Abstract
The presence of engineered nanoparticles (ENPs) in soil systems can modify their properties and the availability of nutrients. This study evaluated the effect of 1% CuO or ZnO ENPs on the physicochemical properties and on the phosphorus (P) adsorption–desorption processes of a volcanic ash soil (Lautaro; LAU). The dynamics of P were conducted through kinetic and isotherm batch experiments. The results showed that LAU soil with 1% CuO or ZnO ENPs increased pHH2O (from 5.67 to 6.03 and 6.82, respectively), electrical conductivity (from 0.119 to 0.143 and 0.150 dS m−1, respectively), Zn availability (597.7 times higher for LAU with 1% ZnO ENPs in relation to soil without ENPs), and Cu availability (41.8 times higher for LAU with 1% CuO ENPs in relation to soil without ENPs). Moreover, the presence of ENPs decreased Brunauer, Emmett, and Teller specific surface area. The adsorption kinetic studies of P on LAU soil without and with 1% ENPs fitted well to the Elovich model (r2 ≥ 0.923), which indicated a chemiadsorption mechanism, whereas the adsorption isotherms were described by Langmuir–Freundlich model (r2 ≥ 0.939). The desorption percentage was LAU > LAU + 1% CuO–ENPs > LAU + 1% ZnO–ENPs, demonstrating an increased stability of the P–soil surface binding with 1% ENPs. Co–existing NO3−, SeO42−, and SO42− anions did not generate a steric hindrance between P and LAU soil binding. Finally, both ENPs could alter the quality of the soil due to changes in their physicochemical properties and decrease the availability of P in volcanic ash soils.
1. Introduction
Phosphorus (P) in agricultural soils is an essential nutrient as it enables optimal crop growth [1]. However, in volcanic ash-derived soils, which cover approximately 124 million hectares or 0.84% of the world’s land surface [2] and are characteristic of countries like Japan, Indonesia, Spain, Italy, USA, Portugal, Chile, Ecuador, New Zealand, West Samoa, and Taiwan [3], there is a low concentration of available P. In this situation, farmers must add excess P fertilizers (e.g., monoammonium phosphate, diammonium phosphate, and triple superphosphate) for optimal crop development [4]. Nevertheless, of all the P applied, only 10–20% is taken up by crops, while the rest is fixed to the soil and remains part of the P–bank characteristic of these soils [4,5]. This is due to volcanic ash soils (VAS; Andisol and Ultisol) having a high Al and Fe content with which P forms precipitates (AlPO4 and FePO4) and adsorbing amorphous minerals such as allophane (>50%), imogolite, and ferrihydrite through a ligand exchange mechanism [4,6].
In recent years, studies to increase P availability in VAS have multiplied [7,8,9]. Yet, there is a paucity of vital research into the factors/phenomena that may decrease P availability in these soils. In this sense, an important field of study is about understanding the impact on soil quality (e.g., physical, chemical, and biological soil properties) and nutrient availability by emerging pollutants such as pharmaceuticals, dyes, phenols, pesticides, care products, microplastics, and engineered nanoparticles (ENPs) [10]. In particular, ENPs are materials smaller than 100 nm [11] that exhibit high specific surface areas and higher reactivity than bulk materials with the same chemical composition [12]. These features have improved antimicrobial, electrical, magnetic, catalytic, and photocatalytic properties over conventional materials [13,14,15,16]. Consequently, incorporating ENPs into healthcare products, cosmetics, and food packaging materials is becoming increasingly common and widespread [17]. Approximately 11,171 nano-sized consumer products from 68 countries were on the market by the start of 2024 [18]. This situation has led to excessive and recurrent use of ENPs, which has caused them to be released into agricultural soils through the reuse of sewage sludge from wastewater treatment plants and nanoagrochemicals (e.g., fertilizers, herbicides, insecticides, and pesticides), or directly through contaminant removal [19]. In this context, it has been suggested that these materials are generating a new form of pollution in the environment, called nanopollution, which is invisible, highly toxic, and considered very difficult to manage and control [20].
The use of ENPs, particularly CuO and ZnO–based ENPs, is of great environmental concern due to their various applications in the agricultural sector to increase soil fertility, crop productivity, and food quality [21,22]. Several studies have reported that adding ZnO and CuO ENPs increases crop growth and yield by stimulating photosynthesis and plant respiration [23,24]. On the other hand, environmental studies on the impact of CuO and ZnO ENPs on soils have focused on understanding their effects on microbiota, transformations, and transport [25,26]. In relation to this, it has been determined that, depending on the concentrations, size, shape, and surface coating of CuO and ZnO ENPs in the soil, they can decrease microbiological activity as well as have a long residence time in soils and leach into the groundwater [6]. In addition, CuO and ZnO ENPs can release Cu2+ and Zn2+ cations, respectively, forming new complex–precipitate compounds with anions such as phosphate (PO43−), selenate (SeO42−), and sulfate (SO42−), affecting their availability in soils [6,27]. Specifically, in VAS, it has been determined that metallic Cu or Ag ENPs can alter the microbiology, pH, and specific surface area, resulting in a change in the availability of contaminants, herbicide, and nutrients [28,29,30]. In this context, Parada et al. [28] determined that in VAS, the adsorption of atrazine was increased due to the presence of Cu–ENPs. Similarly, Suazo-Hernández et al. [6] determined that Cu–ENPs were oxidized in VAS and subsequently released Cu2+ into the solution, forming co-precipitates with P (Cu3(PO4)2), increasing P retention.
The adsorption–desorption processes in soils control the availability of nutrients and are dependent on factors such as organic matter (OM) content, mineralogy, ionic strength, pH, surface coverage, cation type, nature of the sorbent, residence time, and the presence of external substrates, such as amendments and contaminants [31]. To date, no research has evaluated the changes caused by ZnO and CuO ENPs on the physicochemical properties of VAS and P availability. Therefore, assessing the impact of ENPs on soil is crucial for preserving soil health and maintaining the quality of ecosystem services. With this background, the main goal of the present work was to evaluate the effect of ZnO and CuO ENPs on the physicochemical properties and P adsorption–desorption processes of a VAS.
2. Materials and Methods
2.1. Chemicals Used
The reagents used were potassium dihydrogen phosphate (KH2PO4, 99.99% purity), sodium hydroxide (NaOH; 99.99% purity), 37% hydrochloric acid (HCl), sodium chloride (NaCl; ≥99% purity), potassium dichromate (K2Cr2O7), 95.0–97.0% sulfuric acid (H2SO4), ammonium acetate (NH4CH3CO; 99.99% purity), diethylenetriaminepentaacetic acid (DTPA; ≥99% purity) analytical-grade (Merck), and double distilled water (conductivity ≤ 1.0 μS m−1).
2.2. ENPs
The CuO–ENP nanopowder < 50 nm (CAS number 1317–38–0), and ZnO–ENP nanopowder < 100 nm (CAS number 1314–13–2) used were purchased through Sigma-Aldrich (St. Louis, MO, USA) Chemicals. The CuO–ENP exhibited a specific surface area of 29 m2 g−1, while ZnO–ENP exhibited a specific surface area of 10–25 m2 g−1.
2.3. Volcanic Ash Soil Collection
A volcanic ash-derived soil belonging to Andisol order (Soil Taxonomy) from Lautaro (LAU) was collected in southern Chile (38°34′ S, 72°27′ W); it was taken from the top 20 cm of the soil and passed through a <2 mm mesh sieve and kept at room temperature until use.
