The Ultimate Fate of Reactive Dyes Absorbed onto Polymer Beads: Feasibility and Optimization of Sorbent Bio-Regeneration under Alternated Anaerobic–Aerobic Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. RR-Degrading Biomass
2.2.1. Acclimatization and Maintenance
2.2.2. Biodegradation Kinetic Tests
2.3. Preliminary Abiotic Tests
2.3.1. Sorption and Desorption Experiments
2.3.2. Polymer Loading
2.4. Bio-Regeneration Study
2.5. Analysis
3. Results and Discussion
3.1. Preliminary Tests with Polymer
3.2. RR-Degrading Biomass Acclimatization and Characterization
3.3. Bio-Regeneration
3.3.1. Abiotic Control Tests
3.3.2. Bio-Regeneration Tests
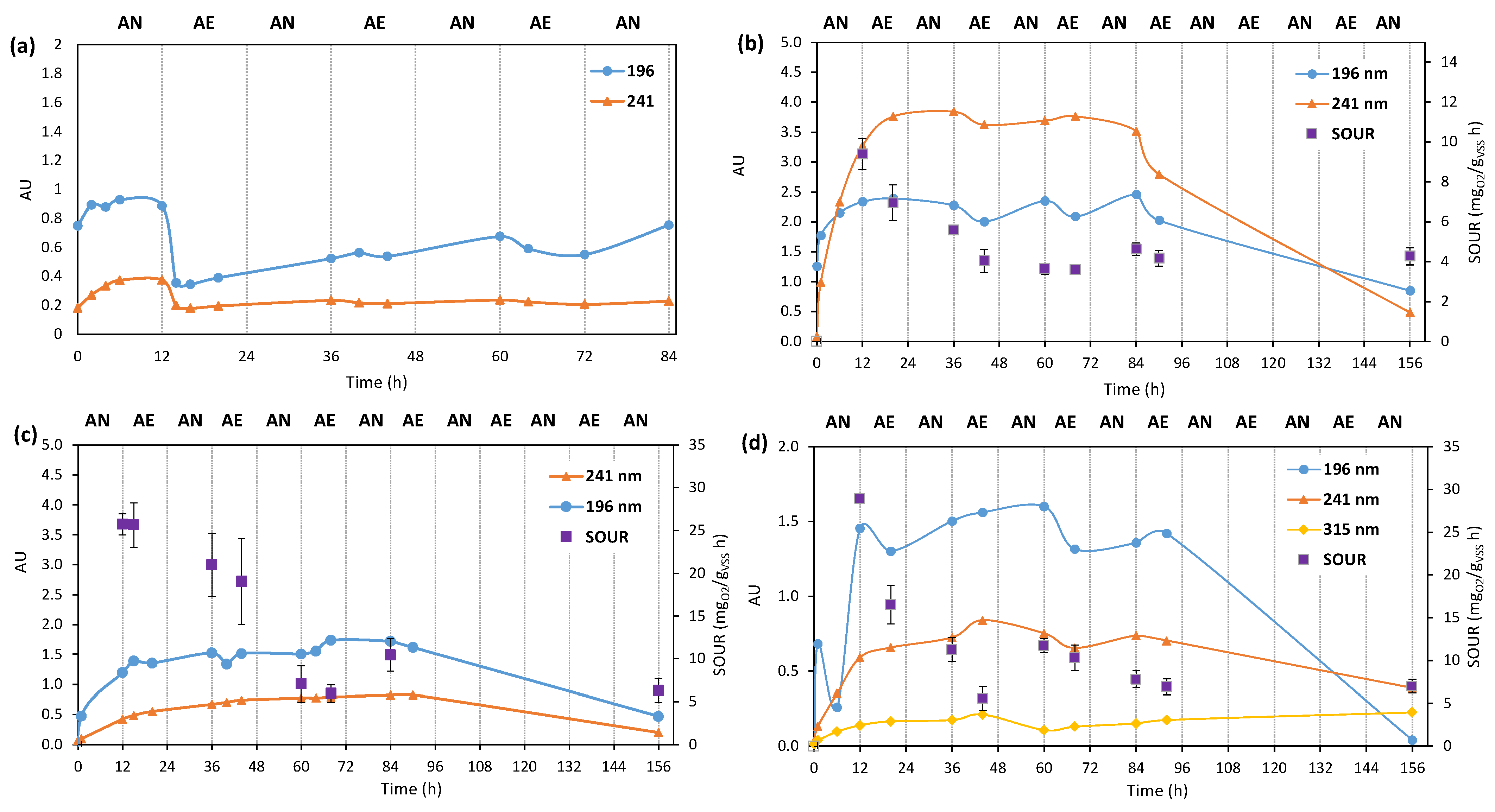
3.3.3. Fate of Biodegradation Products
3.3.4. Comparison with Other Bio-Regeneration Studies
4. Conclusions
- -
- Alternated anaerobic–aerobic conditions of the bio-regeneration environment guaranteed excellent performance with regeneration efficiencies of 91–98% and biodegradation efficiencies of the target dye up to 89%.
