Colorimetric Measurement
Colorimetric assays, which are based on the formation of a coloured reaction product whose absorbance is proportional to analyte concentration, are widely used due to their simplicity, reliability, stability, and relatively low cost. The relationship between light absorbance (A) and analyte concentration (c) is governed by the Beer–Lambert law:
where A is the absorbance, ε is the molar absorptivity of the chromophore, l is the optical pathlength through the sample, and c is the analyte concentration []. For colorimetry-detection-based systems, this means that the intensity of the colour developed is positively correlated with the analyte concentration, provided the other variables are constant. As an example, our recently tested microfluidic droplet-based system showed an excellent calibration regression across a broad range covering three orders of magnitude of ammonium ion concentrations (Figure 3) [].
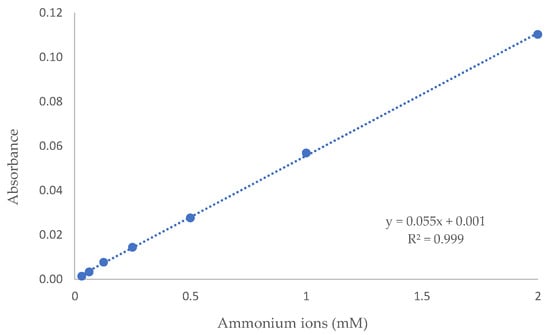
Figure 3.
Results of the calibration of an automated droplet microfluidic system for ammonium ion concentrations (by modified Berthelot’s reaction) in water [].
3.2. Optical Detection Setups for Microfluidic Analysis
Measuring the absorbance in field-deployable microfluidic systems requires precise optical integration with the microfluidic chip []. For the light source, the light-emitting diodes (LEDs) are favoured for their low-cost, stability, small size, and availability across various wavelengths suitable for different chromogenic reactions []. As detectors, photodiodes (PDs) or photomultiplier tubes (PMTs) are commonly used to measure the transmitted light intensity. PDs are compact and cost-effective, while PMTs are more costly and bulkier but offer higher sensitivity for low light levels, which can be important when dealing with diluted environmental samples or short optical pathlengths []. Image sensor technologies such as charge-coupled devices (used in digital cameras) can capture images for colorimetric analysis but may have lower throughput for quantitative absorbance of individual droplets compared to PD/PMT setups, unless specialised high-speed imaging is used []. Optical fibres are frequently used to deliver light to, and collect light from, the microchannel at the droplet interrogation point, ensuring precise alignment [,]. Microlenses can be integrated to focus the light beam onto the droplet or to collimate the transmitted light.
A significant challenge in microfluidic absorbance measurements is the inherently short optical pathlength (often equivalent to the droplet diameter or channel height, typically 50–200 µm), which can limit sensitivity. Strategies to increase effective pathlength in droplet-based systems include removing oil and converting the droplets into a single-phase aqueous flow which can be measured within a U-shape channel [], designing channels that flatten droplets in the detection zone, or employing more complex optical configurations like liquid-core waveguides [] or multi-pass cells (e.g., internal reflectance), although these are more challenging to implement robustly for droplet systems in the field (Figure 4). In our recently developed systems, a flow cell with UT7 polytetrafluoroethylene (PTFE) tubing is commonly used with its optimal pathlength of 0.7 mm [].
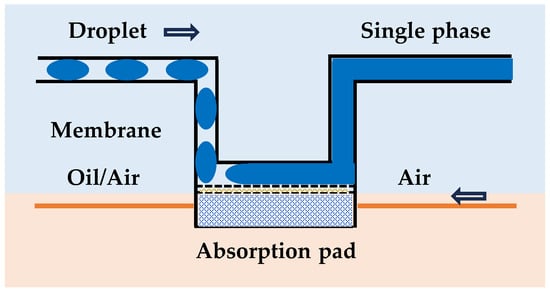
Figure 4.
Strategies for the design of different optical configurations of pathlengths: cross-sectional view of the U-shape channel with membrane and absorption pad [].
As droplets pass the detector, a series of transient absorbance signals (peaks) are generated. Sophisticated algorithms are required for peak detection, baseline correction, noise filtering, and calculation of absorbance values, which are then correlated with analyte concentrations using calibration curves established with standard solutions []. Baseline correction requires monitoring blanks, such as oil separation droplets. Ideally, calibration standards could be introduced frequently to measure accuracy (Figure 5).
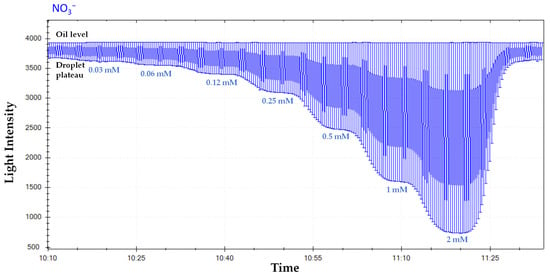
Figure 5.
Screenshot displaying raw light intensity data for nitrate ion standards obtained for calibrating the sensor. The raw light intensity signal shows the oil levels, and the droplet plateaus correspond to different concentration of nitrate standard solutions in increasing order (0.03–2 mM).
4. Application of Microfluidics to Nitrogen Species Analysis in Environmental Waterbodies
Unlike pure aquatic matrices, adapting standard colorimetric chemistries for N-species to microfluidic platforms for environmental water analysis involves optimising reactions for small volumes and rapid timescales and, critically, addressing the diverse and often challenging matrices. These introduce challenges and uncertainties in maintaining precision, sensitivity, and accuracy. Table 1 summarises developed microfluidic systems based on colorimetric or spectrophotometric analyses of N-species in environmental waterbodies with their analytical performances, including detection time, reagent consumption, detection range, and limits of detection (LOD) for each species.

Table 1.
Review of microfluidic analysis for in situ monitoring of ammonium, nitrite, and nitrate ions in environmental waterbodies.
4.1. Ammonium (NH4+) Species
The Berthelot reaction (or its modified version with a less toxic salicylate variant) is the most common colorimetric method for NH4+ detection [,,,,,]. NH4+ reacts with hypochlorite to form monochloramine, which then reacts with phenol (or salicylate) in an alkaline medium, typically catalysed by nitroprusside, to form a blue indophenol dye (λmax ≈ 630–660 nm) [,]. In our recently developed system for in situ monitoring of NH4+ ions in natural waters (Figure 6), the droplet-based microfluidic system showed the main advantages of high measurement frequency (10 s per measurement) and low reagent consumption (0.3 µL per measurement) (Table 1).