2.4. Characterization of Volcanic Ash Soil and ENPs
Phosphorus available was extracted with sodium bicarbonate (0.5 M NaHCO3 at pH 8.50) [32]. The initial soil pH was determined using a ratio of 1:2.5 soil:double distilled water. To measure electrical conductivity (EC), a ratio of 1:5 soil:solution was used. The OM percentage (%) was measured using 20 mL of K2Cr2O7, while H2SO4 was used as the oxidizing agent [33]. The cation exchange capacity was calculated as the sum of Na, K, Mg and Ca, extracted with NH4CH3CO2 solution pH = 7.0 (1000 mmol L−1) [33] and measured by atomic absorption spectroscopy (AAS; iCE TM 3300, Thermo Scientific, Waltham, MA, USA). Exchangeable Al was extracted with KCl (1000 mmol L−1) and measured by AAS. Effective cation exchange capacity (ECEC) was calculated as the sum of the exchangeable base cations plus the exchangeable Al. Micronutrients (Zn and Cu) bioavailability in the soil were extracted with DTPA and measured by AAS. To establish the isoelectric point (IEP), 20 mg of the LAU soil and ENPs samples were suspended in 10 mmol L−1 NaCl and then measured using a Zeta Meter ZM–77 (Malvern Instruments, Worcestershire, UK). The specific surface area of soil and ENPs was obtained using the Brunauer, Emmett and Teller (BET) theory [34]. 0.6 g of samples were degassed for 4 h at 105 °C [6,29,30], and then analysis was conducted using N2 gas at −196 °C in the relative pressure range (P/P0) of 0.05–0.9. Surface area measurements were made with a Quantachrome Nova 1000e analyzer (Quantachrome Instruments, Boynton Beach, FL, USA). The average pore volume and size diameter were obtained using the Barrett–Joyner–Halenda model [35].
2.5. Batch Ad–Desorption Studies
Batch experiments were conducted to investigate the adsorption capacity of P as dihydrogen phosphate (H2PO4−) by adsorption onto 0.5 g of a LAU soil without and with ZnO–ENPs or CuO–ENPs. The P concentrations adsorbed (qt, mmol kg−1) onto the LAU soil without and with ENPs were determined by Equation (1) as follows:
where C0, and Ct are the initial and time t or equilibrium concentrations of P (mmol L−1), respectively, V is the volume (L), and w is the mass (kg) of soil used.
2.5.1. Effect of Doses
To study the effect of doses of ZnO–ENPs or CuO–ENPs in the P adsorption on LAU soil without and with ENPs (0.2–2.0%, w/w) were mixed with 20 mL of a solution of 6.47 mmol L−1 of P pH 5.5 ± 0.1 by adding HCl or NaOH, and ionic strength 10 mmol L−1 NaCl in polypropylene tubes and stirred at 200 rpm using an orbital shaker for 1440 min at 20 ± 2 °C.
2.5.2. Effect of pH
To study the pH effect in the P adsorption on LAU, LAU + 1% CuO–ENPs (%, w/w) and LAU + 1% ZnO–ENPs (%, w/w) systems were mixed with 20 mL of stock solution of 6.47 mmol L−1 of P from pH 4.5 ± 0.1 to 10.5 ± 0.1 by adding HCl or NaOH, and ionic strength 10 mmol L−1 NaCl, which were stirred at 200 rpm using an orbital shaker for 1440 min at 20 ± 2 °C.
2.5.3. Adsorptions Kinetic Studies
For the kinetic study of P on LAU, LAU + 1% CuO–ENPs (%, w/w) and LAU + 1% ZnO–ENPs (%, w/w) systems were mixed with 20 mL of stock solution of 6.47 mmol L−1 of P, ionic strength 10 mmol L−1 NaCl, and pH 5.5 ± 0.1 by adding HCl or NaOH, and P suspensions were stirred at 200 rpm at 20 ± 2 °C and taken from soil in time intervals between 0 and 1440 min (0, 2.5, 5, 10, 30, 60, 120, 180, 360, 720, 1080, and 1440 min). The data obtained from experimental kinetics studies were fitted to the pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich, and Weber–Morris models (Table 1).

Table 1.
The kinetic models used for the description of P adsorption.
2.5.4. Adsorptions Isotherm Studies
Adsorption isotherms of P on LAU, LAU + 1% CuO–ENP (%, w/w) and LAU + 1% ZnO–ENP (%, w/w) systems were obtained mixed 20 mL of stock solution of concentrations 0.04–6.47 mmol L−1 to ionic strength 10 mmol L−1 NaCl, and pH 5.5 ± 0.1 by adding HCl or NaOH. The suspensions were stirred at 200 rpm in an orbital shaker at 20 ± 2 °C for 1440 min. The data obtained from adsorption isotherms were fitted to the Langmuir, Freundlich, and Langmuir–Freundlich (L–F) mathematical models (Table 2).

Table 2.
The isotherm models used for the description of phosphorus adsorption.
2.5.5. Desorption Studies
Desorption studies from systems were studied, where the LAU, LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs was added to 20 mL of a solution of 6.47 mmol L−1 P at pH 5.5 ± 0.1 by adding HCl or NaOH, and 20 ± 2 °C in 10 mmol L−1 NaCl (background electrolyte). The suspensions were stirred at 200 rpm in an orbital shaker at 20 ± 2 °C for 1440 min. The supernatant was separated from the solid through centrifugation at 12,000 rpm for 15 min and was filtered using a 0.45 μm syringe filters. Subsequently, 10 mL of 10 mmol L−1 NaCl of pH 5.5 ± 0.1 was added to HCl or NaOH. At 20 ± 2 °C without P, it was replaced four times by 10 mL of the previous solution (successively) and every time, polypropylene tubes were shaken at the same condition previously mentioned.
The P desorption percentage was calculated using Equation (2), where P desorbed is the amount of nutrient desorbed by NaCl (mmol kg−1) and P adsorbed is the amount of nutrient adsorbed by the solids before NaCl treatment (mmol kg−1).
2.5.6. Co-Existence Anions
The effect of co-existence of NO3−, SO42−, and SeO42− anions on P adsorption was studied using different concentrations of those anions (0.40, 2.43, 4.05, and 6.47 mmol L−1) and a fixed P concentration of 6.47 mmol L−1 at pH 5.5 ± 0.1 by adding HCl or NaOH at 20 ± 2 °C, and ionic strength 10 mmol L−1 NaCl. The solution was stirred at 200 rpm using an orbital shaker at 20 ± 2 °C for 1440 min.
2.5.7. Phosphorus Quantification
The samples from adsorption–desorption studies were centrifuged at 12,000 rpm using a Sorvall Model RC-5B Plus centrifuge (Newtown, CT, USA) for 15 min and filtered through 0.45 μm syringe filters. The P amount in the supernatant was determined at 880 nm [37] using a spectrophotometer Rayleigh UV-2601 (BRAIC Co., Ltd., Beijing, China).
2.6. Data Analysis
The analysis of the adsorption–desoprtion data was conducted, and figures were drawn using the software program Origin 9.0. Standard solutions and reagent blanks were included with each batch experiment. The adsorption–desorption studies were performed in triplicate. The data obtained from experimental kinetics and isotherms adsorption studies were evaluated through the coefficient of determination (r2) parameter.