- -
- The highest PWR, 9%, ensured outstanding results for all contamination levels, without a remarkable loss in performance, even for the highest ones, with satisfactory biodegradation efficiencies always ≥75%.
- -
- The fate of aromatic amines in polymer bio-regeneration was investigated by using an integrated approach combining direct UV-vis spectral analysis with COD measurements and SOUR calculations. Results confirmed the successful biodegradation of the reactive dye without significant accumulation of by-products.
- -
- Promising results of this study suggest that the status of the proposed technology is ready for upscaling at the pilot scale.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ding, W.; Wang, J.; Diao, G. Removal of Azo Dyes from Water by Combined Techniques of Adsorption, Desorption, and Electrolysis Based on a Supramolecular Sorbent. Ind. Eng. Chem. Res. 2013, 52, 2403–2411. [Google Scholar] [CrossRef]
- Vadivel, V.K.; Cikurel, H.; Mamane, H. Removal of Indigo Dye by CaCO3/Ca(OH)2 Composites and Resource Recovery. Ind. Eng. Chem. Res. 2021, 60, 10312–10318. [Google Scholar] [CrossRef]
- Galloni, M.G.; Bortolotto, V.; Falletta, E.; Bianchi, C.L. PH-Driven Selective Adsorption of Multi-Dyes Solutions by Loofah Sponge and Polyaniline-Modified Loofah Sponge. Polymers 2022, 14, 4897. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Hernandez, R.; Sanchez-Montero, M.J.; Izquierdo, C. Regeneration of Carbonaceous Adsorbents. Part II: Chemical, Microbiological and Vacuum Regeneration. Microporous Mesoporous Mater. 2015, 202, 277–296. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Bacterial Regeneration in Biological Activated Carbon Systems. Process Saf. Environ. Prot. 1998, 76, 177–182. [Google Scholar] [CrossRef]