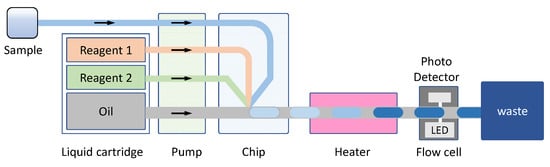
Figure 6.
Schematic diagram of the microfluidic droplet-based system designed for continuous monitoring of ammonium ions in water samples (adapted from []). From left to right: The aqueous sample (via a filter), was pumped and mixed with reagents and oil, stored in fluid pouches, by a peristaltic pump into a microfluidic chip to generate droplets. The droplets reacted in a temperature-controlled the heater unit forming the coloured (blue) compound detected by an in-line photo detector (with an LED light source).
Several other research groups have also developed non-droplet optical-based sensors for NH4+ monitoring in aquatic media. For instance, Khongpet et al. (2019), though focused on freshwater samples, demonstrated an inexpensive microfluidic hydrodynamic sequential injection method with a detection limit of 19 µM, using a sensitive Berthelot’s method []. Systems for environmental waters must often achieve lower detection limits (µg/L or sub-µM levels) compared to wastewater applications []. Cho et al. (2018) fabricated small colorimetric ammonia gas sensors by filtration of modified Berthelot’s reagent on porous paper for the analysis of wastewater or seawater []. Nxumalo et al. (2020) described a µPAD for continuous quantification of NH4+ in industrial wastewater using Nessler’s reagent (potassium tetraiodomercurate(II) and potassium hydroxide), which has a high environmental impact and, hence, is not preferred for field use []. Peters et al. (2019) developed a more suitable field screening system based on the colour change of bromothymol blue or nitrazine yellow for total NH4+ monitoring in freshwater []. Salee et al. (2025) recently developed a new gel-based sensing platform for LED-based colorimetric NH4+ flow-analysis detection in water using Berthelot’s reagents, highlighting stability, accuracy, and high sensitivity (LOD = 1.5 µM) using a higher sample/reagent volume range (50–200 mL) [].
Challenges in environmental waters may arise because of very low ammonium levels in natural unpolluted waters, demanding high sensitivity of the developed methods. In estuarine and marine waters, high salt (saline and brackish) concentrations can affect reaction rates, reagent solubility, and optical properties (refractive index changes) and can cause salt precipitation in microchannels if evaporation occurs [,]. Specific calibration matrices matching sample salinity are essential. Dissolved organic matter (DOM) such as humic and fulvic acids can impart colour to the water (interfering with absorbance readings) or react with reagents, causing chemical interference []. Moreover, the natural buffering capacity of some waters can influence the optimal pH for Berthelot’s reaction.
4.2. Nitrite (NO2−) Species
The Griess reaction is the standard method for determination of total NO2− ion levels. Nitrite reacts with an aromatic amine (e.g., sulphanilamide) under acidic conditions to form a diazonium salt, which then couples with a second aromatic compound (e.g., N-(1-naphthyl) ethylenediamine dihydrochloride, known as NEDA·2HCl) to produce a stable, intensely coloured azo dye (pink, λmax ≈ 540–550 nm) [,,,].
The rapid kinetics and high molar absorptivity of the Griess reaction make it well-suited for microfluidics. Man et al. (2024) demonstrated a good concentration gradient colorimetric detection chip designed for nitrite in environmental water samples (river, lakes, etc.) with good sensitivity and high recovery (>95%) []. Portable systems, sometimes smartphone-based, have also been developed for rapid nitrite field measurements [,]. A recent strategy for NO2− detection is based on a smartphone app using photooxidation []. This reaction, operated on an Android phone, was proven by the injection of sodium nitrite solution as the real sample.
Some microfluidic systems are valuable for tracking nitrification hotspots or anoxic zones where nitrite can accumulate. Beaton et al. (2011) developed an early in situ microfluidic colorimetric sensor for the determination of total NO2− in seawater samples []. This microfluidic device based on a poly (methyl methacrylate) (PMMA) microchip was operated in situ for 57 h and made 375 discrete measurements with low reagent consumption. Although the developed systems require the on-site addition of some reagents after loading the sample onto the device, they have several advantages in terms of speed, simplicity, selectivity, and sensitivity. With some further modifications, the developed system can be a good candidate for the simultaneous detection of other inorganic N-species, such as NH4+ and NO3−.
Low-cost fabricated non-droplet-based microfluidic devices (µPADs with or without integrated smartphones) were employed for quantitative colorimetric analysis of NO2− across a broad concentration range with satisfactory accuracy and reproducibility [,,,]. Shi et al. (2018) demonstrated a robust method for the determination of NO2− ions by colorimetry in the microfluidic network []. It was shown that for lower concentrations of NO2− ions, the errors measured were lower than 1.16% and the method is therefore suitable for different application scenarios with complex water quality and testing environments.
Like the monitoring of NH4+, high salinity can affect the kinetics of the Griess reaction and optical baselines. Therefore, salinity-matched calibrations are crucial in these systems []. Strong oxidants or reductants, if present, can also interfere, though this is generally less common in typical surface waters than in some industrial discharges.
4.3. Nitrate (NO3−) Species
Direct colorimetric determination of nitrate is challenging. Most methods involve the reduction of nitrate to nitrite, followed by nitrite quantification using the Griess reaction []. Several reduction methods are typically used, among which cadmium (Cd) reduction by passing the sample through a column of copper-plated Cd granules is traditional, but Cd is toxic and the efficiency of the column is reduced over time []. Nevertheless, miniaturised Cd reactors have been integrated into microfluidic chips (often continuous flow upstream) []. Jayawardane et al. (2014) used zinc microparticles (University of Melbourne, Australia) for on-chip reduction of NO3− in a µPAD []. Vanadium-(III) chloride (VCl3) reduction is based on the reduction of nitrates to nitrites at elevated temperatures (60–80 °C) [,,]. While avoiding Cd, integrating controlled and stable heating for VCl3 reduction in portable microfluidic systems can be complex. Using smartphone-based detection, a simple method based on the VCl3 reduction-Griess reaction was established for on-site NO3− analysis in natural water samples with various salinities []. The proposed method showed a good linearity for concentrations of NO3− up to 20 µM. While on-chip enzymatic reactors have been explored, enzyme stability, cost, and potential inhibition and hence reduction in reaction stability of matrix components are challenges and sources of uncertainty [].