3. Results and Discussion
3.1. Impact of CuO and ZnO ENPs on Soil Physicochemical Properties
The initial physicochemical properties of the LAU soil, such as pH, EC, OM%, available nutrient content, BET-specific surface area, and pore volume, which are an important indicator of soil fertility, were analyzed before starting the experiments (Table 3) and after the addition of 1% Cu and ZnO ENPs. The initial LAU soil had an acidic reaction (5.67), medium EC value (0.119 dS m−1), and high OM content (15%). In addition, there was a low content of Zn bioavailable (1.32 mg kg−1) and Cu bioavailable (2.08 mg kg−1). On the other hand, after adding 1% of Cu and ZnO ENPs, the LAU soil showed an increase in pH (6.03, and 6.82, respectively) (Table 3), being higher in the presence of ZnO–ENPs than CuO–ENPs. This phenomena could be due to the consumption of the H+ from the solution by the ENPs or to a hydration process of the ENPs (Equations (3) and (4), respectively), where M can be Cu or Zn [38].

Table 3.
Physicochemical properties of Lautaro (LAU) soil without and with 1% ENPs.
The EC, which is a measure of the number of salts, of the LAU soil with 1% ENPs showed an increase in the values, being 1.26 higher with 1% ZnO–ENPs, and 1.20 for with 1% CuO–ENPs than LAU soil alone (Table 3). This result suggests the greater dissolution of ZnO–ENPs and it is in concordance with the high pH values observed. Moreover, these results were consistent with the number of times the concentration of bioavailable metal has increased since, with 1% CuO–ENPs, the concentration of Cu bioavailable in the LAU soil was 41.82 times higher than the LAU soil without ENPs. Meanwhile, with 1% ZnO–ENPs, the concentration of Zn bioavailable in the LAU soil increased 597.73 times compared to the soil without ENPs. Similar outcomes of variation in pH and available Zn after adding ZnO–ENPs to a soil were observed by Verma et al. [39].
Finally, the LAU soil had a BET-specific surface area value of 23.026 m2 g−1 and an average pore volume of 0.022 cm3 g−1. After the addition of 1% ENPs, there was a decrease in these values, being lower with CuO–ENPs (9.030 m2 g−1, and 0.007 cm3 g−1) than with ZnO–ENPs (12.201 m2 g−1, and 0.009 cm3 g−1) (Table 3). These results showed that the presence of 1% ENPs accounts for the reduction in the active surface, and pore volume of the LAU soil. All the changes noted in the physicochemical properties of the LAU soil can indirectly affect P adsorption–desorption processes, which will be analyzed in the following sections.
3.2. Effect of the Doses
Figure 1a shows that the LAU soil without ENPs presented a P adsorption of 116.79 mmol kg−1 (46.03%). Following the addition of ENPs in the range of 0.2 to 2%, P adsorption increased for CuO–ENPs from 117.56 mmol kg−1 (45.82%) to 157.36 mmol kg−1 (59.20%) and for ZnO–ENPs from 119.61 mmol kg−1 (46.62%) to 196.12 mmol kg−1 (78.55%).
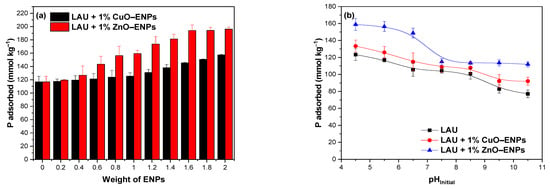
Figure 1.
(a) Effect of doses of CuO–ENPs or ZnO–ENPs on the phosphorus (P) adsorption on Lautaro (LAU) soil and (b) pHInitial effect of the solution on the P adsorption without and with 1% CuO–ENPs or ZnO–ENPs on LAU soil.
These results suggested that both ENPs favored P adsorption after coming in contact with the LAU soil, which may be related to incorporating related groups such as ≡Cu–OH and ≡Zn–OH present on surface of both ENPs or cations such as Cu2+ and Zn2+, which had a high affinity for P [6,40]. In addition, CuO and ZnO ENPs had a differential effect on the increase of P adsorption in the LAU soil, being higher with 1% ZnO ENPs than with 1% CuO ENPs. These results can be related mainly to the higher solubility of the ZnO ENPs than CuO ENPs, which is supported by the EC and pH values determined and not with the surface charge (Figure 2) and BET-specific surface area (which was higher for CuO–ENPs (29 m2 g−1) compared to ZnO–ENPs (10–25 m2 g−1).
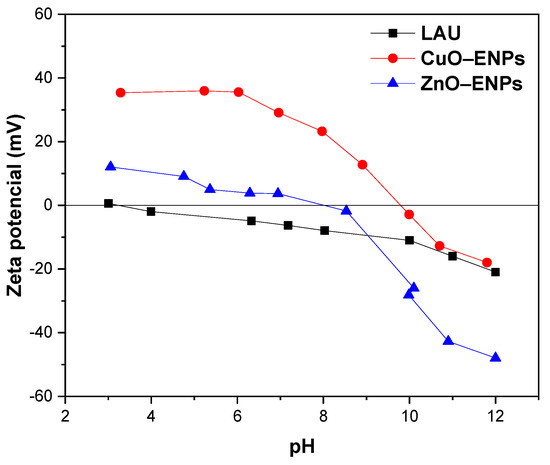
Figure 2.
pH–zeta potential curves (mV) obtained for Lautaro (LAU) soil, Cu–ENPs, and ZnO–ENPs.
3.3. Effect of pH
The pH had an important role of P adsorption on VAS because affects the ionization surface charge and the speciation of P (Figure 1b). In fact, Figure 1b showed that is evident that P adsorption depended on the pH of the solution. In relation to this, the amount of P adsorption in LAU soil without ENPs at pH 4.5 was 123.35 mmol kg−1 (49.13%), and decreased linearly with increasing pH, reaching at pH 10.5 an P adsorption of 77.08 mmol kg−1 (30.03%). The negative charge of LAU soil gradually increased with increasing pH (Figure 2), and in consequence the repulsive force between negatively charged soil surface and P anion increased. At the same time, Figure 1b shows that with 1% CuO or ZnO ENPs from pH 4.5 to 10.5, the P adsorption on LAU soil was between 1.04 and 1.45 times higher than LAU soil without ENPs. This was because, concerning acidic pH, cations (Cu2+ or Zn2+) could be released from ENPs into solution and form complexes/co–precipitates with P [6]. In addition, these cations could adsorb on the soil surface, either on the functional groups containing ≡OH or on the soil minerals, acting as a cation bridge favoring the binding of P [41]. At basic pH, P adsorption likely drops due to a lower released of cations from ENPs [42]. In addition, P can be adsorbed on surface active site of both CuO or ZnO ENPs [43,44]. Similar behavior for P adsorption in VAS with Cu–ENPs was previously reported by Suazo-Hernández et al. [6,29].
3.4. Adsorption Kinetic Studies
The influence of contact time on P adsorption on the different systems studied was shown in Figure 3a.

Figure 3.
(a) Phosphorus (P) adsorption kinetics at pH 5.5 ± 0.1 of the solution in the presence of 1% CuO or ZnO ENPs on Lautaro (LAU) soil modelled by (b) pseudo-first-order (PFO), and pseudo-second-order (PSO); (c) Elovich, and, (d) Weber–Morris models.
The amount of adsorbed P increased from 57.91 to 115.60 mmol kg−1, 58.97 to 126.91 mmol kg−1, and 73.20 to 176.78 mmol kg−1 from 2.5 min to 1440 min for LAU, LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems, respectively. The greater adsorption of P on LAU + 1% CuO–ENPs, and LAU + 1% ZnO–ENPs systems compared with LAU alone was mainly due to the presense and high reactivity of ENPs. In addition, Figure 3a illustrated that P adsorption on LAU, LAU + 1% CuO–ENP, and LAU + 1% ZnO–ENP systems reached adsorption equilibrium at 720 min. This indicated that long contact times had a significant influence on P adsorption in VAS as well as the presence of 1% ENPs did not change the behavior.