- Mosca Angelucci, D.; Tomei, M.C. Regeneration Strategies of Polymers Employed in Ex-Situ Remediation of Contaminated Soil: Bioregeneration versus Solvent Extraction. J. Environ. Manag. 2015, 159, 169–177. [Google Scholar] [CrossRef]
- Mosca Angelucci, D.; Stazi, V.; Tomei, M.C. Sustainable Approach for Removal of Remazol Red Using Absorptive Polymers: Optimization of Process Performance and Sorbent Regeneration. Int. J. Environ. Sci. Technol. 2024, 21, 2383–2394. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Bozic, A.L. Environmental Aspects on the Photodegradation of Reactive Triazine Dyes in Aqueous Media. J. Photochem. Photobiol. A Chem. 2013, 252, 131–144. [Google Scholar] [CrossRef]
- Tomei, M.C.; Mosca Angelucci, D.; Daugulis, A.J. Sequential Anaerobic-Aerobic Decolourization of a Real Textile Wastewater in a Two-Phase Partitioning Bioreactor. Sci. Total Environ. 2016, 573, 585–593. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of Azo Dyes by Anaerobic–Aerobic Sequential Biological Treatment: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Tomei, M.C.; Soria Pascual, J.; Mosca Angelucci, D. Analysing Performance of Real Textile Wastewater Bio-Decolourization under Different Reaction Environments. J. Clean. Prod. 2016, 129, 468–477. [Google Scholar] [CrossRef]
- Williams, T.M.; Unz, R.F. The Nutrition of Thiothrix, Type 021n, Beggiatoa and Leucothrix Strains. Water Res. 1989, 23, 15–22. [Google Scholar] [CrossRef]
- Mata, A.M.T.; Pinheiro, H.M.; Lourenço, N.D. Effect of Sequencing Batch Cycle Strategy on the Treatment of a Simulated Textile Wastewater with Aerobic Granular Sludge. Biochem. Eng. J. 2015, 104, 106–114. [Google Scholar] [CrossRef]
