Detection and Screening of Organic Contaminants in A Riverine System of Georgia Using Non-Targeted Analysis
Abstract
1. Introduction
- What specific organic contaminants are prevalent in the water of the North Oconee River watershed in Athens-Clarke County, Georgia, given its significance as a drinking water source?
- Are chemical and biological transformations occurring within the North Oconee River watershed system, potentially altering the composition or toxicity of organic pesticides and related compounds?
2. Materials and Method
2.1. Sample Collection and Preservation
2.2. Extraction of Water Samples
2.3. Microbial Degradation Study of Malathion in Different Environmental Conditions
2.4. Instrumental Analysis
2.5. Non-Targeted Data Analysis
- Found in all the samples collected during a sampling event.
- Not found in any of the field blanks or, if found in a field blank, the peak area should be three times larger in the samples.
- Has a similarity score (as calculated by the Mass Hunter qualitative software, v 10.0, Agilent Technologies®, USA) to a reference spectrum in the NIST library of ≥500.
- Three or more molecular ions in the sample should match with the NIST library to hit a peak for a given analyte.
2.6. Quality Assurance and Quality Control (QA/QC)
3. Results and Discussion
3.1. Physiological Parameter Dynamics in River Water
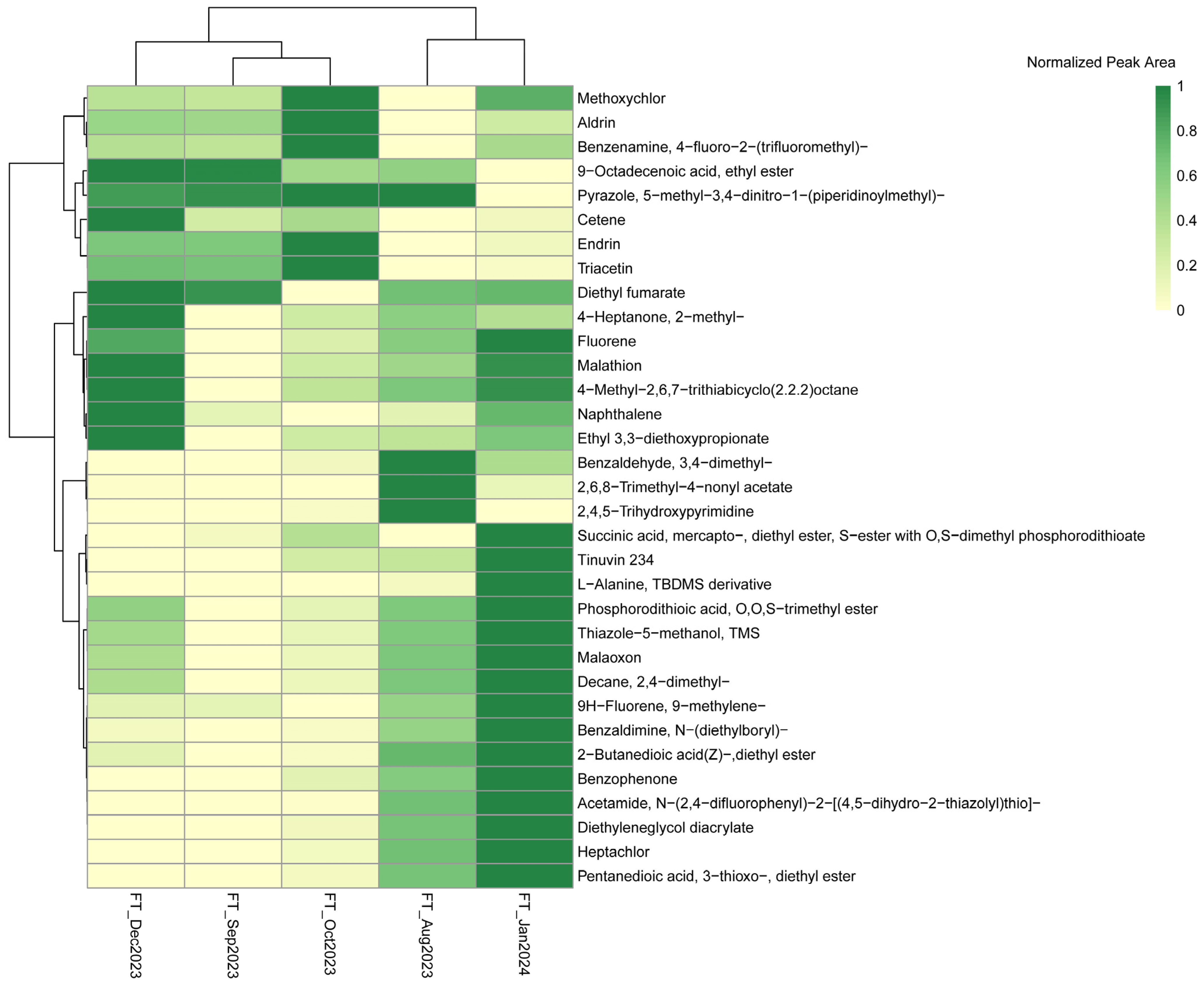
3.2. Extent of Organic Contaminants Detected in River Water
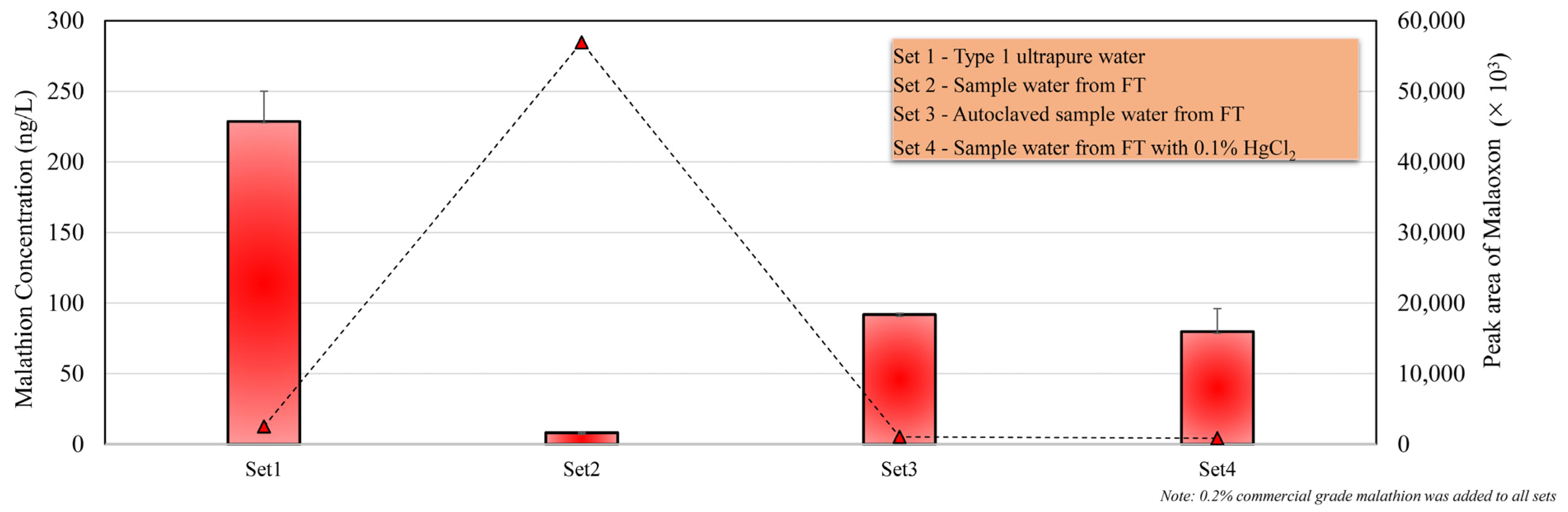