To explore the potential rate-controlling step during P adsorption process, the desorption kinetics data were fitted to the PFO, PSO, Elovich, and Weber–Morris adsorption models (Figure 3b–d). The parameters obtained are showed in Table 4.

Table 4.
Pseudo-second-order (PSO), pseudo-first-order (PFO), Elovich, and Weber–Morris parameters (±standard error) obtained from phosphorus adsorption kinetics at pH 5.5 ± 0.1 for Lautaro (LAU) soil in the absence and presence of 1% CuO or ZnO ENPs.
3.4.1. Pseudo-First-Order and Pseudo-Second-Order Models
The PSO model for the LAU, LAU + 1% CuO–ENP, and LAU + 1% ZnO–ENP systems presented r2 values closer to 1 compared to the PFO model (Table 4), indicating that the overall rate of the P adsorption process on the different systems would be controlled by chemiadsorption forming bidentate complexes [45]. The h parameter, that can be related to the initial adsorption rate, may be attributed to chemical and/or hydrogen bonding between P and the surface hydroxyls of the soils at time ≈ 0 [46]. In this context, the h value presented the following trend LAU > LAU + 1% CuO–ENPs > LAU + 1% ZnO–ENPs, reaching values of 27.12 mmol kg−1 min−1, 24.30 mmol kg−1 min−1, and 1.11 mmol kg−1 min−1, respectively. The decreased in the value of h due to the presence of 1% CuO–ENPs or ZnO–ENPs at t→0, may be associated with blocking adsorption sites. In this sense, Julich and Gäth, [47] informed that CuO–ENPs due to the high surface reactivity they showed a strong affinity by positive surface groups like ≡Al–OH and ≡Fe–OH contained in agricultural soils collected from Hesse (Germany). In a similar way, Chang et al. [48] suggested that due to Ca2+ increasing the ionic strength of soil and compressing the double electric layer of CuO–ENPs, which reduced the repulsive force between ENPs, CuO–ENPs were aggregated/deposited on soil surface of agricultural soils collected from China. Meanwhile, the equilibrium rate constant (k2) of P adsorption showed the same sequence as h values. However, the PSO model was not the best one to describe the P adsorption process on the systems studied, due to r2 ≤ 0.879.
3.4.2. Elovich Model
The Elovich model indicates that the adsorption of an analyte occurs on a heterogeneous surface through chemiadsorption and without interactions between the adsorbed species [49]. This model has been used to explain P adsorption on different types of soils without and with ENPs [29,30]. In this context, Table 4 showed for different systems that the Elovich chemiadsorption model presented an r2 value closer to 1 (r2 ≥ 0.923) than the PSO, and PFO models, which supported the good ability of the model to explain P adsorption in LAU soil in the presence and absence of 1% ENPs. Similar behavior has been reported for P adsorption in VAS without and with ENPs [29,50]. For three systems, the constant kinetic α ranged between 202.34 kg−1 mmol, and 873.95 mmol kg−1 min−1 and the desorption parameter β varied between 0.06 kg−1 mmol and 0.10 kg−1 mmol. Higher values of α than β indicated a higher adsorption rate than desorption rate, which showed the high feasibility of the adsorption process for the different systems [51]. Finally, the Elovich model described chemiadsorption and, therefore, suggested the potential for formation of chemical bonds between the P and surface–active sites of the systems (i.e., ≡Zn–OH, ≡Cu–OH, ≡Al–OH, and ≡Fe–OH).
3.4.3. Weber–Morris Model
The Weber–Morris model has been used to identify the steps during the P diffusion process on the LAU soil without and with ENPs [52]. The research data of P adsorption show that the plots usually do not pass through the origin, so the adsorption process is carried out through a more complex mechanism [52,53]. In this case, the line did not pass through the origin, suggesting that the intraparticle diffusion model was not the only rate–controlling step. In this sense, the plot of qt vs. t1/2 for the process of P adsorption on LAU soil without and with 1% ENPs was divided into three stages: (i) boundary layer diffusion of solute molecules, where occurred diffusion to the sorbent surface (ii) intraparticle diffusion, where occurred the solute diffusion within the internal mesopores and micropores and (iii) a solute sorption in the interior sorbent surface, where a rapid uptake occurs (Figure 3d) [54]. The r2 values for the film diffusion segments were very high (r2 ≥ 0.979), with the execution of LAU + 1% ZnO–ENPs (r2 = 0.752), as well as for intraparticle diffusion (r2 ≥ 0.977). The film diffusion rate constant, Kint1, increased with ENPs, being higher for ZnO–ENPs than for CuO–ENPs, indicating that P transport via the liquid film was more favorable with ZnO–ENPs. Additionally, the Kint2 values, which were calculated from the slopes from the second stage, suggest that this process is slower for LAU and LAU + 1% CuO–ENPs than the initial Kint1 period, likely due to the high porosity of these systems [55]. The C value indicates the thickness of the boundary layer and for the systems, and, in particular, the C2 value exhibited the following next trend; LAU + 1% CuO–ENPs > LAU > LAU + 1% ZnO–ENPs, reaching values of 78.11 mmol kg−1, 73.05 mmol kg−1 and 3.98 mmol kg−1, respectively. Therefore, the reduction in this value after adding ZnO–ENPs accounts for these ENPs working against the boundary layer diffusion effect, unlike the effect caused by CuO–ENPs.
Based on kinetic models, it can be mentioned that the fitted of suitability for the P adsorption process on the systems in this study were Elovich and Weber–Morris models. These results suggested a similar mechanism for P adsorption kinetic on LAU + 1% CuO–ENPs > LAU > LAU + 1% ZnO–ENPs systems. However, a good model fit, does not necessarily mean that the underlying mechanisms of the model are correct; rather, the experimental data are consistent with the model because to P adsorption on VAS is a complex process and it is even more in the presence of ENPs, which is governed by many factors including type of ENPs, and changes generated in soil properties like pH, and EC by ENPs presence, further research is needed to determine the underlying mechanisms involved, particularly in ENPs amended soil.
3.5. Adsorption Isotherm Studies
The dependence of the amount of adsorbed P and the concentration at equilibrium is shown in Figure 4a. The amount of adsorbed P increased significantly in the presence of 1% CuO–ENPs and ZnO–ENPs in the LAU soil, indicating their marked effect on P binding to the soil. Moreover, the experimental data of adsorption isotherms of P on LAU soil and LAU + 1% CuO–ENPs described an L–type curve and for LAU + 1% ZnO–ENPs was H–curve. This suggested that both the soil and ENPs had high affinity for P [56]. Although for LAU + 1% ZnO–ENP system the curve also indicated that the adsorption system was dominated by chemiadsorption mechanism [57]. The amounts of P adsorbed in the LAU soil without and with CuO–ENPs and ZnO–ENPs were 104.74 mmol kg−1 (40.96%), 120.94 mmol kg−1 (47.13%), and 147.39 mmol kg−1 (58.26%), respectively, which correspond to the maximum experimental adsorption.

Figure 4.
(a) Phosphorus (P) adsorption isotherms at pH 5.5 ± 0.1 in the absence and presence of 1% CuO or ZnO ENPs on Lautaro (LAU) soil and (b) P adsorption isotherms in the absence and presence of 1% CuO or ZnO ENPs on LAU soil modelled by Langmuir, Freundlich, and Langmuir–Freundlich models.