Microfluidic portable devices often involve an upstream microreactor for reduction (e.g., a packed bed of Cd or Zn) followed by generation for the Griess reaction [,]. Ensuring consistent reduction efficiency over time, especially with natural water particulates that can clog reducers, is a major challenge for long-term autonomous deployments. For example, Plant et al. (2023) developed an in situ UV spectrophotometry-based method for NO3− analysis in seawater, with updated temperature correction as an important instrumental component in the experimental methodology [].
Khanfar et al. (2017) developed microfluidic chips to detect nitrate in different water samples []. A novel method with a designed PMMA device showed reduced power consumption (~40%) for NO3− using the Griess reagent at 35 °C []. The use of a nutrient sensor deployed within an autonomous underwater vehicle (AUV) to collect NO3− data in a shelf sea environment showed the possibility of capturing high-frequency events of spring phytoplankton []. Rapid NO3− determination was realised using a portable device based on innovative 3D double micro-structured associated reactors [] (Figure 7). This system allowed for different water samples’ analysis with low reagent consumption (26.8 µL per sample), good reproducibility, and low RSD (0.5–1.38%).

Figure 7.
Portable device for nitrate detection with the main interface of the App on a smartphone and the main body assembled with PDMS chip, printed circuit board (PCB), and PMMA detection chip suitable for the long-term spectrophotometric measurements. Reprinted with permission [].
Challenges in environmental water analysis comprise reducer longevity and fouling due to particulates and biofilm growth that can rapidly deactivate or clog micro-reducers. Reduction efficiency ensuring complete reduction of NO3− to NO2− without over-reduction to NH4+ or N2O, especially across varying sample salinities and DOM content, is challenging. This is especially the case for low concentrations, achieving sensitive NO3− detection after dilution by reagent addition given the potential inefficiencies in reduction.
4.4. Total Dissolved Nitrogen (TDN) and Total Nitrogen (TN)
Total dissolved nitrogen (TDN) (e.g., for filtered sample) and total nitrogen (TN) (e.g., for unfiltered sample) analysis involves converting all N (organic and inorganic) compounds into a single measurable form, usually NO3− or NH4+. This typically requires an initial, aggressive oxidative digestion step []. Digestion methods can be performed by alkaline persulphate after heating with potassium persulphate (K2S2O8) and sodium hydroxide (NaOH) often under pressure at higher temperatures (100–120 °C) that converts N to NO3− []. UV irradiation in the presence of an oxidant (e.g., persulphate or titanium dioxide, TiO2) can oxidise organic N to NO3− [,]. This is often preferred for in situ systems due to less aggressive reagent requirements.
Integrating high pressure and temperature and persulphate digestion into conventional polydimethylsiloxane (e.g., PDMS) microfluidic devices is highly problematic due to material incompatibility and sealing []. In contrast, UV-based micro-digesters are more amenable to microfluidic integration [,]. Often, digestion is performed off-chip or in a separate robust microfluidic module upstream of NO3−/NO2− detection. For example, Lin et al. (2021) developed a flow injection analysis (FIA) system using on-line UV digestion for TDN in natural waters []. Seamlessly coupling such digesters with downstream droplet systems for aqueous environmental matrices remains an active research area. The major areas of challenge, and hence the primary hurdle in microfluidic systems, are the robust, efficient, and compact on-chip digestion processes for diverse environmental samples and preventing interference from the digestion reagents in subsequent colorimetric steps.
4.5. Organic Nitrogen
Dissolved organic nitrogen (DON) and PON are significant components of the organic N pool in many ecosystems. Direct colorimetric methods for bulk DON/PON in microfluidics are rare. Typically, DON is determined by the difference in TDN (NH4+ + NO2− + NO3−). Recently, Uhlikova et al. (2024) developed a µPAD for the speciation of dominant inorganic N-species (NH4+ and NO3−) in environmental waters []. This system showed stability during 24 h at ambient temperature. Therefore, the development of accurate microfluidic methods for medium- and long-term TDN and dissolved inorganic nitrogen (DIN) monitoring are crucial for DON estimation.
4.6. Multiplexed Detection of Nitrogen Species
Environmental studies often require simultaneous quantification of multiple N-species to understand transformation pathways and nutrient stoichiometry. Strategies for this are to use parallel microfluidic channels, each dedicated to a specific analyte with its distinct colorimetric reaction and detector []; sequential reagent addition if chemistries are compatible; or spectral deconvolution if chromophores have distinct absorbance maxima []. Nightingale et al. (2019) developed a system for simultaneous monitoring of NO3− and NO2− in natural waters [] (Figure 8). This droplet-based microfluidic sensor device showed high measurement frequency and low fluid consumption, at a rate of 2.8 mL per day.
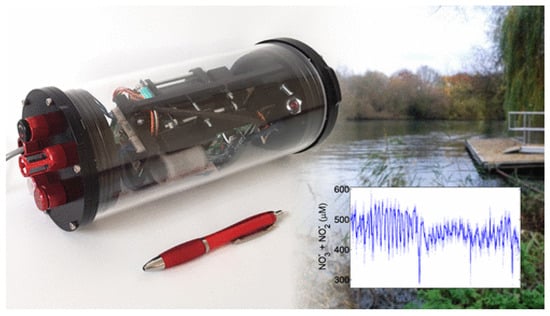
Figure 8.
Photograph of a field-deployable microfluidic sensor (slightly larger than a pen) with integrated fluidics, heater, flow cells, and control electronics for simultaneous monitoring of total nitrate and nitrite in situ [].
Altahan et al. (2022) demonstrated robust sensors for long-term simultaneous measurements of NO3−, NO2−, phosphate (PO43−), and silicic acid in seawater [] (Figure 9). The analyser performed well in the field during a 46-day deployment on a pontoon with a water supply from a depth of 1 m.

