Dissolved Iron and Organic Matter in Boreal Rivers across a South–North Transect
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Chemical Analyses
3. Results and Discussion
3.1. Main Physical and Hydrochemical Characteristics
3.2. Characterization of Dissolved Organic Matter
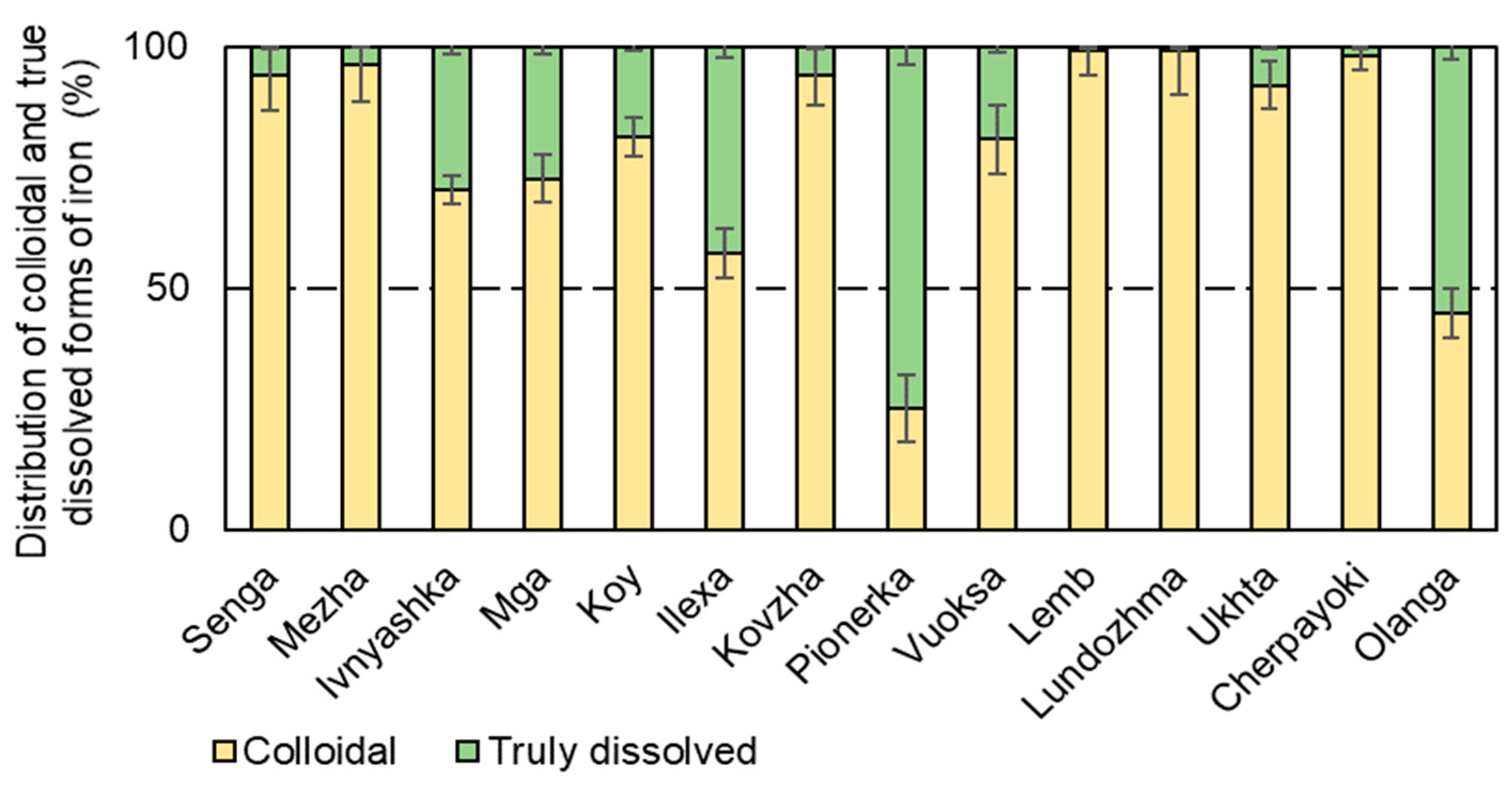
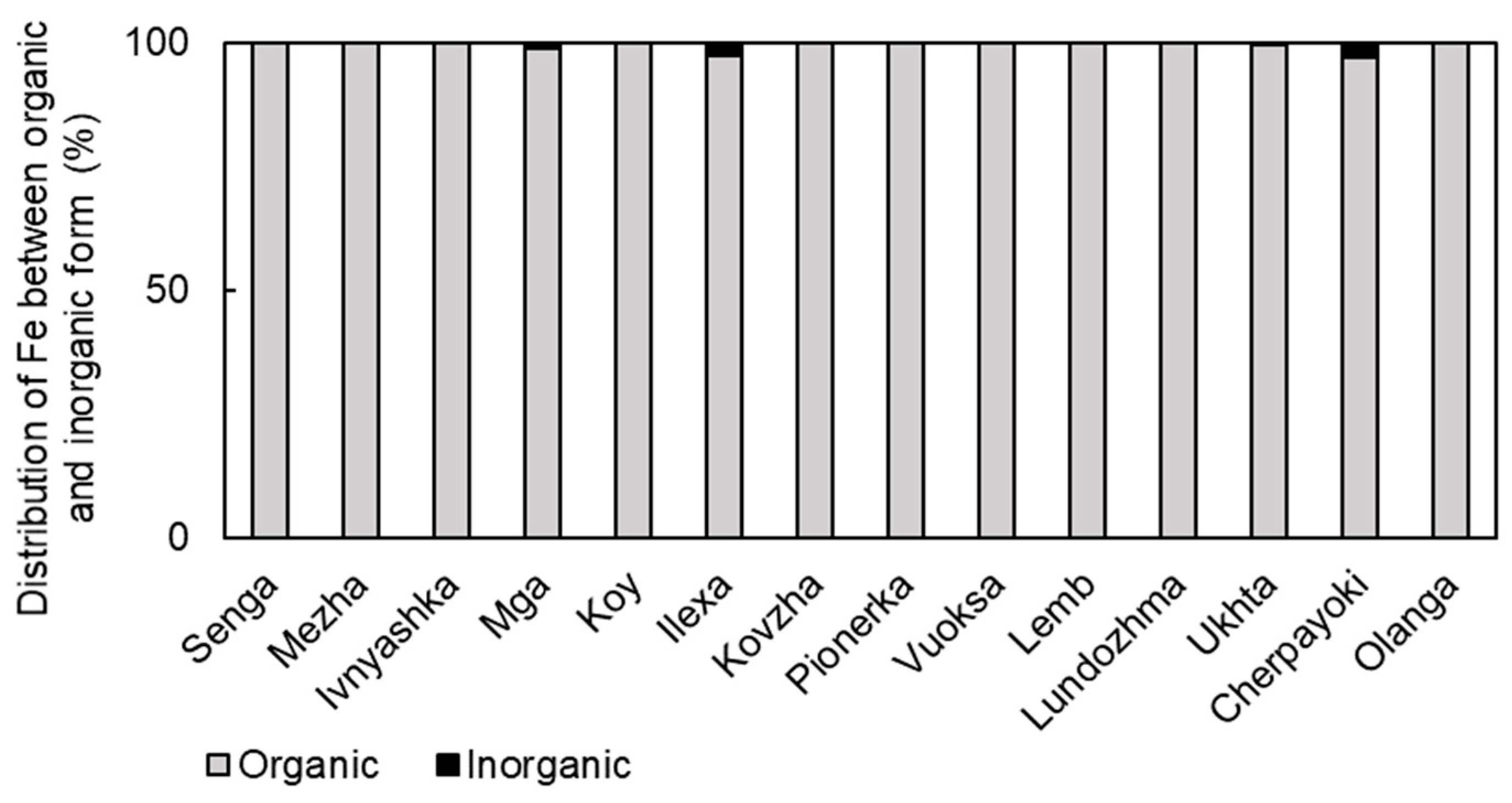
3.3. Forms of Iron Presence in the Studied Water Bodies
3.4. Comparison with Results of Previous Studies of Boreal River Waters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the Carbon Cycle: From Local Processes to Global Implications—A Synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Williamson, C.E.; Morris, D.P.; Pace, M.L.; Olson, O.G. Dissolved Organic Carbon and Nutrients as Regulators of Lake Ecosystems: Resurrection of a More Integrated Paradigm. Limnol. Oceanogr. 1999, 44, 795–803. [Google Scholar] [CrossRef]
- Solomon, C.T.; Jones, S.E.; Weidel, B.C.; Buffam, I.; Fork, M.L.; Karlsson, J.; Larsen, S.; Lennon, J.T.; Read, J.S.; Sadro, S.; et al. Ecosystem Consequences of Changing Inputs of Terrestrial Dissolved Organic Matter to Lakes: Current Knowledge and Future Challenges. Ecosystems 2015, 18, 376–389. [Google Scholar] [CrossRef]