To explain the affinity soil–P (KL, n, and KL–F) and the maximum adsorption capacity (qmax) and to understand the implications that ENPs can have on these parameters, the experimental data were modeled using the Langmuir, Freundlich, and Langmuir–Freundlich isotherm models (Figure 4b) and the data are tabulated in Table 5.

Table 5.
Langmuir, Freundlich, and Langmuir–Freundlich parameters (±standard error) for phosphorus adsorption isotherms at pH 5.5 ± 0.1 in the absence and presence of 1% CuO or ZnO ENPs on Lautaro (LAU) soil.
3.5.1. Langmuir and Freundlich Models
The Langmuir model is based on the P adsorption occurring on a homogeneous surface, so that the adsorption sites are similar, unlike the Freundlich model, which considers a heterogeneous surface with adsorption sites of differing energy [58]. Table 5 showed that for the LAU and LAU + 1% CuO–ENP soil, there were minimal differences between the r2 values obtained from the two models; therefore, P adsorption can be described by both models, suggesting that adsorption is of a cooperative type. On the other hand, the Langmuir model for the LAU + 1% ZnO–ENP system showed a value of r2 = 0.938, which was much higher than the value of r2 = 0.877 obtained from the Freundlich model, suggesting that the P adsorption on LAU soil in the presence of those ENPs occurrs in a homogeneous surface. According to the Langmuir model, there was a clear increase of the qmax with the ENPs, reaching values of 139.13 mmol kg−1, 132.64 mmol kg−1, and 117.22 mmol kg−1 for LAU + 1% ZnO–ENPs, LAU + 1% CuO–ENPs and LAU systems, respectively. The differences in the effect produced between both ENPs on P adsorption may be related to the higher solubility of ZnO–ENPs compared to CuO–ENPs [42]. As a result, there was a greater possibility of forming complexes and precipitates of Zn2+ with P. In the study realized by Lv et al. [59] was reported the formation of Zn3(PO4)2 precipitates (Ksp = 9.1 × 10−31). Similarly, Suazo-Hernández et al. [6] reported the formation of Cu3PO4 precipitates (Ksp = 1.40 × 10−37). Therefore, despite the smaller size of CuO–ENPs and higher BET–specific surface area value (<50 nm and 29 m2 g−1, respectively) compared to ZnO–ENPs (<100 nm, and 10–25 m2 g−1, respectively), they have a lower reactivity. This behavior contributes to reducing the dissolution process of CuO–ENPs [60], which was evidenced by the lower EC, and pH values and adsorption of P. In addition, P could be adsorbed on CuO–ENPs [43] and ZnO–ENPs [44] through specific Lewis acid–base interaction or ligand exchange mechanism, where P serves as Lewis base to donate electron pairs to bind Cu and Zn atoms, respectively, and inner-sphere complexes can be formed. While the adsorption affinity (KL), which represents the ability of the system to adsorb P from the solution, showed values of 1.75 L mmol−1, 2.08 L mmol−1 and 24.79 L mmol−1 for LAU, LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems, respectively. Therefore, 1% ZnO–ENPs increased P adsorption affinity on the LAU soil more than 1% CuO–ENPs.
3.5.2. Langmuir–Freundlich Model
The L–F model considers adsorption occurring on a heterogeneous surface [61]. The L–F model at low adsorbate concentrations reduces to the Freundlich isotherm model, while at high concentrations it approaches the Langmuir isotherm model [62]. The value of r2 obtained from L–F model for the LAU and LAU + 1% CuO–ENP systems was 0.995, and for the LAU + 1% ZnO–ENP system was r2 = 0.939, suggesting that P adsorption followed an adsorption mechanism on a heterogeneous surface. These data were consistent with the studies conducted by [28,30,63]. In particular, Suazo-Hernández et al. [30] determined that P adsorption on different fractions of an Andisol soil with 1% Cu–ENPs fitted to the L–F model, which could be related to a heterogenization of the soil surface produced by ENPs. In addition, KF–L parameter showed a similar tendency that KL parameter. According to these results, it can be mentioned that the best fitted for P adsorption isotherm data for the three systems studied was L–F model.
3.6. Desorption Studies
Desorption studies can indicate how strong P is attached to LAU soil and evaluate the effect of 1% CuO or ZnO ENPs on P–soil binding. In this study, NaCl (10 mmol L−1) at pH 5.5 was selected to desorb P from the different systems (Figure 5).

Figure 5.
Phosphorus (P) desorption percentage obtained from Lautaro (LAU) soil without and with 1% CuO–ENPs or ZnO–ENPs using 10 mmol L−1 NaCl as the extracting agent.
Figure 5 showed that P desorption from the LAU soil was higher than for LAU 1% + CuO–ENPs or ZnO–ENPs systems with. The P desorption percentage after four treatments showed the following sequence LAU > LAU + 1% CuO–ENPs > LAU + 1% ZnO–ENPs with values of 44.59%, 19.14%, and 14.59%, respectively. In other words, the desorption for the LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems was 0.43 and 0.33 times lower that LAU soil alone. This behavior makes sense if we consider that after adding 1% CuO–ENPs or ZnO–ENPs, surface groups, such as ≡Cu–OH or ≡Zn–OH, are being incorporated, which have been shown to have a high affinity for P. Taghipour and Jalali, ref. [64] reported that the binding between the active sites of Al2O3 and TiO2 ENPs and adsorbed P is so strong that P adsorption from soils was relatively irreversible. The results obtained from desorption studies are related to the trend obtained in the KL and KF–L parameters of the Langmuir and L–F models, respectively.
3.7. Competitive Effects
Different anions can co–existing with P in soil solution, which can compete for active sites of LAU soil, LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems with P and affect their availability. The conventional anions in soil solutions such as NO3−, SeO42−, and SO42− were selected in experiment at 0.40, 2.43, 4.05, and 6.47 mmol L−1, representing low, medium, and high concentration levels. The effects of anions with different concentration on P adsorption capacity on LAU soil in the presence and absence of 1% CuO or ZnO ENPs ENPs were tested at pH 5.5 and the results were depicted in Figure 6.
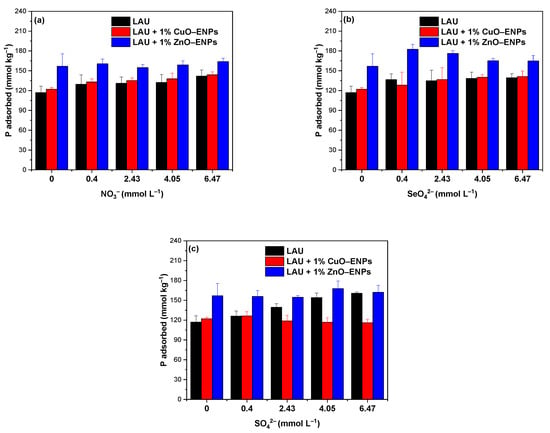
Figure 6.
Competitive studies of phosphorus (P) adsorption under the absence and presence of 1% CuO–ENPs or ZnO–ENPs on Lautaro (LAU) soil with (a) nitrate (NO3−), (b) selenate (SeO42−), and (c) sulfate (SO42−).