3.3. Chemical and Biological Degradation of Malathion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duttagupta, S.; Mukherjee, A.; Bhattacharya, A.; Bhattacharya, J. Wide exposure of persistent organic pollutants (PoPs) in natural waters and sediments of the densely populated Western Bengal basin, India. Sci. Total Environ. 2020, 717, 137187. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Hollender, J.; Schymanski, E.L.; Singer, H.P.; Ferguson, P.L. Nontarget screening with high resolution mass spectrometry in the environment: Ready to go? Environ. Sci. Technol. 2017, 51, 11505–11512. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Peter, K.T.; Gipe, A.D.; Zhao, H.; Hou, F.; Wark, D.A.; Khangaonkar, T.; Kolodziej, E.P.; James, C.A. Suspect and nontarget screening for contaminants of emerging concern in an urban estuary. Environ. Sci. Technol. 2019, 54, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Oxygenated polycyclic aromatic hydrocarbons in the surface water environment: Occurrence, ecotoxicity, and sources. Environ. Int. 2022, 163, 107232. [Google Scholar] [CrossRef] [PubMed]
- Muscalu, A.M.; Górecki, T. Comprehensive two-dimensional gas chromatography in environmental analysis. TrAC Trends Anal. Chem. 2018, 106, 225–245. [Google Scholar] [CrossRef]
- Begum, A.; Alam, S.N.; Jalal Uddin, M. Management of pesticides: Purposes, uses, and concerns. In Pesticide Residue in Foods: Sources, Management, and Control; Springer: Berlin/Heidelberg, Germany, 2017; pp. 53–86. [Google Scholar]
- Casteel, C.A.; Ballew, M.D. Water Resources Activities, Georgia District, 1985; US Geological Survey: Reston, VA, USA, 1986.