- Aygun, A.; Nas, B.; Sevimli, M.F. Treatment of Reactive Dyebath Wastewater by Electrocoagulation Process: Optimization and Cost-Estimation. Korean J. Chem. Eng. 2019, 36, 1441–1449. [Google Scholar] [CrossRef]
- Saleh, M.; Alterkaoui, A.; Ozdemir, N.C.; Arslan, H.; Bilici, Z.; Dizge, N. Adsorption of Phosphate Ions and Reactive Red 180 from Aqueous Solution Using Thermally Activated Lemon Peels Waste. Int. J. Environ. Sci. Technol. 2024, 21, 1683–1696. [Google Scholar] [CrossRef]
- Tomei, M.C.; Mosca Angelucci, D.; Daugulis, A.J. Self-Regenerating Tubing Bioreactor for Removal of Toxic Substrates: Operational Strategies in Response to Severe Dynamic Loading Conditions. Sci. Total Environ. 2020, 723, 138019. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Somasiri, W.; Li, X.F.; Ruan, W.Q.; Jian, C. Evaluation of the Efficacy of Upflow Anaerobic Sludge Blanket Reactor in Removal of Colour and Reduction of COD in Real Textile Wastewater. Bioresour. Technol. 2008, 99, 3692–3699. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Effects of Alkalinity and Co-Substrate on the Performance of an Upflow Anaerobic Sludge Blanket (UASB) Reactor through Decolorization of Congo Red Azo Dye. Bioresour. Technol. 2005, 96, 633–643. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Alparslan, S. Application of Anaerobic-Aerobic Sequential Treatment System to Real Textile Wastewater for Color and COD Removal. Enzym. Microb. Technol. 2005, 36, 273–279. [Google Scholar] [CrossRef]