In Figure 6a, a slight increase in P adsorption for LAU soil can be observed. LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems with increasing concentrations of NO3− anions indicated a clear synergistic effect, unlike what was observed in the presence of SeO42− and SO42− anions (Figure 6b,c, respectively), where for LAU soil, LAU + 1% CuO–ENPs and LAU + 1% ZnO–ENPs systems, they produced an increase, or did not change the P adsorption in relation to systems without co–existing anions. Therefore, the effect of SeO42− and SO42− anions is concentration–dependent and they can act as a synergistic, or expectant anions. Various investigations have provided information on the synergistic effect of NO3− on P, as well as the dual effect of SeO42− and SO42− [65,66]. In general, the presence of NO3−, SeO42−, and SO42− anions for LAU soil, LAU + 1% CuO–ENPs, and LAU + 1% ZnO–ENPs systems did not generate a steric hindrance between P and the soil surface and suggests that the P–LAU soil binding was not only through chemistry adsorption mechanism, as it was previously determined from isotherm and kinetic studies, but also highly selective, which was more favored in the presence of 1% ZnO–ENPs.
4. Implications of Environmental Risk
The extensive application of CuO–ENPs and ZnO–ENPs would introduce an increasing amount of these types of ENPs into the environment, resulting in a substantial number of CuO–ENPs and ZnO–ENPs inevitably accumulating in agricultural VAS or uptake and transported to all parts of the plant, allowing them to enter the food chain [67]. The result of the present study indicates that CuO–ENP and ZnO–ENP changed the physicochemical properties of VAS. At the same time, the solubility and surface groups of ENPs played a most important role in the adsorption of P in soil than surface charge, and BET–specific surface area.
Particularly, it was found that the binding of CuO–ENP and ZnO–ENP to soil surface significantly decreased the BET–specific surface area, and pore volume of LAU soil but they could generate an increased in the concentration of Cu2+ and Zn2+ cations in the solution, which increased the qmax of P, KL, and KL–F values. A similar study also showed that Cu–ENPs increased P adsorption capacity in soils due to increased Cu2+ concentration in solution and increased surface area [29]. Therefore, once agricultural soils derived from volcanic ash are contaminated with CuO–ENPs and ZnO–ENPs, the soils sequester nutrients becoming unavailable to plant uptake. Finally, future studies should explore the release rate of Cu1+/2+ and Zn2+ from CuO–ENPs and ZnO–ENPs into terrestrial systems and the potential contamination of groundwater. This last is highly relevant because Cu2+ and Zn2+ are heavy metals and they are non-biodegradable, persistent, potentially toxic [68,69]; therefore, Cu2+ and Zn2+ could alter the quality of the soil, affecting plants, microbial communities, and consequently, human health.
5. Conclusions
The characterization of the Lautaro (LAU) soil showed that 1% CuO–ENPs or ZnO–ENPs generated a change in physicochemical properties. Phosphorus (P) adsorption on LAU decreased with increasing pH without and with ENPs, while the presence of 1% of CuO–ENPs or ZnO–ENPs produced an increase in the adsorption of P in all pH range. The adsorption kinetics studies of P in soils without and with 1% CuO–ENPs or ZnO–ENPs fitted well to the Elovich model (r2 ≥ 0.923) and indicated a chemiadsorption mechanism. The adsorption isotherms on the LAU soil in the presence and absence of ENPs were described by the Langmuir–Freundlich model (r2 ≥ 0.939). The P desorption percentage showed the following sequence LAU > LAU + 1% CuO–ENPs > LAU + 1% ZnO–ENPs and suggested an increased stability of the P–soil surface binding. Co-existing NO3−, SeO42−, and SO42− anions did not generate a steric hindrance between P and LAU soil binding. Finally, ENPs could alter the physicochemical properties and decreased availability of P in volcanic ash soils (VAS). Therefore, farmers in VAS must be careful about using nanotechnology in agricultural practices because they can increase P retention and affect agricultural crop production.
Author Contributions
Conceptualization, J.S.-H., M.d.l.L.M., S.C., L.C. and A.R.; methodology, J.S.-H., S.R. and E.S.-S.; software, J.S.-H.; validation, M.d.l.L.M., B.F. and J.S.-Y.; formal analysis, J.S.-H.; M.d.l.Á.S. and A.R.; investigation, J.S.-H. and E.S.-S.; resources, J.S.-H. and M.d.l.L.M.; data curation, J.S.-H.; writing—original draft preparation, J.S.-H., S.R., A.R., M.d.l.Á.S. and J.S.-Y.; writing—review and editing, J.S.-H., E.S.-S., B.F., S.C., L.C. and A.R.; visualization, J.S.-H., M.d.l.Á.S., S.C., L.C. and A.R; supervision, M.d.l.L.M. and A.R.; project administration, J.S.-H.; funding acquisition, J.S.-H. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo de Chile (ANID) grant number FONDECYT Postdoctoral Grant N° 3230179.
Data Availability Statement
The raw data of this article will be made available by the authors on request.
Acknowledgments
Special thanks to Technological Bioresource Nucleus (BIOREN-UFRO) and Soil and Plant Laboratory. Eulàlia Sans-Serramitjana acknowledges the Agencia Nacional de Investigación y Desarrollo (ANID) from the Chilean Government FOVI Project N° 230053. This work was partially funded by the Research Directorate of Universidad de La Frontera.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Janes-Bassett, V.; Blackwell, M.S.A.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A Meta-Analysis of Phosphatase Activity in Agricultural Settings in Response to Phosphorus Deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Francelino, M.R.; Thomazini, A.; Schaefer, C.E.G.R.; Anjos, L.H.C.; Pereira, M.G.; Lyra, G.B. The Thermal Regime and Mineralogical Attributes of Highland Volcanic-Ash Soils from the Cotopaxi Volcano, Ecuador: Absent Permafrost and Little Pedogenesis. Geoderma Reg. 2022, 29, e00496. [Google Scholar] [CrossRef]
- Dahlgren, R.A.; Saigusa, M.; Ugolini, F.C. The Nature, Properties and Management of Volcanic Soils. Adv. Agron. 2004, 82, 113–182. [Google Scholar] [CrossRef]
- Borie, F.; Aguilera, P.; Castillo, C.; Valentine, A.; Seguel, A.; Barea, J.M.; Cornejo, P. Revisiting the Nature of Phosphorus Pools in Chilean Volcanic Soils as a Basis for Arbuscular Mycorrhizal Management in Plant P Acquisition. J. Soil Sci. Plant Nutr. 2019, 19, 390–401. [Google Scholar] [CrossRef]
- Velásquez, G.; Ngo, P.T.; Rumpel, C.; Calabi-Floody, M.; Redel, Y.; Turner, B.L.; Condron, L.M.; de la Luz Mora, M. Chemical Nature of Residual Phosphorus in Andisols. Geoderma 2016, 271, 27–31. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Urdiales, C.; Poblete-Grant, P.; Pesenti, H.; Cáceres-Jensen, L.; Sarkar, B.; Bolan, N.; de la Luz Mora, M. Effect of Particle Size of Nanoscale Zero–Valent Copper on Inorganic Phosphorus Adsorption–Desorption in a Volcanic Ash Soil. Chemosphere 2023, 340, 139836. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Velásquez, G.; Gianfreda, L.; Saggar, S.; Bolan, N.; Rumpel, C.; Mora, M.L. Improving Bioavailability of Phosphorous from Cattle Dung by Using Phosphatase Immobilized on Natural Clay and Nanoclay. Chemosphere 2012, 89, 648–655. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Hannet, G.; Singh, K.; Fidelis, C.; Farrar, M.B.; Muqaddas, B.; Bai, S.H. Effects of Biochar, Compost, and Biochar-Compost on Soil Total Nitrogen and Available Phosphorus Concentrations in a Corn Field in Papua New Guinea. Environ. Sci. Pollut. Res. 2021, 28, 27411–27419. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Cacciamali, A.; Pascucci, L.; Villa, R.; Dotti, S. Engineered Nanoparticles Toxicity on Adipose Tissue Derived Mesenchymal Stem Cells: A Preliminary Investigation. Res. Vet. Sci. 2022, 152, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Asha, A.B.; Narain, R. Nanomaterials Properties. In Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 343–359. ISBN 9780128168066. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Li, R.; Shen, X.; Zhang, J.; Jiang, Q.; Wang, L.; Zhang, Y. Tailoring Biochar Supported Iron Nanoparticles to Activate Persulfate for Atrazine Degradation in Soil. J. Environ. Chem. Eng. 2024, 12, 111967. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.K.; Sudarsan, S.; Trofimov, E.A. High-Entropy Oxide (CeGdHfPrZr)O2 Nanoparticles as Reusable Photocatalyst for Wastewater Remediation. Surf. Interfaces 2024, 51, 104815. [Google Scholar] [CrossRef]
- Solymos, K.; Babcsányi, I.; Ariya, B.; Gyulavári, T.; Ágoston, Á.; Szamosvölgyi, Á.; Kukovecz, Á.; Kónya, Z.; Farsang, A.; Pap, Z. Photocatalytic and Surface Properties of Titanium Dioxide Nanoparticles in Soil Solutions. Environ. Sci. Nano 2024, 11, 1204–1216. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Fawole, O.A. Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review. Biomolecules 2023, 13, 1092. [Google Scholar] [CrossRef]
- STATNANO Nanotechnology Products Database. Available online: https://statnano.com (accessed on 1 September 2024).