- Kalinkina, N.; Tekanova, E.; Korosov, A.; Zobkov, M.; Ryzhakov, A. What Is the Extent of Water Brownification in Lake Onego, Russia? J. Great Lakes Res. 2020, 46, 850–861. [Google Scholar] [CrossRef]
- George, G. The Impact of Climate Change on European Lakes; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-90-481-2944-7. [Google Scholar]
- Klante, C.; Larson, M.; Persson, K.M. Brownification in Lake Bolmen, Sweden, and Its Relationship to Natural and Human-Induced Changes. J. Hydrol. Reg. Stud. 2021, 36, 100863. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Pumpanen, J.; Keinänen, M.; Laudon, H.; Ojala, A.; Palviainen, M.; Kiirikki, M.; Neitola, K.; Berninger, F. Assessment of a Portable UV–Vis Spectrophotometer’s Performance for Stream Water DOC and Fe Content Monitoring in Remote Areas. Talanta 2021, 224, 121919. [Google Scholar] [CrossRef] [PubMed]
- Hongve, D.; Riise, G.; Kristiansen, J.F. Increased Colour and Organic Acid Concentrations in Norwegian Forest Lakes and Drinking Water—A Result of Increased Precipitation? Aquat. Sci. 2004, 66, 231–238. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Karlsson, J. Nonlinear Response of Dissolved Organic Carbon Concentrations in Boreal Lakes to Increasing Temperatures. Limnol. Oceanogr. 2009, 54, 2513–2519. [Google Scholar] [CrossRef]
- Eikebrokk, B.; Vogt, R.D.; Liltved, H. NOM Increase in Northern European Source Waters: Discussion of Possible Causes and Impacts on Coagulation/Contact Filtration Processes. Water Supply 2004, 4, 47–54. [Google Scholar] [CrossRef]
- Erlandsson, M.; Buffam, I.; Fölster, J.; Laudon, H.; Temnerud, J.; Weyhenmeyer, G.A.; Bishop, K. Thirty-Five Years of Synchrony in the Organic Matter Concentrations of Swedish Rivers Explained by Variation in Flow and Sulphate. Glob. Chang. Biol. 2008, 14, 1191–1198. [Google Scholar] [CrossRef]
- Finland’s Sixth National Communication under the United Nations Framework Convention on Climate Change; Ministry of the Environment and Statistics Finland: Helsinki, Finland, 2013; p. 314.
- Assessment Report on Climate Change and Its Consequences in Russian Federation; Federal Service for Hydrometeorology and Environmental Monitoring: Moscow, Russia, 2008; p. 54.