- Lotito, A.M.; De Sanctis, M.; Di Iaconi, C.; Bergna, G. Textile Wastewater Treatment: Aerobic Granular Sludge vs Activated Sludge Systems. Water Res. 2014, 54, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Firmino, P.I.M.; da Silva, M.E.R.; Cervantes, F.J.; dos Santos, A.B. Colour Removal of Dyes from Synthetic and Real Textile Wastewaters in One- and Two-Stage Anaerobic Systems. Bioresour. Technol. 2010, 101, 7773–7779. [Google Scholar] [CrossRef] [PubMed]
- Jonstrup, M.; Kumar, N.; Murto, M.; Mattiasson, B. Sequential Anaerobic-Aerobic Treatment of Azo Dyes: Decolourisation and Amine Degradability. Desalination 2011, 280, 339–346. [Google Scholar] [CrossRef]
- Pinheiro, H.M.; Touraud, E.; Thomas, O. Aromatic Amines from Azo Dye Reduction: Status Review with Emphasis on Direct UV Spectrophotometric Detection in Textile Industry Wastewaters. Dye. Pigment. 2004, 61, 121–139. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Phugare, S.S.; Patil, P.S.; Jadhav, J.P. Biochemical Degradation Pathway of Textile Dye Remazol Red and Subsequent Toxicological Evaluation by Cytotoxicity, Genotoxicity and Oxidative Stress Studies. Int. Biodeterior. Biodegrad. 2011, 65, 733–743. [Google Scholar] [CrossRef]
- Kalyani, D.C.; Telke, A.A.; Dhanve, R.S.; Jadhav, J.P. Ecofriendly Biodegradation and Detoxification of Reactive Red 2 Textile Dye by Newly Isolated Pseudomonas sp. SUK1. J. Hazard. Mater. 2009, 163, 735–742. [Google Scholar] [CrossRef]
- Al-Amrani, W.A.; Lim, P.E.; Seng, C.E.; Wan Ngah, W.S. Bioregeneration of Mono-Amine Modified Silica and Granular Activated Carbon Loaded with Acid Orange 7 in Batch System. Bioresour. Technol. 2012, 118, 633–637. [Google Scholar] [CrossRef]
- Al-Amrani, W.A.; Lim, P.E.; Seng, C.E.; Wan Ngah, W.S. Operational Factors Affecting the Bioregeneration of Mono-Amine Modified Silica Loaded with Acid Orange 7. Water Res. 2012, 46, 6419–6429. [Google Scholar] [CrossRef]