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent Advances in the Applications of Nano-Agrochemicals for Sustainable Agricultural Development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef]
- Biswas, J.K.; Sarkar, D. Nanopollution in the Aquatic Environment and Ecotoxicity: No Nano Issue! Curr. Pollut. Rep. 2019, 5, 4–7. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shaghaleh, H.; Hamoud, Y.A.; Holford, P.; Shao, H.; Qi, W.; Hashmi, M.Z.; Wu, T. Zinc Oxide Nanoparticles: Potential Effects on Soil Properties, Crop Production, Food Processing, and Food Quality. Environ. Sci. Pollut. Res. 2021, 28, 36942–36966. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Meetei, T.T.; Devi, Y.B.; Panda, S.K.; Upadhyaya, H. Zinc Oxide Nanoparticles (ZnO-NPs): A Promising Nanoparticle in Renovating Plant Science. Acta Physiol. Plant. 2021, 43, 136. [Google Scholar] [CrossRef]
- Zafar, H.; Aziz, T.; Khan, B.; Mannan, A.; Rehman, R.U.; Zia, M. CuO and ZnO Nanoparticle Application in Synthetic Soil Modulates Morphology, Nutritional Contents, and Metal Analysis of Brassica Nigra. ACS Omega 2020, 5, 13566–13577. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, L.; Živčák, M.; Barboricova, M.; Kovár, M.; Filaček, A.; Ferencova, J.; Vysoká, D.M.; Brestič, M. Effect of Copper Oxide and Zinc Oxide Nanoparticles on Photosynthesis and Physiology of Raphanus sativus L. under Salinity Stress. Plant Physiol. Biochem. 2024, 206, 108281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Pan, B.; Zhang, X.; Zhang, H.; Steinberg, C.E.W.; Qiu, H.; Vijver, M.G.; Peijnenburg, W.J.G.M. Application of Low Dosage of Copper Oxide and Zinc Oxide Nanoparticles Boosts Bacterial and Fungal Communities in Soil. Sci. Total Environ. 2021, 757, 143807. [Google Scholar] [CrossRef]
- Lv, W.; Geng, H.; Zhou, B.; Chen, H.; Yuan, R.; Ma, C.; Liu, R.; Xing, B.; Wang, F. The Behavior, Transport, and Positive Regulation Mechanism of ZnO Nanoparticles in a Plant-Soil-Microbe Environment. Environ. Pollut. 2022, 315, 120368. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, N.; Tian, K.; Liu, S.; Ge, F. Accelerated Effects of Nano-ZnO on Phosphorus Removal by Chlorella Vulgaris: Formation of Zinc Phosphate Crystallites. Sci. Total Environ. 2018, 635, 559–566. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Diez, M.C.; Cea, M.; Sant’Ana da Silva, A.; Rodríguez-Rodríguez, C.E.; Tortella, G.R. Combined Pollution of Copper Nanoparticles and Atrazine in Soil: Effects on Dissipation of the Pesticide and on Microbiological Community Profiles. J. Hazard. Mater. 2019, 361, 228–236. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Klumpp, E.; Arancibia-Miranda, N.; Poblete-Grant, P.; Jara, A.; Bol, R.; de la Luz Mora, M. Describing Phosphorus Sorption Processes on Volcanic Soil in the Presence of Copper or Silver Engineered Nanoparticles. Minerals 2021, 11, 373. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Klumpp, E.; Arancibia-Miranda, N.; Jara, A.; Poblete-Grant, P.; Sepúlveda, P.; Bol, R.; de la Luz Mora, M. Combined Effect of Soil Particle Size Fractions and Engineered Nanoparticles on Phosphate Sorption Processes in Volcanic Soils Evaluated by Elovich and Langmuir-Freundlich Models. J. Soil Sci. Plant Nutr. 2022, 22, 3685–3696. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Olsen, S.; Sommers, L. Phosphorus. In Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties; ASA Publisher: Monroe, MI, USA, 1982; Volume 9, pp. 403–430. [Google Scholar]
- Sadzawka, R.A.; Carrasco, R.M.A.; Grez, Z.R.; Mora de la Luz, M.G.; Flores, P.H.; Neaman, A. Métodos de Análisis Recomendados Para Suelos Chilenos; Comisión de Normalización y Acreditación (CNA): Santiago, Chile, 2006. [Google Scholar]
- Antônio, D.C.; Caldeira, C.L.; Freitas, E.T.F.; Delbem, I.D.; Gasparon, M.; Olusegun, S.J.; Ciminelli, V.S.T. Effects of Aluminum and Soil Mineralogy on Arsenic Bioaccessibility. Environ. Pollut. 2021, 274, 116482. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Qiao, S.; Zhou, J. Magnetic Fe0/Iron Oxide-Coated Diatomite as a Highly Efficient Adsorbent for Recovering Phosphorus from Water. Chem. Eng. J. 2021, 412, 128696. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Gao, X.; Rodrigues, S.M.; Spielman-Sun, E.; Lopes, S.; Rodrigues, S.; Zhang, Y.; Avellan, A.; Duarte, R.M.B.O.; Duarte, A.; Casman, E.A.; et al. Effect of Soil Organic Matter, Soil pH, and Moisture Content on Solubility and Dissolution Rate of CuO NPs in Soil. Environ. Sci. Technol. 2019, 53, 4959–4967. [Google Scholar] [CrossRef] [PubMed]
- Verma, Y.; Singh, S.K.; Jatav, H.S.; Rajput, V.D.; Minkina, T. Interaction of Zinc Oxide Nanoparticles with Soil: Insights into the Chemical and Biological Properties. Environ. Geochem. Health 2021, 44, 221–234. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, X.; Wu, H.; Cen, J.; Ji, Y. Efficient Phosphate Removal by Dendrite-like Halloysite-Zinc Oxide Nanocomposites Prepared via Noncovalent Hybridization. Appl. Clay Sci. 2021, 213, 106232. [Google Scholar] [CrossRef]
- Wu, P.; Cui, P.; Du, H.; Alves, M.E.; Zhou, D.; Wang, Y. Long-Term Dissolution and Transformation of ZnO in Soils: The Roles of Soil PH and ZnO Particle Size. J. Hazard. Mater. 2021, 415, 125604. [Google Scholar] [CrossRef]
- Jośko, I.; Dobrzyńska, J.; Dobrowolski, R.; Kusiak, M.; Terpiłowski, K. The Effect of pH and Ageing on the Fate of CuO and ZnO Nanoparticles in Soils. Sci. Total Environ. 2020, 721, 137771. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Akhzari, D. The Removal of Phosphate from Aqueous Solutions Using Two Nano-Structures: Copper Oxide and Carbon Tubes. Clean Technol. Environ. Policy 2016, 18, 817–827. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, S.; Liu, Z.; Liu, J.; Huo, M.; Yang, W. Study of Phosphate Removal from Aqueous Solution by Zinc Oxide. J. Water Health 2015, 13, 704–713. [Google Scholar] [CrossRef]