Figure 9.
Deployment setup of the AutoLAB analyser (Envirotech LLC, Chesapeake, VA, USA) in a weather-proof aluminium container for simultaneous determination of nitrate plus nitrite in seawater (adapted from []).
Developing compact, robust microfluidic multiplexed sensors capable of delivering near-real-time and accurate results in field conditions remains a key but challenging technological and scientific goal. Achieving this requires overcoming barriers in fluid control, signal cross-talk, and miniaturised optical integration, all within a compact, low-power platform suitable for decentralised deployment.
5. Advantages and Overcoming Limitations in Environmental Water Analysis
5.1. Enhanced Analytical Capabilities for Environmental Science
Portable and potentially autonomous systems enable denser sampling in space and time, capturing episodic events, diel cycles, and fine-scale heterogeneity in nutrient distributions crucial for ecological modelling [,]. Reduced logistical burden for small-scale fieldwork, low power consumption, and minimal reagent requirements simplify deployment in remote locations, e.g., on buoys, AUVs, or for manual transects [,]. Lower reagent costs per analysis and potential for automation can make extensive monitoring programs more feasible [].
5.2. Addressing Complex Environmental Matrix Effects
As explained, applying microfluidic colorimetry to diverse environmental waters offers substantial benefits but also faces unique matrix-specific challenges. Environmental waters are far more variable than controlled laboratory solutions or even treated wastewater []. Algae, detritus, and sediments in rivers, lakes, and coastal waters can clog microchannels and cause significant light scattering, interfering with absorbance measurements []. DOM, including humic and fulvic acids which are ubiquitous in natural waters, can impart colour (background absorbance) and fluorescence, complex with analytes or reagents, or foul surfaces [,,,]. Background correction includes using a reference wavelength, sample pre-treatment (e.g., solid-phase extraction for DOM removal, though this adds complexity), development of colorimetric chemistries less susceptible to DOM interference, or advanced chemometric data processing.
Salinity is a major challenge for estuarine and marine applications. High and variable salt content can affect reaction kinetics and equilibria; alter reagent solubility and stability; change the refractive index of the aqueous phase, impacting optical measurements if not properly accounted for in microfluidic systems (e.g., lens effects); or lead to salt precipitation and channel clogging, especially if evaporation occurs or if reagents are incompatible with high salt concentrations. Use of salt-matched calibration standards corresponding to the sample salinity range [], development of salt-tolerant reagent formulations, careful material selection to avoid corrosion, and robust fluidic design to prevent clogging are some of the most recent issues developed.
Many pristine or oligotrophic waters have very low nutrient levels (nM to low µM range). Achieving adequate sensitivity with short optical pathlengths is demanding. Mitigation of this can be achieved by using highly sensitive chromogenic reagents (high molar absorptivity), optimisation of reaction conditions for maximum colour yield, on-chip pre-concentration techniques (e.g., solid-phase extraction, electrophoresis) prior to coloured-compound formation, or exploration of alternative detection methods with higher intrinsic sensitivity (e.g., fluorescence, though this review focuses on colorimetry).
Biofouling for long-term in situ deployments and biofilm formation on chip surfaces and in tubing can alter flow characteristics, consume analytes, or release interfering substances []. This is often mitigated by the use of antifouling coatings, periodic flushing with cleaning agents or biocides, optimised flow regimes, or integration of UV sterilisation.
5.3. Robustness and Field Deployment Considerations
Device durability is important as devices must withstand physical handling, temperature fluctuations, and potentially corrosive environments. Materials like glass or robust polymers such as cyclic olefine copolymer (COC) or polyetheretherketone (PEEK) are often preferred over PDMS for field systems []. Critically, power management for autonomous deployments should minimise the power consumption of pumps, valves, detectors, and control electronics [,]. Essentially, calibration stability implies maintained calibration accuracy over time and across varying environmental conditions (e.g., temperature changes affecting reaction rates or detector performance). Automated on-chip recalibration routines or temperature compensation mechanisms are needed. Long-term reagent stability and storage of liquid reagents on-chip or in portable cartridges can be an issue. Lyophilised reagents reconstituted on-demand or in situ reagent generation are potential solutions.
6. Future Perspectives and Emerging Trends
6.1. Advanced Materials and Manufacturing
The field of microfluidic colorimetry for environmental N analysis is continually evolving, driven by technological advancements and the growing demand for better environmental intelligence. Beyond PDMS, the use of glass, thermoplastics like COC, and fluoropolymers will increase for field-deployable systems due to better chemical resistance, optical properties, and mechanical robustness [,]. Additive manufacturing (3D printing) is enabling rapid prototyping of complex 3D fluidic networks and integrated components [].
6.2. Enhanced On-Chip Integration and Autonomy
The trend is towards fully autonomous “sample-to-answer” systems. This includes an integrated upstream sample preparation system comprising robust on-chip modules for filtration, particulate removal, dilution, and, importantly for environmental samples, analyte pre-concentration. Autonomous operation includes integration with microcontrollers, low-power pumps and valves, and telemetry for unattended long-term deployments on buoys, moorings, or AUVs [,]. This includes self-diagnosis and automated recalibration. “Smart” reagent cartridges are disposable cartridges containing stable reagents, calibration standards, and waste reservoirs to simplify field operation and reduce contamination risks.
6.3. Data Science and Sensor Networks
On-board data processing (edge computing) and machine learning (ML) algorithms allow for real-time quality control, advanced signal processing, interference correction, and predictive alerts for unusual N levels. Data integration includes wireless communication (e.g., satellite) for transmitting data from distributed sensor networks to central databases, enabling large-scale, real-time environmental monitoring and modelling [,].
Continued development of low-cost, user-friendly smartphone-based readers are in development for citizen science applications and rapid field screening by non-specialists [,].