- Al-Amrani, W.A.; Lim, P.E.; Seng, C.E.; Wan Ngah, W.S. Bioregeneration of Azo Dyes-Loaded Mono-Amine Modified Silica in Batch System: Effects of Particle Size and Biomass Acclimation Condition. Chem. Eng. J. 2014, 251, 175–182. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Hernández-Montoya, V.; Ramírez-López, E.M.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Effect of Surface Chemistry of Carbons from Pine Sawdust for the Adsorption of Acid, Basic and Reactive Dyes and Their Bioregeneration Using Pseudomona Putida. Ecol. Eng. 2016, 95, 112–118. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Luo, J.; Duan, C.; Hu, H.; Qian, G. Enhanced Decolourisation of Methylene Blue by LDH-Bacteria Aggregates with Bioregeneration. Chem. Eng. J. 2014, 242, 187–194. [Google Scholar] [CrossRef]
- Prpich, G.P.; Rehmann, L.; Daugulis, A.J. On the Use, and Reuse, of Polymers for the Treatment of Hydrocarbon Contaminated Water Via a Solid-Liquid Partitioning Bioreactor. Biotechnol. Prog. 2008, 24, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Estévez, S.; Mosca Angelucci, D.; Moreira, M.T.; Tomei, M.C. Techno-Environmental and Economic Assessment of Color Removal Strategies from Textile Wastewater. Sci. Total Environ. 2024, 913, 169721. [Google Scholar] [CrossRef] [PubMed]
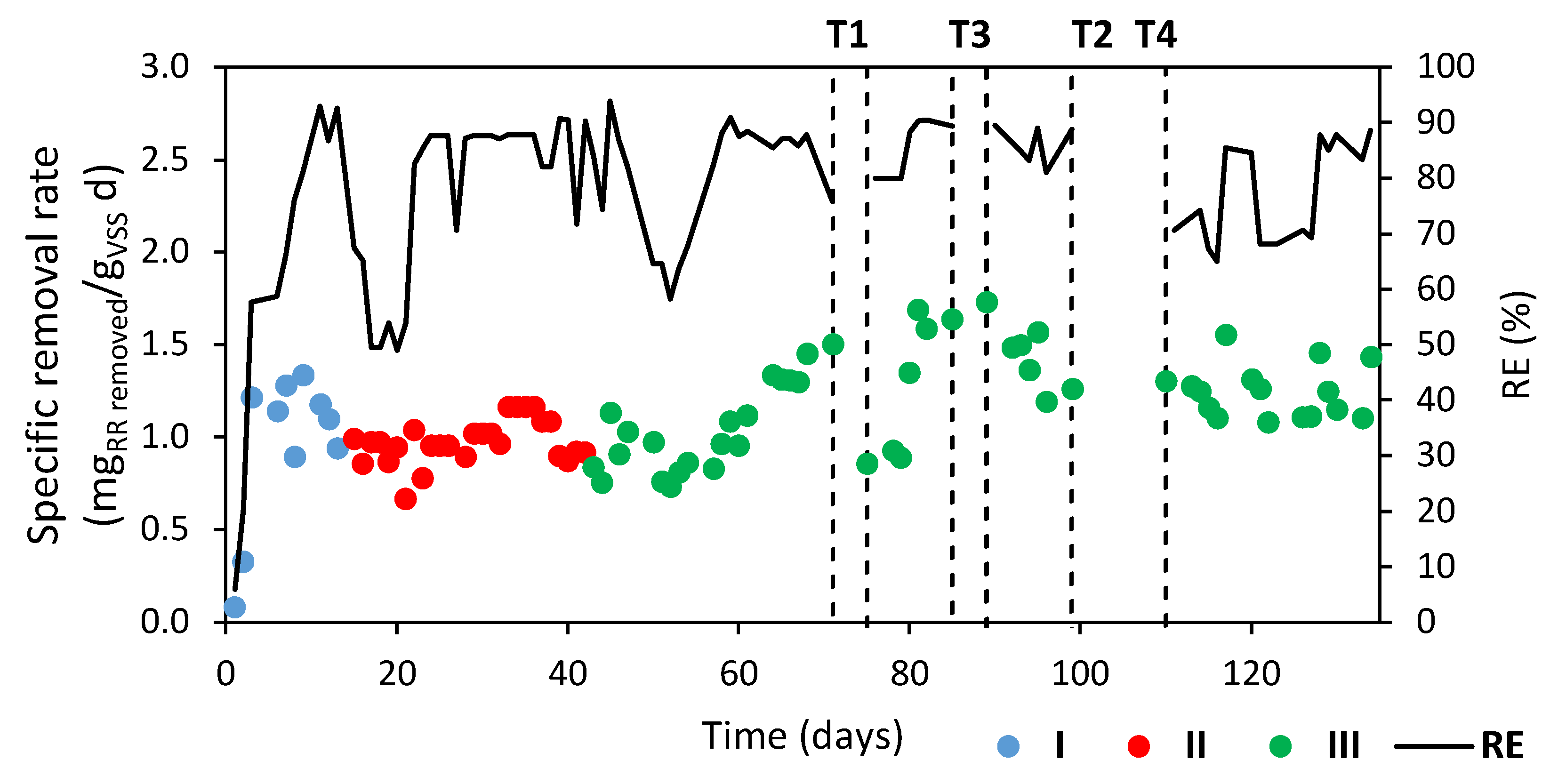
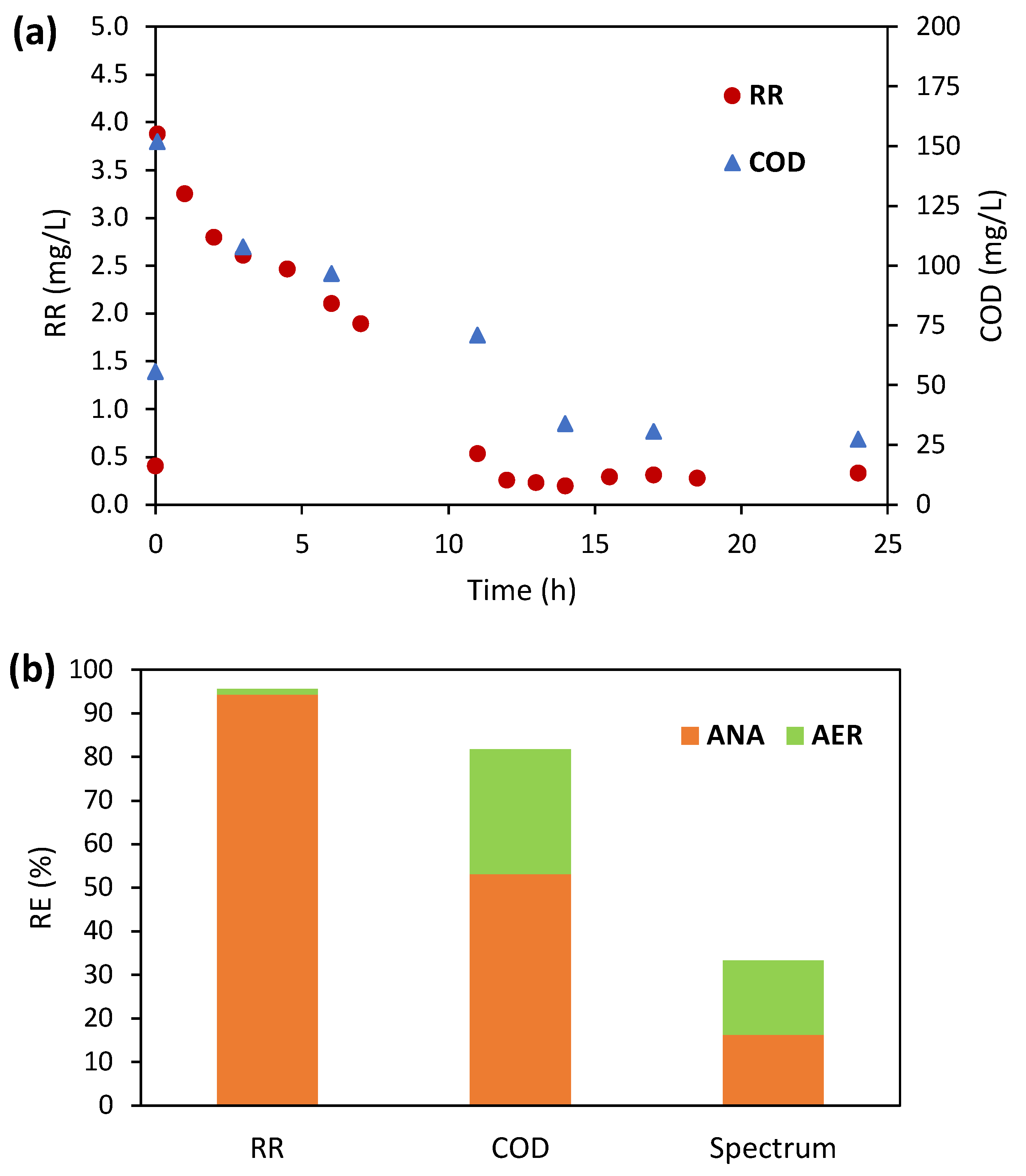
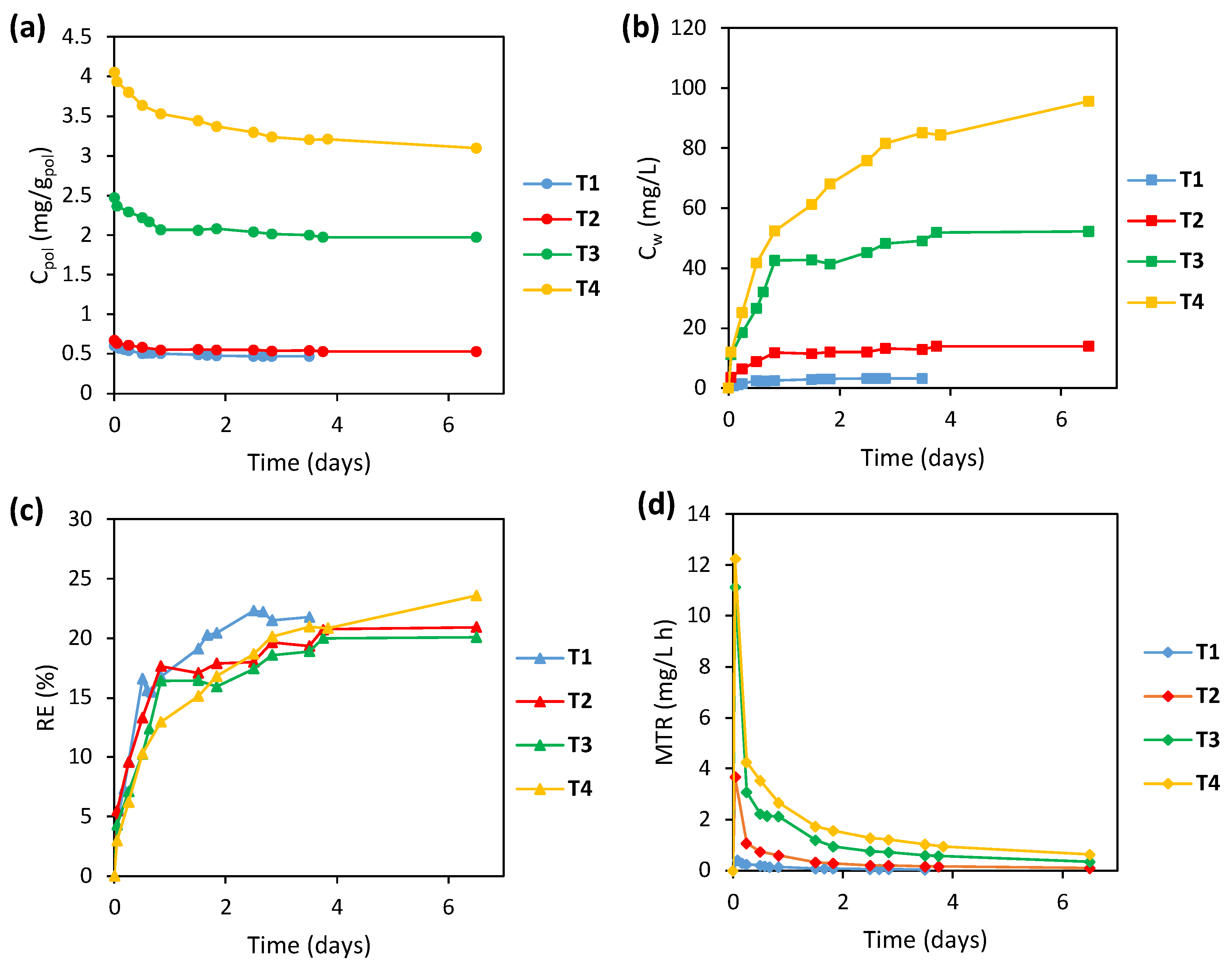

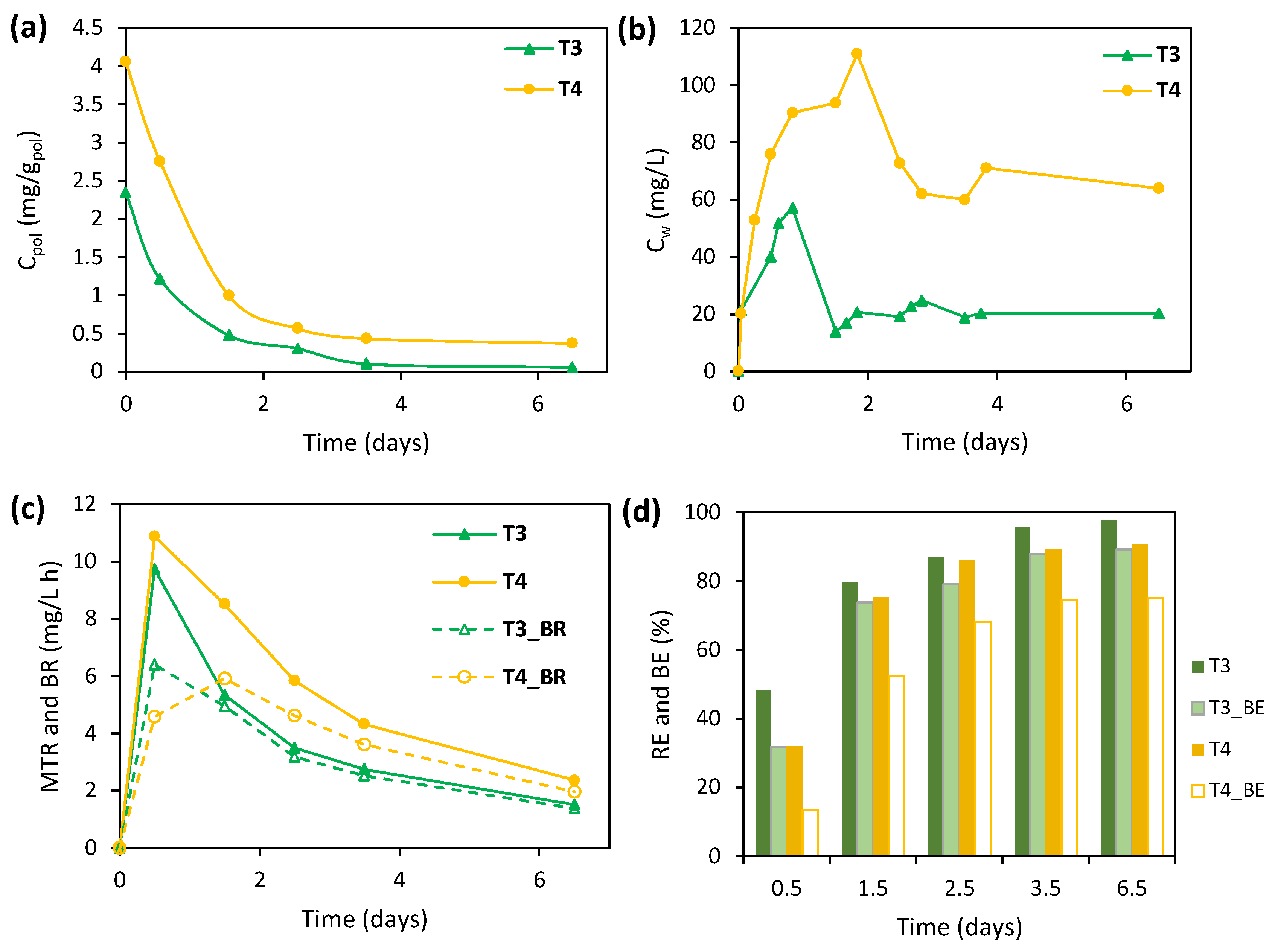
| Experimental Stage | Period (Days) | X Concentration (gVSS/L) | RR Concentration (mg/L) | COD Concentration (mg/L) | SA/RR 1 |
|---|---|---|---|---|---|
| I | 1–14 | 2.61 ± 0.10 | 5–12 | 139–335 | 50:1 |
| II | 15–42 | 2.64 ± 0.33 | 10–12 | 207–295 | 40:1 |