- Strahan, C.M. Clarke County, Ga. and the City of Athens; CP Byrd, Printer: Atlanta, GA, USA, 1893. [Google Scholar]
- Fisher, D.S.; Steiner, J.L.; Endale, D.M.; Stuedemann, J.A.; Schomberg, H.H.; Franzluebbers, A.J.; Wilkinson, S.R. The relationship of land use practices to surface water quality in the Upper Oconee Watershed of Georgia. For. Ecol. Manag. 2000, 128, 39–48. [Google Scholar] [CrossRef]
- Shrestha, S.; Dwivedi, P.; McKay, S.K.; Radcliffe, D. Assessing the Potential Impact of Rising Production of Industrial Wood Pellets on Streamflow in the Presence of Projected Changes in Land Use and Climate: A Case Study from the Oconee River Basin in Georgia, United States. Water 2019, 11, 142. [Google Scholar] [CrossRef]
- Kolde, R.; Kolde, M.R. Package ‘pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Berkani, M.; Almomani, F.; Dragoi, E.N. Data mining for pesticide decontamination using heterogeneous photocatalytic processes. Chemosphere 2021, 270, 129449. [Google Scholar] [CrossRef] [PubMed]
- Poomagal, S.; Sujatha, R.; Kumar, P.S.; Vo, D.V. A fuzzy cognitive map approach to predict the hazardous effects of malathion to environment (air, water and soil). Chemosphere 2021, 263, 127926. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Hosseinzadeh, S.; Khataee, A.; Dragoi, E.N. The concentration of persistent organic pollutants in water resources: A global systematic review, meta-analysis and probabilistic risk assessment. Sci. Total Environ. 2021, 796, 149000. [Google Scholar] [CrossRef]
- Bray, J.; Miranda, A.; Keely-Smith, A.; Kaserzon, S.; Elisei, G.; Chou, A.; Nichols, S.J.; Thompson, R.; Nugegoda, D.; Kefford, B.J. Sub-organism (acetylcholinesterase activity), population (survival) and chemical concentration responses reinforce mechanisms of antagonism associated with malathion toxicity. Sci. Total Environ. 2021, 778, 146087. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, J.; Singh, K. Biodegradation of malathion by Brevibacillus sp. strain KB2 and Bacillus cereus strain PU. World J. Microbiol. Biotechnol. 2012, 28, 1133–1141. [Google Scholar] [CrossRef]
- Geed, S.R.; Kureel, M.K.; Shukla, A.K.; Singh, R.S.; Rai, B.N. Biodegradation of malathion and evaluation of kinetic parameters using three bacterial species. Resour.-Effic. Technol. 2016, 2, S3–S11. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health Part C 2019, 37, 288–329. [Google Scholar] [CrossRef]