6.4. Novel Chemistries and Detection Schemes
More sensitive, selective, and matrix-tolerant chromogenic reagents are needed to be specifically tailored for microfluidic analysis of environmental samples (e.g., stable at varying salinities, less prone to DOM interference). Although N-species are dominant in freshwater ecosystems, some other important chemical parameters, in particular P-ions (e.g., PO43−) or C are limiting nutrients in eutrophication processes and should be analysed simultaneously using a specially designed multiplex microfluidic system. Currently, one of the goals of our research group is the design of new systems that would allow simultaneous monitoring of two or more nutrients (e.g., different N- and P-ions), which is of great importance when it comes to rivers and lakes. While this review focuses on colorimetry, coupling microfluidics with other sensitive detection modes (e.g., electrochemical sensors or highly sensitive fluorescence assays) for ultra-trace analysis of N-species will expand the capabilities of such hybrid technologies in the future [].
6.5. Standardisation and Commercialisation
Robust, validated, and commercially available microfluidic N sensors are needed for widespread adoption by environmental agencies and researchers. This requires standardised performance metrics and validation protocols ensuring the comparability and reliability of data from different devices and laboratories. For example, the use of internal standards is likely to be required to meet environmental regulatory compliance. Cost-effectiveness implies further reductions in the cost of components, e.g., chips, sensors, and consumables. User-friendly interfaces make devices easy to operate and maintain by non-specialists. Several companies are emerging in the broader field of microfluidic water quality sensors, and N-specific systems are gradually becoming more mature [,].
7. Conclusions
Microfluidic colorimetric analysis offers a promising technology that can revolutionise the way we monitor N-species in diverse environmental waterbodies. The inherent advantages of miniaturisation include rapid analysis, dramatically reduced sample and reagent consumption, enhanced portability, and potential for high-throughput automation directly address many limitations of traditional laboratory-based methods. Significant advances have been made in adapting classical colorimetric chemistries for species of NH4+, NO2−, and NO3− to non-droplet and droplet-based microfluidic platforms, and pioneering efforts are tackling the challenges of on-chip digestion for total N and pre-concentration for ultra-trace analysis.
However, the transition from laboratory prototypes to widely deployed, robust, and autonomous field sensors for complex and variable environmental matrices is ongoing. Key challenges remain, particularly in mitigating interference from suspended solids, DOM, and salinity; ensuring long-term stability and antifouling capabilities for in situ deployments; and achieving the extremely low detection limits often required for oligotrophic waters.
Continued innovation in materials science, microfabrication, integrated sample preparation, reagent stability, data analytics, and smart system design will be crucial in overcoming these hurdles. As microfluidic colorimetric sensors become more sensitive, reliable, cost-effective, and user-friendly, they will empower scientists, environmental managers, and policymakers with unprecedented capabilities for high-resolution spatiotemporal monitoring. This will lead to a deeper understanding of N-species biogeochemistry, improved detection of environmental pollution events, more effective management of aquatic ecosystems, and ultimately, better protection of our vital water resources in a rapidly changing world.
Author Contributions
Conceptualisation, J.M.; writing—original draft preparation, J.M.; writing—review and editing, J.M., J.L., S.A. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
During the preparation of this manuscript, the authors used GenAI for the purposes of literature review. The authors have reviewed and edited the output and take full responsibility for the content of this publication. The authors are grateful to the Academic Editors and Reviewers for all their advice.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABS | Acrylonitrile butadiene styrene |
| AUV | Autonomous underwater vehicle |
| BTB | Bromothymol blue |
| COC | Cyclic olefine copolymer |
| DEP | Dielectrophoresis |
| DO | Dissolved oxygen |
| DOM | Dissolved organic matter |
| DON | Dissolved organic nitrogen |
| HAB | Harmful algal bloom |
| LED | Light-emitting diode |
| LOC | Lab-on-chip |
| ML | Machine learning |
| N | Nitrogen |
| NEDA·2HCl | N-(1-naphthyl) ethylenediamine dihydrochloride |
| NH4+ | Ammonium ion |
| NO2− | Nitrite ion |
| NO3− | Nitrate ion |
| NY | Nitrazine yellow |
| µPAD | Microfluidic paper-based device |
| PCB | Printed circuit board |
| PEEK | Polyetheretherketone |
| PD | Photodiode |
| PDMS | Polydimethylsiloxane |
| PLA | Polylactic acid |
| PMMA | Polymethyl methacrylate |
| PMT | Photomultiplier tube |
| PO43− | Phosphate ion |
| PON | Particulate organic nitrogen |
| PTFE | Polytetrafluoroethylene |
| SAW | Surface acoustic wave |
| TDN | Total dissolved nitrogen |
| TN | Total nitrogen |
| UV | Ultraviolet |
| VCl3 | Vanadium-(III) chloride |
References
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Sipler, R.A.; Bronk, D.A. Dynamics of dissolved organic nitrogen. In Biogeochemistry of Marine Dissolved Organic Matter, 2nd ed.; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 127–232. [Google Scholar]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar]
- Chesapeake Bay Foundation. Available online: www.cbf.org (accessed on 23 June 2025).
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Gulf of Mexico hypoxia, aka “The Dead Zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675–678. [Google Scholar] [CrossRef]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October Establishing a Framework for Community Action in the Field of Water Policy. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0060:en:NOT (accessed on 24 June 2025).
- U.S. Environmental Protection Agency (EPA). Clean Water Act Section 303(d): Impaired Waters and Total Maximum Daily Loads (TMDLs). Available online: https://www.epa.gov/tmdl (accessed on 24 June 2025).
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater. Available online: https://www.standardmethods.org/doi/book/10.2105/SMWW.2882 (accessed on 24 June 2025).