| III | 43–134 | 2.79 ± 0.63 | 10–12 | 141–185 | 20:1 |
| Test | Day | X Concentration (gVSS/L) | Amount of Polymer (g) | PWR (v/v %) | RR Polymer Concentration (mg/gpol) |
|---|---|---|---|---|---|
| T1 | 71–75 | 1.40 | 4.771 | 2 | 0.602 |
| T2 | 99–103 | 1.65 | 20.613 | 9 | 0.671 |
| T3 | 85–89 | 1.60 | 20.591 | 9 | 2.347 |
| T4 | 106–110 | 1.55 | 20.065 | 9 | 4.054 |
| Test | Volumetric Initial Loading (mgRR/Lreactor) | Biomass Initial Loading (mgRR/gVSS) | RE (%) | MTR (mgRR/L d) | BE (%) | BR (mgRR/L d) | BR/MTR (%) |
|---|---|---|---|---|---|---|---|
| T1 | 14.55 | 10.39. | 95.22 | 0.163 | 83.39 | 0.146 | 89.8 |
| T2 | 69.05 | 41.85 | 93.02 | 0.412 | 78.38 | 0.348 | 84.4 |
| T3 | 240.92 | 150.57 | 97.62 | 1.512 | 89.21 | 1.382 | 91.4 |
| T4 | 405.32 | 261.50 | 90.77 | 2.367 | 75.08 | 1.959 | 82.8 |
| Test | A(196)t=0 | A(196)end | A(241)t=0 | A(241)end | CODt=0 | CODend |
|---|---|---|---|---|---|---|
| T1 | 0.749 | 0.754 | 0.1818 | 0.2297 | 17.4 | 11.8 |
| T2 | 1.2542 | 0.8482 | 0.0841 | 0.4877 | 152.7 | 103.0 |
| T3 | 0.0961 | 0.4709 | 0.0682 | 0.2003 | 164.1 | 144.4 |
| T4 | 0.122 | 0.0462 | 0.0358 | 0.3878 | 16.8 | 163.0 |
| Dyes | Sorbent Material | C0 (mg/gpol) | Regeneration Rate (gsorbent/L d) | Inoculum
Concentration (mgVSS/gsorbent) | RE (Time) % (d) | Reference |
|---|---|---|---|---|---|---|
| AO7 | GAC | GAC: 53–192 | 0.065 | 420 | GAC: 0–15 (11) | [28] |
| MAMS | MAMS: 51–96 | MAMS: 98–77 (11) | ||||
| AO7 | MAMS | 93–102 | 0.04–0.125 | 140–700 | 39–78 (4–12) | [29] |
| AO7 | MAMS | AO7: 12–202 a | A07: 0.025–0.07 a | 420 | A07: 54–100 a (7–20 b) | [30] |
| AY9 | AY9: 12–118 a | AY9: 0.014–0.025 a | AY9: 100 (20–35 b) | |||