- Bolster, C.H. Kinetics of Phosphorous Sorption to Biochar-Amended Soils. Chemosphere 2023, 345, 140523. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; Fu, Q.L.; Xiong, J.W.; Hong, C.; Hu, H.Q.; Violante, A. Adsorption of Phosphate onto Ferrihydrite and Ferrihydrite-Humic Acid Complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Julich, D.; Gäth, S. Sorption Behavior of Copper Nanoparticles in Soils Compared to Copper Ions. Geoderma 2014, 235–236, 127–132. [Google Scholar] [CrossRef]
- Chang, M.; Liu, Y.; Xu, M.; Li, H.; Li, S. Particle Morphology and Soil Properties Affect the Retention of Copper Oxide Nanoparticles in Agricultural Soils. Environ. Geochem. Health 2024, 46, 281. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Choque-Quispe, Y.; Solano-Reynoso, A.M.; Ramos-Pacheco, B.S.; Zamalloa-Puma, M.M.; Álvarez-López, G.J.; Zamalloa-Puma, A.; Choque-Quispe, K.; Alzamora-Flores, H. Multimetal Removal in Aqueous Medium Using a Potato Starch/Nopal Mucilage Copolymer: A Study of Adsorption Kinetics and Isotherms. Results Eng. 2023, 18, 101164. [Google Scholar] [CrossRef]
- Pigna, M.; Jara, A.A.; de la Luz Mora, M.; Violante, A. Effect Of pH, Phosphate and/or Malate on Sulfate Sorption on Andisols. Rev. la Cienc. del suelo y Nutr. Veg. 2007, 7, 662–673. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Nebytov, A.E.; Yurkshtovich, T.L.; Golub, N.V. Fabrication of Oxidized Bacterial Cellulose by Nitrogen Dioxide in Chloroform/Cyclohexane as a Highly Loaded Drug Carrier for Sustained Release of Cisplatin. Carbohydr. Polym. 2020, 248, 116745. [Google Scholar] [CrossRef]
- Costigan, E.M.; Oehler, M.A.; MacRae, J.D. Phosphorus Recovery from Recirculating Aquaculture Systems: Adsorption Kinetics and Mechanism. J. Water Process Eng. 2022, 49, 102992. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Yang, D.; Xiang, J.; Chen, Y. Removal and Recovery of Phosphorus from Secondary Effluent Using Layered Double Hydroxide-Biochar Composites. Sci. Total Environ. 2022, 844, 156802. [Google Scholar] [CrossRef]
- Ðurović-Pejčev, R.; Radmanović, S.; Tomić, Z.P.; Kaluđerović, L.; Đorđević, T. Characterization of the Clomazone Sorption Process in Four Agricultural Soils Using Different Kinetic Models. Environ. Sci. Process. Impacts 2023, 25, 542–553. [Google Scholar] [CrossRef]
- Marković-Nikolić, D.Z.; Cakić, M.D.; Petković, G.; Nikolić, G.S. Kinetics, Thermodynamics and Mechanisms of Phosphate Sorption onto Bottle Gourd Biomass Modified by (3-Chloro-2-Hydroxypropyl) Trimethylammonium Chloride. Prog. React. Kinet. Mech. 2019, 44, 267–285. [Google Scholar] [CrossRef]
- Tamungang, N.E.B.; Mvondo-Zé, A.D.; Ghogomu, J.N.; Mofor, N.A. Evaluation of Phosphorus Sorption Characteristics of Soils from the Bambouto Sequence (West Cameroon). Int. J. Biol. Chem. Sci. 2016, 10, 860. [Google Scholar] [CrossRef]
- Angkawijaya, A.E.; Santoso, S.P.; Bundjaja, V.; Soetaredjo, F.E.; Gunarto, C.; Ayucitra, A.; Ju, Y.H.; Go, A.W.; Ismadji, S. Studies on the Performance of Bentonite and Its Composite as Phosphate Adsorbent and Phosphate Supplementation for Plant. J. Hazard. Mater. 2020, 399, 123130. [Google Scholar] [CrossRef]
- Yang, J.; Xin, X.; Zhang, X.; Zhong, X.; Yang, W.; Ren, G.; Zhu, A. Effects of Soil Physical and Chemical Properties on Phosphorus Adsorption-Desorption in Fluvo-Aquic Soil under Conservation Tillage. Soil Tillage Res. 2023, 234, 105840. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Han, W.; Zhang, J.; Yang, K.; Christie, P. Dissolution and Microstructural Transformation of ZnO Nanoparticles under the Influence of Phosphate. Environ. Sci. Technol. 2012, 46, 7215–7221. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution of ZnO Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of Nitrate and Phosphate Adsorption on Al-Modified Biochar: Influence of Al Content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Brazesh, B.; Mousavi, S.M.; Zarei, M.; Ghaedi, M.; Bahrani, S.; Hashemi, S.A. Biosorption. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 587–628. ISBN 9780128188057. [Google Scholar]
- Sun, W.; Jiang, B.; Wang, F.; Xu, N. Effect of Carbon Nanotubes on Cd(II) Adsorption by Sediments. Chem. Eng. J. 2015, 264, 645–653. [Google Scholar] [CrossRef]
- Taghipour, M.; Jalali, M. Effect of Nanoparticles on Kinetics Release and Fractionation of Phosphorus. J. Hazard. Mater. 2015, 283, 359–370. [Google Scholar] [CrossRef]
- Almasri, D.A.; Saleh, N.B.; Atieh, M.A.; McKay, G.; Ahzi, S. Adsorption of Phosphate on Iron Oxide Doped Halloysite Nanotubes. Sci. Rep. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Q.; Zhao, L.; Wu, W. Enhanced Simultaneous Removal of Nitrate and Phosphate Using Novel Solid Carbon Source/Zero-Valent Iron Composite. J. Clean. Prod. 2021, 289, 125757. [Google Scholar] [CrossRef]
- He-Yi, Z.; Wen-Hao, S. Classification, Uptake, Translocation, and Detection Methods of Nanoparticles in Crop Plants: A Review. Environ. Sci. 2024, 11, 1847–1870. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Chandra Ghosh, G.; Hossain, M.R.; Islam, M.S.; Habib, A.; Zaman, S.; Bosu, H.; Nice, M.S.; Haldar, M.; Khan, A.S. Human Health Risk and Receptor Model-Oriented Sources of Heavy Metal Pollution in Commonly Consume Vegetable and Fish Species of High Ganges River Floodplain Agro-Ecological Area, Bangladesh. Heliyon 2022, 8, e11172. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, Y.; Yang, C.; Dong, L. Monitoring of Cu2+ Release from Controllably Synthesized Nano-Copper Pesticides. Environ. Pollut. Bioavailab. 2024, 36, 2300477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).