- Strobl, R.O.; Robillard, P.D. Network design for water quality monitoring of surface freshwaters: A review. J. Environ. Manag. 2008, 87, 639–648. [Google Scholar] [CrossRef]
- Johnson, K.S.; Needoba, J.A.; Riser, S.C.; Showers, W.J. Chemical sensor networks for the aquatic environment. Chem. Rev. 2007, 107, 623–640. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Beaton, A.D.; Mowlem, M.C. Trends in microfluidic systems for in situ chemical analysis of natural waters. Sens. Actuators B Chem. 2015, 221, 1398–1405. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Portable smartphone-based colorimetric system for simultaneous on-site microfluidic paper-based determination and mapping of phosphate, nitrite and silicate in coastal waters. Environ. Monit. Assess. 2022, 194, 190. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem. Soc. Rev. 2017, 46, 6210–6226. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D printed heaters for microfluidic applications: Ammonium analysis within environmental water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- Cho, Y.B.; Jeong, S.; Chun, H.; Kim, Y.S. Selective colorimetric detection of dissolved ammonia in water via modified Berthelot’s reaction on porous paper. Sens. Actuators B Chem. 2018, 256, 167–175. [Google Scholar] [CrossRef]
- Nxumalo, N.L.; Madikizela, L.M.; Kruger, H.G.; Onwubu, S.C.; Mdluli, P.S. Development of a paper-based microfluidic device for the quantification of ammonia in industrial wastewater. Water SA 2020, 46, 506–513. [Google Scholar] [CrossRef]
- Peters, J.J.; Almeida, M.I.G.S.; Šraj, L.O.; McKelvie, I.D.; Kolev, S.D. Development of a micro-distillation microfluidic paper-based analytical device as a screening tool for total ammonia monitoring in freshwaters. Anal. Chim. Acta 2019, 1079, 120–128. [Google Scholar] [CrossRef]
- Patey, M.D.; Rijkenberg, M.J.A.; Statham, P.J.; Stinchcombe, M.C.; Achterberg, E.P.; Mowlem, M.C. Determination of nitrate and phosphate in seawater at nanomolar concentrations. Trends Anal. Chem. 2010, 27, 169–182. [Google Scholar] [CrossRef]
- Šraj, L.O.C.; Almeida, M.I.G.S.; Swearer, S.E.; Kolev, S.D.; McKelvie, I.D. Analytical challenges and advantages of using flow-based methodologies for ammonia determination in estuarine and marine waters. Trends Anal. Chem. 2014, 59, 83–92. [Google Scholar] [CrossRef]
- Vidal, E.; Lorenzetti, A.S.; Lista, A.G.; Domini, C.E. Micropaper-based analytical device (µPAD) for the simultaneous determination of nitrite and fluoride using a smartphone. Microchem. J. 2018, 143, 467–473. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Colorimetric determination of nitrate after reduction to nitrite in a paper-based dip strip. Chem. Proc. 2021, 5, 9. [Google Scholar] [CrossRef]
- Beaton, A.D.; Schaap, A.M.; Pascal, R.; Hanz, R.; Martincic, U.; Cardwell, C.L.; Morris, A.; Clinton-Bailey, G.; Saw, K.; Hartman, S.E.; et al. Lab-on-Chip for in situ analysis of nutrients in the deep sea. ACS Sens. 2022, 7, 89–98. [Google Scholar] [CrossRef]
- Uhlikova, N.; Almeida, M.I.G.S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the speciation of inorganic nitrogen species. Talanta 2024, 271, 125671. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Warren, B.M.; Makris, K.; Evans, G.W.H.; Papadopoulou, E.; Coleman, S.; Niu, X. A droplet microfluidic-based sensor for simultaneous in situ monitoring of nitrate and nitrite in natural waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Kurniawan, A.; Kao, C.-Y.; Wang, M.-J. Single step and mask-free 3D wax printing of microfluidic paper-based analytical devices for glucose and nitrite assays. Talanta 2019, 194, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Gomez, I.; Ortega-Muñoz, M.; Salinas-Castillo, A.; Álvarez-Bermejo, J.A.; Ariza-Avidad, M.; de Orbe-Payá, I.; Santoyo-Gonzalez, F.; Capitan-Vallvey, L.F. Tetrazine-based chemistry for nitrite determination in a paper microfluidic device. Talanta 2016, 160, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Dudala, S.; Dubey, S.K.; Goel, S. Fully integrated, automated, and smartphone enabled point-of-source portable platform with microfluidic device for nitrite detection. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1518–1524. [Google Scholar] [CrossRef]
- Solvas, X.C.; deMello, A. Droplet microfluidics: Recent developments and future applications. Chem. Commun. 2011, 47, 1936–1942. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef]
- Cramer, C.; Fischer, P.; Windhab, E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004, 59, 3045–3058. [Google Scholar] [CrossRef]
- Baret, J.C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Evans, G.W.H.; Xu, P.; Kim, B.J.; Hassan, S.; Niu, X. Phased peristaltic micropumping for continuous sampling and hardcoded droplet generation. Lab Chip 2017, 17, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lunn, J.; Yeung, K.; Dhandapani, S.; Carter, L.; Roose, T.; Shaw, L.; Nightingale, A.; Niu, X. Droplet microfluidic-based in situ analyzer for monitoring free nitrate in soil. Environ. Sci. Technol. 2024, 58, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Link, D.R.; Grasland-Mongrain, E.; Duri, A.; Sarrazin, F.; Cheng, Z.D.; Cristobal, G.; Marquez, M.; Weitz, D.A. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. Engl. 2006, 45, 2556–2560. [Google Scholar] [CrossRef]
- Fu, T.; Ma, Y. Bubble formation and breakup dynamics in microfluidic devices: A review. Chem. Eng. Sci. 2015, 135, 343–372. [Google Scholar] [CrossRef]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. Engl. 2003, 42, 768–772. [Google Scholar] [CrossRef]
- Song, H.; Ismagilov, R.F. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc. 2003, 125, 14613–14619. [Google Scholar] [CrossRef]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.M.; Link, D.R.; Weitz, D.A. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef]