- Worrall, F.; Harriman, R.; Evans, C.D.; Watts, C.D.; Adamson, J.; Neal, C.; Tipping, E.; Burt, T.; Grieve, I.; Monteith, D.; et al. Trends in Dissolved Organic Carbon in UK Rivers and Lakes. Biogeochemistry 2004, 70, 369–402. [Google Scholar] [CrossRef]
- Estlander, S.; Pippingsköld, E.; Horppila, J. Artificial Ditching of Catchments and Brownification-Connected Water Quality Parameters of Lakes. Water Res. 2021, 205, 117674. [Google Scholar] [CrossRef] [PubMed]
- Horppila, J.; Nurminen, L.; Rajala, S.; Estlander, S. Making Waves: The Sensitivity of Lakes to Brownification and Issues of Concern in Ecological Status Assessment. Water Res. 2024, 249, 120964. [Google Scholar] [CrossRef] [PubMed]
- Asmala, E.; Carstensen, J.; Räike, A. Multiple Anthropogenic Drivers behind Upward Trends in Organic Carbon Concentrations in Boreal Rivers. Environ. Res. Lett. 2019, 14, 124018. [Google Scholar] [CrossRef]
- Ledesma, J.L.J.; Futter, M.N.; Laudon, H.; Evans, C.D.; Köhler, S.J. Boreal Forest Riparian Zones Regulate Stream Sulfate and Dissolved Organic Carbon. Sci. Total Environ. 2016, 560–561, 110–122. [Google Scholar] [CrossRef] [PubMed]
- de Wit, H.A.; Valinia, S.; Weyhenmeyer, G.A.; Futter, M.N.; Kortelainen, P.; Austnes, K.; Hessen, D.O.; Räike, A.; Laudon, H.; Vuorenmaa, J. Current Browning of Surface Waters Will Be Further Promoted by Wetter Climate. Environ. Sci. Technol. Lett. 2016, 3, 430–435. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Ekström, S.M. Increasing Iron Concentrations in Surface Waters – a Factor behind Brownification? Biogeosciences 2012, 9, 1465–1478. [Google Scholar] [CrossRef]
- Sarkkola, S.; Nieminen, M.; Koivusalo, H.; Laurén, A.; Kortelainen, P.; Mattsson, T.; Palviainen, M.; Piirainen, S.; Starr, M.; Finér, L. Iron Concentrations Are Increasing in Surface Waters from Forested Headwater Catchments in Eastern Finland. Sci. Total Environ. 2013, 463–464, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-H.; Hoikkala, L.; Kasurinen, V.; Tiirola, M.; Kortelainen, P.; Vähätalo, A.V. The Effect of Iron on the Biodegradation of Natural Dissolved Organic Matter. J. Geophys. Res. Biogeosci. 2016, 121, 2544–2561. [Google Scholar] [CrossRef]
- Heikkinen, K.; Saari, M.; Heino, J.; Ronkanen, A.-K.; Kortelainen, P.; Joensuu, S.; Vilmi, A.; Karjalainen, S.-M.; Hellsten, S.; Visuri, M.; et al. Iron in Boreal River Catchments: Biogeochemical, Ecological and Management Implications. Sci. Total Environ. 2022, 805, 150256. [Google Scholar] [CrossRef]
- Shapiro, J. The Relation of Humic Color to Iron in Natural Waters. Int. Ver. Für Theor. Und Angew. Limnol. Verhandlungen 1966, 16, 477–484. [Google Scholar] [CrossRef]
- Hunter, K.A.; Boyd, P.W. Iron-Binding Ligands and Their Role in the Ocean Biogeochemistry of Iron. Environ. Chem. 2007, 4, 221–232. [Google Scholar] [CrossRef]
- Gledhill, M.; Buck, K. The Organic Complexation of Iron in the Marine Environment: A Review. Front. Microbiol. 2012, 3, 18807. [Google Scholar] [CrossRef]
- Drozdova, O.Y.; Aleshina, A.R.; Tikhonov, V.V.; Lapitskiy, S.A.; Pokrovsky, O.S. Coagulation of Organo-Mineral Colloids and Formation of Low Molecular Weight Organic and Metal Complexes in Boreal Humic River Water under UV-Irradiation. Chemosphere 2020, 250, 126216. [Google Scholar] [CrossRef] [PubMed]