- Kralj, M.B.; Černigoj, U.; Franko, M.; Trebše, P. Comparison of photocatalysis and photolysis of malathion, isomalathion, malaoxon, and commercial malathion—Products and toxicity studies. Water Res. 2007, 41, 4504–4514. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Yan, X.; Duan, J.; Saint, C.P.; Beecham, S. Transformation pathway and toxicity assessment of malathion in aqueous solution during UV photolysis and photocatalysis. Chemosphere 2019, 234, 204–214. [Google Scholar] [CrossRef]
- Badr, A.M. Organophosphate toxicity: Updates of malathion potential toxic effects in mammals and potential treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef] [PubMed]
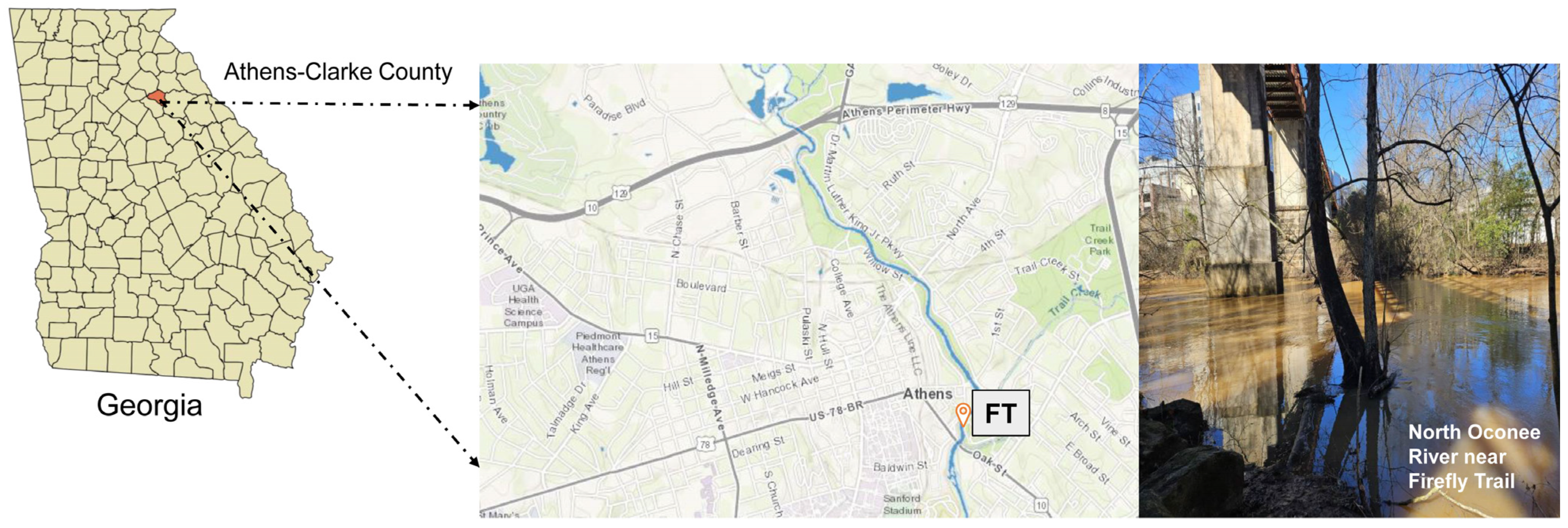

| Sample Site | Date | Latitude and Longitude | Temp (°C) | pH | ORP | DO (ppm) | Conductivity (µS/cm) | TDS (ppm) | Salinity (PSU) | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|---|---|---|
| Firefly Trail (FT) | 08/25/2023 (E*1) | 33.956652 N, 83.367263 W | 31.2 | 7.19 | 212 | 2.3 | 179 | 78 | 0.005 | 26.5 |
| 09/19/2023 (E2) | 30.8 | 7.2 | 218.8 | 3.1 | 114 | 73 | 0.004 | 27.1 | ||
| 10/19/2023 (E3) | 13.87 | 7.26 | 159.4 | 4.8 | 156 | 69 | 0.08 | 31.4 | ||
| 12/12/2023 (E4) | 8.1 | 6.9 | 190 | 2.9 | 120 | 101 | 0.08 | 25 | ||
| 30/1/2024 (E5) | 7.3 | 6.43 | 179.2 | 6.1 | 100 | 45 | 0.05 | 29 |
| GC | |
| Agilent 8860 GC System with Auto-Injector and Tray | |
| Inlet | |
| Split/Splitless inlet | |
| Mode | Pulsed splitless |
| Injection pulse pressure | 50 psi until 0.7 min |
| Purge flow to split vent | 50 psi until 0.75 min |
| Injection volume | 1 µL |
| Inlet temperature | 280 °C |
| Carrier gas | Helium |
| Inlet liner | Agilent low pressure-drop (LPD) with glass wool |
| Oven | |
| Initial oven temperature | 80 °C |
| Initial oven hold | 1.5 min |
| Ramp rate 1 | 40 °C/min |
| Final temperature 1 | 120 °C |
| Final hold 1 | 0 min |
| Ramp rate 2 | 10 °C/min |
| Final temperature 2 | 300 °C |
| Final hold 2 | 4 min |
| Total run time | 24.5 min |
| Post run time | 1.5 min |
| Equilibration time | 0.25 min |
| Column | |
| Type | Agilent J&W HP-5 ms Ultra Inert |
| Length | 30 m |
| Diameter | 0.25 mm |
| Film thickness | 0.25 µm |
| Control mode | Constant flow |
| Flow | 1.374 mL/min |
| Inlet connection | Split/Splitless |
| Outlet connection | MSD |
| MSD | |
| Model | Agilent 5977B MSD |
| Tune file | CUSTOM.U |
| Mode | Scan and SIM |
| Scan range | 45 to 550 amu |
| Solvent delay | 4 min |
| Quad temperature | 150 °C |
| Source temperature | 280 °C |
| Transfer line temperature | 280 °C |
| SIM (m/z) | 93.0, 125.0, 173.0 |
| Chemical Categories | Name of the Analyte |
|---|---|
| Organophosphates | Malathion |
| Malaoxon | |
| Phosphorodithioic acid, O, O, S-trimethyl ester (also can be categorized under ester) | |