- Franke, T.; Abate, A.R.; Weitz, D.; Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 2009, 9, 2625–2627. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Johnson, K.S.; Coletti, L.J.; Jannasch, H.W.; Sakamoto, C.M.; Swift, D.D.; Riser, S.C. Long-term nitrate measurements in the ocean using the in situ ultraviolet spectrophotometer: Sensor integration into the APEX profiling float. J. Atmos. Ocean. Technol. 2007, 30, 1854–1866. [Google Scholar] [CrossRef]
- Bhuiyan, W.T.; Milinovic, J.; Warren, B.; Liu, Y.; Nightingale, A.M.; Niu, X. Automated droplet-based microfluidic analyser for in-situ monitoring of ammonium ions in river water. ACS ES T Water 2025, 5, 4387–4394. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Makris, K.; Bhuiyan, W.T.; Harvey, T.J.; Niu, X. Easily fabricated monolithic fluoropolymer chips for sensitive long-term absorbance measurement in droplet microfluidics. RSC Adv. 2020, 10, 30975–30981. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cheng, J.; Zou, Y.; Guo, J.; Guo, J. Detection and analysis of droplets in microfluidic devices: A review. IEEE Sens. J. 2024, 24, 33881–33902. [Google Scholar] [CrossRef]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef]
- Geng, Z.; Miao, Y.; Zhang, G.; Liang, X. Colorimetric biosensor based on smartphone: State-of-art. Sens. Actuators A Phys. 2023, 349, 114056. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y. Development and application of analytical detection techniques for droplet-based microfluidics. Anal. Chim. Acta 2020, 1113, 66–84. [Google Scholar] [CrossRef]
- Lu, B.; Lunn, J.; Nightingale, A.M.; Niu, X. Highly sensitive absorbance measurement using droplet microfluidics integrated with an oil extraction and long pathlength detection flow cell. Front. Chem. 2024, 12, 1394388. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Oveys, H.; Fan, X. Liquid-core optical ring-resonator sensors. Opt. Lett. 2006, 31, 1319–1321. [Google Scholar] [CrossRef]
- Khongpet, W.; Pencharee, S.; Puangpila, C.; Hartwell, S.K.; Lapanantnoppakhun, S.; Jakmunee, J. A compact hydrodynamic sequential injection system for consecutive on-line determination of phosphate and ammonium. Microchem. J. 2019, 147, 403–410. [Google Scholar] [CrossRef]
- Man, Y.; Yu, K.; Tan, H.; Jin, X.; Tao, J.; Pan, L. A microfluidic colorimetric system for rapid detection of nitrite in surface water. J. Hazard. Mater. 2024, 465, 133133. [Google Scholar] [CrossRef]
- Beaton, A.D.; Sieben, V.J.; Floquet, C.F.A.; Waugh, E.M.; Bey, S.A.K.; Ogilvie, I.R.G.; Mowlem, M.C.; Morgan, H. An automated microfluidic colourimetric sensor applied in situ to determine nitrite concentration. Sens. Actuators B Chem. 2011, 156, 1009–1014. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.; da Rocha, Z.; Seabra, A.; Valdés, F.; Alonso-Chamarro, J. Highly integrated autonomous lab-on-a-chip device for on-line and in situ determination of environmental chemical parameters. Lab Chip 2018, 18, 1884–1890. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, H.L.; Zhu, X.Q.; Zhu, J.M.; Zuo, Y.F.; Yang, Y.; Jiang, F.H.; Sun, C.J.; Zhao, W.H.; Han, X.T. Optofluidic differential colorimetry for rapid nitrite determination. Lab Chip 2018, 18, 2994. [Google Scholar] [CrossRef]
- Khanfar, M.F.; Al-Faqheri, W.; Al-Halhouli, A. Low cost lab on chip for the colorimetric detection of nitrate in mineral water products. Sensors 2017, 17, 2345. [Google Scholar] [CrossRef]
- Murphy, B.J.; Luy, E.A.; Panzica, K.L.; Johnson, G.; Sieben, V.J. An energy efficient thermally regulated optical spectroscopy cell for lab-on-chip devices: Applied to nitrate detection. Micromachines 2021, 12, 861. [Google Scholar] [CrossRef]
- Vincent, A.G.; Pascal, R.W.; Beaton, A.D.; Walk, J.; Hopkins, J.E.; Woodward, E.M.S.; Mowlem, M.; Lohan, M.C. Nitrate drawdown during a shelf sea spring bloom revealed using a novel microfluidic in situ chemical sensor deployed within an autonomous underwater glider. Mar. Chem. 2018, 205, 29–36. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, J.; Hu, X.; Chen, L.; Zuo, Y.; Yang, Y.; Jiang, F.; Sun, C.; Zhao, W.; Han, X. Rapid nitrate determination with a portable lab-on-chip device based on double microstructured assisted reactors. Lab Chip 2021, 21, 1109. [Google Scholar] [CrossRef]
- Cogan, D.; Cleary, J.; Fay, C.; Rickard, A.; Jankowski, K.; Phelan, T.; Bowkett, M.; Diamond, D. The development of an autonomous sensing platform for the monitoring of ammonia in water using a simplified Berthelot method. Anal. Methods 2014, 6, 7606–7614. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Y.; Zhang, Y.; Lin, H. Determination of ammonia nitrogen in natural waters: Recent advances and applications. Trends Environ. Anal. Chem. 2019, 24, e00073. [Google Scholar] [CrossRef]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Yuan, M.; Trinh, M.V.; Chen, Y.; Lu, Y.; Wang, L.; Cheng, S.; Li, Z.; Santikunaporn, M.; Asavatesanupap, C. Steam stripping for recovery of ammonia from wastewater using a high-gravity rotating packed bed. Environments 2024, 11, 206. [Google Scholar] [CrossRef]
- Salee, P.; Nitiyanontakit, S.; Tungkijanansin, N.; Varanusupakul, P.; Unob, F. A new gel-based platform for an LED-based colorimetric flow-analysis detection of ammonia in water. Microchem. J. 2025, 215, 114352. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, X.; Niu, W.; Zhang, J.; Shen, Y.; Li, H.; Chen, J. Evaluation of matrix effects on automated nutrient determination in seawater using segmented flow analysis. Microchem. J. 2025, 212, 113459. [Google Scholar] [CrossRef]
- Beaton, A.D.; Cardwell, C.L.; Thomas, R.S.; Sieben, V.J.; Legiret, F.E.; Waugh, E.M.; Statham, P.J.; Mowlem, M.C.; Morgan, H. Lab-on-chip measurement of nitrate and nitrite for in situ analysis of natural waters. Environ. Sci. Technol. 2012, 46, 9548–9556. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, L.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.B. The Griess reaction. A survey of the chemistry. Anal. Chem. 1979, 51, 1493–1502. [Google Scholar] [CrossRef]