- Ekström, S.M.; Regnell, O.; Reader, H.E.; Nilsson, P.A.; Löfgren, S.; Kritzberg, E.S. Increasing Concentrations of Iron in Surface Waters as a Consequence of Reducing Conditions in the Catchment Area. J. Geophys. Res. Biogeosci. 2016, 121, 479–493. [Google Scholar] [CrossRef]
- Björnerås, C.; Weyhenmeyer, G.A.; Evans, C.D.; Gessner, M.O.; Grossart, H.-P.; Kangur, K.; Kokorite, I.; Kortelainen, P.; Laudon, H.; Lehtoranta, J.; et al. Widespread Increases in Iron Concentration in European and North American Freshwaters. Glob. Biogeochem. Cycles 2017, 31, 1488–1500. [Google Scholar] [CrossRef]
- Guan, J.; Qi, K.; Wang, J.; Zhuang, J.; Yuan, X.; Yan, B.; Lu, N.; Qu, J. Effects of Conversion from Boreal Natural Wetlands to Rice Paddy Fields on the Dynamics of Total Dissolved Iron during Extreme Precipitation Events. Chemosphere 2020, 242, 125153. [Google Scholar] [CrossRef] [PubMed]
- Knorr, K.-H. DOC-Dynamics in a Small Headwater Catchment as Driven by Redox Fluctuations and Hydrological Flow Paths—Are DOC Exports Mediated by Iron Reduction/Oxidation Cycles? Biogeosciences 2013, 10, 891–904. [Google Scholar] [CrossRef]
- Brezonik, P.L.; Finlay, J.C.; Griffin, C.G.; Arnold, W.A.; Boardman, E.H.; Germolus, N.; Hozalski, R.M.; Olmanson, L.G. Iron Influence on Dissolved Color in Lakes of the Upper Great Lakes States. PLoS ONE 2019, 14, e0211979. [Google Scholar] [CrossRef]
- Xiao, Y.; Riise, G. Coupling between Increased Lake Color and Iron in Boreal Lakes. Sci. Total Environ. 2021, 767, 145104. [Google Scholar] [CrossRef] [PubMed]
- Ejankowski, W.; Lenard, T. Climate Driven Changes in the Submerged Macrophyte and Phytoplankton Community in a Hard Water Lake. Limnologica 2015, 52, 59–66. [Google Scholar] [CrossRef]
- Cuss, C.W.; Ghotbizadeh, M.; Grant-Weaver, I.; Javed, M.B.; Noernberg, T.; Shotyk, W. Delayed Mixing of Iron-Laden Tributaries in Large Boreal Rivers: Implications for Iron Transport, Water Quality and Monitoring. J. Hydrol. 2021, 597, 125747. [Google Scholar] [CrossRef]
- Creed, I.F.; Bergström, A.-K.; Trick, C.G.; Grimm, N.B.; Hessen, D.O.; Karlsson, J.; Kidd, K.A.; Kritzberg, E.; McKnight, D.M.; Freeman, E.C.; et al. Global Change-Driven Effects on Dissolved Organic Matter Composition: Implications for Food Webs of Northern Lakes. Glob. Chang. Biol. 2018, 24, 3692–3714. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.I.; Urrutia-Cordero, P.; Zhang, H.; Ekvall, M.K.; Medeiros, L.R.; Hansson, L.-A. Charophytes Collapse beyond a Critical Warming and Brownification Threshold in Shallow Lake Systems. Sci. Total Environ. 2019, 661, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xing, Y.; Ding, A.; Sun, S.; Cheng, H.; Bian, Z.; Yang, K.; Wang, S.; Zhu, G. Brownification of Freshwater Promotes Nitrogen-Cycling Microorganism Growth Following Terrestrial Material Increase and Ultraviolet Radiation Reduction. Sci. Total Environ. 2022, 853, 158556. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Schott, J. Iron Colloids/Organic Matter Associated Transport of Major and Trace Elements in Small Boreal Rivers and Their Estuaries (NW Russia). Chem. Geol. 2002, 190, 141–179. [Google Scholar] [CrossRef]
- Trick, C.G.; Bill, B.D.; Cochlan, W.P.; Wells, M.L.; Trainer, V.L.; Pickell, L.D. Iron Enrichment Stimulates Toxic Diatom Production in High-Nitrate, Low-Chlorophyll Areas. Proc. Natl. Acad. Sci. USA 2010, 107, 5887–5892. [Google Scholar] [CrossRef] [PubMed]