| AR14 | AR14: 9–127 a | AR14: 0.03–0.05 a | AR14: 100–82 a (11–17 b) | |||
| AB74 | Carbons from pine sawdust | AB74: 110.9 | 1.43 | - | AB74: 6 (7) | [31] |
| BG1 | BG1: 155.1 | BG1: 25 (7) | ||||
| RB4 | RB4: 48.6 | RB4: 3 (7) | ||||
| MB | Layered double hydroxide–B. subtilis | 6–9 | 1.44 | - | 83% (0.5) | [32] |
| AR361 | GAC | 505–535 | - | 0.175 ± 3.38 × 109 (cells/mL) | 0 (GAC) | [6] |
| Brimac | 52% (Brimac) | |||||
| RR | Hytrel 8206 | 0.7–4 | 15.4–15.9 | 15.4–16.0 | 91–98 (6.5) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca Angelucci, D.; Stazi, V.; Tomei, M.C. The Ultimate Fate of Reactive Dyes Absorbed onto Polymer Beads: Feasibility and Optimization of Sorbent Bio-Regeneration under Alternated Anaerobic–Aerobic Phases. Environments 2024, 11, 207. https://doi.org/10.3390/environments11090207
Mosca Angelucci D, Stazi V, Tomei MC. The Ultimate Fate of Reactive Dyes Absorbed onto Polymer Beads: Feasibility and Optimization of Sorbent Bio-Regeneration under Alternated Anaerobic–Aerobic Phases. Environments. 2024; 11(9):207. https://doi.org/10.3390/environments11090207
Chicago/Turabian StyleMosca Angelucci, Domenica, Valentina Stazi, and Maria Concetta Tomei. 2024. "The Ultimate Fate of Reactive Dyes Absorbed onto Polymer Beads: Feasibility and Optimization of Sorbent Bio-Regeneration under Alternated Anaerobic–Aerobic Phases" Environments 11, no. 9: 207. https://doi.org/10.3390/environments11090207
APA StyleMosca Angelucci, D., Stazi, V., & Tomei, M. C. (2024). The Ultimate Fate of Reactive Dyes Absorbed onto Polymer Beads: Feasibility and Optimization of Sorbent Bio-Regeneration under Alternated Anaerobic–Aerobic Phases. Environments, 11(9), 207. https://doi.org/10.3390/environments11090207