- Zhan, Y.; Lin, H.; Xiang, J.; Zhang, B.; Zhao, Z.; Lin, J.; Li, J.; Cheng, Y. A low-cost read-out photoresponsive colorimetric platform for nitrite detection based on smartphone APP. Microchem. J. 2025, 215, 114365. [Google Scholar] [CrossRef]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- Cogan, D.; Fay, C.; Boyle, D.; Osborne, C.; Kent, N.; Cleary, J.; Diamond, D. Development of a low cost microfluidic sensor for the direct determination of nitrate using chromotropic acid in natural waters. Anal. Methods 2015, 7, 5396. [Google Scholar] [CrossRef]
- Schnetger, B.; Lehners, C. Determination of nitrate plus nitrite in small volume marine water samples using vanadium(III)chloride as a reduction agent. Mar. Chem. 2014, 160, 91–98. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.; Evans, G.W.H.; Coleman, S.M.; Niu, X. Nitrate measurement in droplet flow: Gas-mediated crosstalk and correction. Lab Chip 2018, 18, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Li, H.; Bo, G.; Lin, K.; Yuan, D.; Ma, J. On-site detection of nitrate plus nitrite in natural water samples using smartphone-based detection. Microchem. J. 2021, 165, 106117. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Wang, D.; Zheng, M.; Yang, M.; Yang, Y.; Ren, T. Recent advances in microfluidic sensors for nutrients detection in water. Trends Analyt. Chem. 2023, 158, 116790. [Google Scholar] [CrossRef]
- Plant, J.N.; Sakamoto, C.M.; Jonhson, K.S.; Maurer, T.L.; Bif, M.B. Updated temperature correction for computing seawater nitrate with in situ ultraviolet spectrophotometer and submersible ultraviolet nitrate analyzer nitrate sensors. Limnol. Oceanogr. Methods 2023, 21, 581–593. [Google Scholar] [CrossRef]
- Valderrama, J.C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 1981, 10, 109–122. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, C.; Wang, Y.; Wu, S.; Jin, Y.; Yang, M.; Liu, H. Effects of nitrate and nitrite on the UV/PDS process: Performance and byproduct formation from the perspective of substituents. RSC Adv. 2025, 15, 7480. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, L.; Zhong, G.; Wu, Q.; Liu, J.; Liu, X. A portable analytical system for rapid on-site determination of total nitrogen in water. Water Res. 2021, 202, 117410. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Kalsoom, U.; Farajikhah, S.; Innis, P.C.; Nesterenko, P.N.; Lewis, T.W.; Breadmore, M.C.; Paull, B. Enhanced physicochemical properties of polydimethylsiloxane based microfluidic devices and thin films by incorporating synthetic microdiamond. Sci. Rep. 2017, 8, 15109. [Google Scholar]
- Bittig, H.C.; Maurer, T.L.; Plant, J.N.; Schmechtig, C.; Wong, A.P.S.; Claustre, H.; Trull, T.W.; Bhaskar, T.V.S.U.; Boss, E.; Dall’Olmo, G.; et al. A BGC-Argo Guide: Planning, Deployment, Data Handling and Usage Mission Considerations or the Global. Front. Mar. Sci. 2019, 6, 502. [Google Scholar] [CrossRef]
- Lin, K.; Xu, J.; Guo, H.; Huo, Y.; Zhang, Y. Flow injection analysis method for determination of total dissolved nitrogen in natural waters using on-line ultraviolet digestion and vanadium chloride reduction. Microchem. J. 2021, 164, 105993. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.U.; deMello, A.J. Multiplexed microfluidic systems for high-throughput colorimetric analysis. Lab Chip 2015, 15, 1995–2002. [Google Scholar]
- Johnson, K.S.; Coletti, L.J. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep Sea Res. 2002, 49, 1291–1305. [Google Scholar] [CrossRef]
- Altahan, M.F.; Esposito, M.; Achterberg, E.P. Improvement of on-site sensor for simultaneous determination of phosphate, silicic acid, nitrate plus nitrite in seawater. Sensors 2022, 22, 3479. [Google Scholar] [CrossRef]
- Neumann, K.C.; La, D.; Yoo, H.; Burkepile, D.E. Programmable autonomous water samplers (PAWS): An inexpensive, adaptable and robust submersible system for time-integrated water sampling in freshwater and marine ecosystems. HardwareX 2023, 13, e00392. [Google Scholar] [CrossRef]
- Grand, M.M.; Clinton-Bailey, G.S.; Beaton, A.D.; Schaap, A.M.; Johengen, T.H.; Tamburri, M.N.; Connelly, D.P.; Mowlem, M.C.; Achterberg, E.P. A lab-on-chip phosphate analyzer for long-term in situ monitoring at fixed observatories: Optimization and performance evaluation in estuarine and oligotrophic coastal waters. Front. Mar. Sci. 2017, 4, 255. [Google Scholar] [CrossRef]
- Hudson, N.; Baker, A.; Ward, D.; Reynolds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jiang, Y.; Liang, W.; Zeng, H.; Chen, H.; Zhang, M. Affordable droplet-based flow analyzer with peristaltic micro-pumps for fluorescent ammonium sensing. Talanta Open 2024, 10, 100356. [Google Scholar] [CrossRef]
- Yang, T.; Stavrakis, S.; deMello, A. A high-sensitivity, integrated absorbance and fluorescence detection scheme for probing picoliter-volume droplets in segmented flows. Anal. Chem. 2017, 89, 12880–12887. [Google Scholar] [CrossRef]
- Delaunay, L.; Compère, C.; Lehaitre, M. Biofouling protection for marine environmental sensors. Ocean Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, G.S.; Chiu, D.T. Disposable microfluidic devices: Fabrication, function, and application. Biotechniques 2005, 38, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. Engl. 2016, 55, 3862–3881. [Google Scholar] [CrossRef]
- Ray, P.P. A survey on Internet of Things architectures. J. King Saud Univ. Comput. Inf. Sci. 2018, 30, 291–319. [Google Scholar] [CrossRef]
- Calvo-López, A.; Alonso-Chamarro, J.; Puyol, M. Highly versatile and automated total ammonia nitrogen compact analyzer suitable for different types of water samples. Environ. Sci. Water Res. Technol. 2023, 9, 3366. [Google Scholar] [CrossRef]
- Fluidion: Water Intelligence Company. Available online: www.fluidion.com (accessed on 27 June 2025).
- Sea-Bird Scientific Company. Available online: www.seabird.com (accessed on 27 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).