- Hassellöv, M.; von der Kammer, F. Iron Oxides as Geochemical Nanovectors for Metal Transport in Soil-River Systems. Elements 2007, 4, 401. [Google Scholar] [CrossRef]
- Saari, M.; Rossi, P.M.; Postila, H.; Marttila, H. Predicting Iron Transport in Boreal Agriculture-Dominated Catchments under a Changing Climate. Sci. Total Environ. 2020, 714, 136743. [Google Scholar] [CrossRef] [PubMed]
- Oleinikova, O.V.; Poitrasson, F.; Drozdova, O.Y.; Shirokova, L.S.; Lapitskiy, S.A.; Pokrovsky, O.S. Iron Isotope Fractionation during Bio- and Photodegradation of Organoferric Colloids in Boreal Humic Waters. Environ. Sci. Technol. 2019, 53, 11183–11194. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.W. Broad Diversity of Viable Bacteria in “sterile” (0.2 Microm) Filtered Water. Res. Microbiol. 2004, 155, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, B.; Wang, Y.; Zhang, G.; Jiang, X.; Li, X. Passage and Community Changes of Filterable Bacteria during Microfiltration of a Surface Water Supply. Environ. Int. 2019, 131, 104998. [Google Scholar] [CrossRef] [PubMed]
- Nakai, R. Size Matters: Ultra-Small and Filterable Microorganisms in the Environment. Microbes Environ. 2020, 35, ME20025. [Google Scholar] [CrossRef] [PubMed]
- Ilina, S.M.; Drozdova, O.Y.; Lapitskiy, S.A.; Alekhin, Y.V.; Demin, V.V.; Zavgorodnyaya, Y.A.; Shirokova, L.S.; Viers, J.; Pokrovsky, O.S. Size Fractionation and Optical Properties of Dissolved Organic Matter in the Continuum Soil Solution-Bog-River and Terminal Lake of a Boreal Watershed. Org. Geochem. 2014, 66, 14–24. [Google Scholar] [CrossRef]
- Ilina, S.M.; Lapitskiy, S.A.; Alekhin, Y.V.; Viers, J.; Benedetti, M.; Pokrovsky, O.S. Speciation, Size Fractionation and Transport of Trace Elements in the Continuum Soil Water–Mire–Humic Lake–River–Large Oligotrophic Lake of a Subarctic Watershed. Aquat. Geochem. 2016, 22, 65–95. [Google Scholar] [CrossRef]
- Peacock, M.; Evans, C.D.; Fenner, N.; Freeman, C.; Gough, R.; Jones, T.G.; Lebron, I. UV-Visible Absorbance Spectroscopy as a Proxy for Peatland Dissolved Organic Carbon (DOC) Quantity and Quality: Considerations on Wavelength and Absorbance Degradation. Environ. Sci. Process. Impacts 2014, 16, 1445–1461. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ Ver. 3.1. 2014. Available online: http://vminteq.lwr.kth.se (accessed on 15 February 2020).
- Tipping, E.; Hurley, M.A. A Unifying Model of Cation Binding by Humic Substances. Geochim. Cosmochim. Acta 1992, 56, 3627–3641. [Google Scholar] [CrossRef]
- Tipping, E.; Lofts, S.; Sonke, J. Humic Ion-Binding Model VII: A Revised Parameterisation of Cation-Binding by Humic Substances. Environ. Chem. 2011, 8, 225. [Google Scholar] [CrossRef]
- Lofts, S.; Tipping, E. Assessing WHAM/Model VII against Field Measurements of Free Metal Ion Concentrations: Model Performance and the Role of Uncertainty in Parameters and Inputs. Environ. Chem. 2011, 8, 501–516. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Kleja, D.B. Modeling Salt-Dependent Proton Binding by Organic Soils with the NICA-Donnan and Stockholm Humic Models. Environ. Sci. Technol. 2005, 39, 5372–5377. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D. NIST SRD 46. Critically Selected Stability Constants of Metal Complexes: Version 8.0 for Windows, National Institute of Standards and Technology 2004. Available online: https://doi.org/10.18434/M32154 (accessed on 20 December 2023).