| Succinic acid, mercapto-, diethyl ester, S-ester with O, S-dimethyl phosphorodithioate | |
| Polycyclic Aromatic Hydrocarbons (PAHs) | Naphthalene |
| Fluorene | |
| 9H-Fluorene, 9-methylene- | |
| Organohalides | Aldrin |
| Methoxychlor | |
| Heptachlor | |
| Benzaldehyde, 3,4-dimethyl- | |
| Endrin | |
| Benzenamine, 4-fluoro-2-(trifluoromethyl)- | |
| UV Absorbers | Tinuvin 234 |
| Benzophenone | |
| Plasticizers | Triacetin |
| Boryl Compounds | Benzaldimine, N-(diethylboryl)- |
| Acrylates | Diethyleneglycol diacrylate |
| Esters | Pentanedioic acid, 3-thioxo-, diethyl ester |
| 9-Octadecenoic acid, ethyl ester | |
| Diethyl fumarate | |
| 2-Butanedioic acid(Z)-,diethyl ester | |
| Ethyl 3,3-diethoxypropionate | |
| Aliphatic Compounds | Decane, 2,4-dimethyl- |
| 4-Methyl-2,6,7-trithiabicyclo[2.2.2]octane | |
| 2,6,8-Trimethyl-4-nonyl acetate | |
| Cetene | |
| Amino Acids and Derivatives | L-Alanine, TBDMS derivative |
| Thiazole Derivatives | Thiazole-5-methanol, TMS |
| Pyrazole, 5-methyl-3,4-dinitro-1-(piperidinoylmethyl)- | |
| Ketones | 4-Heptanone, 2-methyl- |
| Pyrimidine | 2,4,5-Trihydroxypyrimidine |
| Carboxyl acid amide | Acetamide, N-(2,4-difluorophenyl)-2-[(4,5-dihydro-2-thiazolyl) thio]- |
| Sampling Events | Firefly Trail (FT) |
| FT_Aug2023 (E1) | 25.640 |
| FT_Sept2023 (E2) | 24.438 |
| FT_Oct2023 (E3) | 31.208 |
| FT_Dec2023 (E4) | 34.424 |
| FT_Jan2024 (E5) | 39.408 |
| Parameters | Firefly Trail (FT) |
| Min | 24.438 |
| Max | 39.408 |
| Geomean | 30.531 |
| Median | 31.208 |
| 1st Quartile | 25.640 |
| 3rd Quartile | 34.424 |
| Std Dev | 5.554 |
| Average | 31.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basapuram, G.; Duttagupta, S.; Dutta, A. Detection and Screening of Organic Contaminants in A Riverine System of Georgia Using Non-Targeted Analysis. Environments 2024, 11, 89. https://doi.org/10.3390/environments11050089
Basapuram G, Duttagupta S, Dutta A. Detection and Screening of Organic Contaminants in A Riverine System of Georgia Using Non-Targeted Analysis. Environments. 2024; 11(5):89. https://doi.org/10.3390/environments11050089
Chicago/Turabian StyleBasapuram, Gayatri, Srimanti Duttagupta, and Avishek Dutta. 2024. "Detection and Screening of Organic Contaminants in A Riverine System of Georgia Using Non-Targeted Analysis" Environments 11, no. 5: 89. https://doi.org/10.3390/environments11050089
APA StyleBasapuram, G., Duttagupta, S., & Dutta, A. (2024). Detection and Screening of Organic Contaminants in A Riverine System of Georgia Using Non-Targeted Analysis. Environments, 11(5), 89. https://doi.org/10.3390/environments11050089