- Selberg, A.; Viik, M.; Ehapalu, K.; Tenno, T. Content and Composition of Natural Organic Matter in Water of Lake Pitkjärv and Mire Feeding Kuke River (Estonia). J. Hydrol. 2011, 400, 274–280. [Google Scholar] [CrossRef]
- Xue, J.-P.; Cuss, C.W.; Noernberg, T.; Javed, M.B.; Chen, N.; Pelletier, R.; Wang, Y.; Shotyk, W. Size and Optical Properties of Dissolved Organic Matter in Large Boreal Rivers during Mixing: Implications for Carbon Transport and Source Discrimination. J. Hydrol. Reg. Stud. 2022, 40, 101033. [Google Scholar] [CrossRef]
- Savenko, A.V.; Savenko, V.S. Trace Element Composition of the Dissolved Matter Runoff of the Russian Arctic Rivers. Water 2024, 16, 565. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace Elements in River Waters. Treatise Geochem. 2004, 5, 225–272. [Google Scholar]
- Drozdova, O.Y.; Karpukhin, M.M.; Dumtsev, S.V.; Lapitskiy, S.A. The Forms of Metals in the Water and Bottom Sediments of the Malaya Sen’ga River (Vladimir Oblast). Mosc. Univ. Geol. Bull. 2021, 76, 336–342. [Google Scholar] [CrossRef]
- Krickov, I.V.; Pokrovsky, O.S.; Manasypov, R.M.; Lim, A.G.; Shirokova, L.S.; Viers, J. Colloidal Transport of Carbon and Metals by Western Siberian Rivers during Different Seasons across a Permafrost Gradient. Geochim. Cosmochim. Acta 2019, 265, 221–241. [Google Scholar] [CrossRef]




| Object Name | River Length 1, km | Watershed Area 1, km2 | MAAT 2, °C | MAP 2, mm/day 1 |
|---|---|---|---|---|
| Senga | 32 | 163 | 3.74 | 1.27 |
| Mezha | 168 | 2630 | 3.66 | 1.55 |
| Ivnyashka | 14.3 | 29 | 2.16 | 1.72 |
| Mga | 93 | 754 | 3.46 | 1.73 |
| Koy | 14 | 27 | 1.85 | 1.72 |
| Ilexa | 4.7 | 32 | 1.46 | 1.72 |
| Kovzha | 108 | 1080 | 1.53 | 1.72 |
| Pionerka | 4.6 | 7.9 | 3.49 | 1.73 |
| Vuoksa | 143 | 68,700 | 3.21 | 1.73 |
| Lemb | 1.8 | 2.1 | 1.79 | 1.72 |
| Lundozhma | 11.1 | 64.2 | 0.97 | 1.73 |
| Ukhta | 47 | 380 | 0.12 | 1.73 |
| Cherpayoki | 13 | 17 | 0.12 | 1.73 |
| Olanga | 67 | 5679 | −0.62 | 1.83 |
| River | pH | κ | HCO3− | Cl− | NO3− | SO42− | Na | Mg | K | Ca | DOC | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μS/cm | mg/L | |||||||||||
| Senga | 7.6 | 94 | 122.9 | 5.1 | 2.8 | 4.8 | 2.0 | 3.9 | 0.7 | 23.0 | 22 | 0.19 |
| Mezha | 7.6 | 36 | 35.3 | 1.2 | 1.1 | 0.6 | 1.0 | 1.8 | 0.4 | 11.7 | 19 | 0.37 |
| Ivnyashka | 7.3 | 78 | 51 | 7.9 | 4.8 | 3.3 | 5.0 | 3.9 | 1.6 | 11.9 | 22 | 0.13 |
| Mga | 7.8 | 244 | 202 | 15.1 | 0.9 | 12.4 | 8.6 | 12.7 | 3.0 | 49.0 | 8 | 0.17 |
| Koy | 7.8 | 214 | 176.2 | 0.6 | n.d. | 1.5 | 2.6 | 10.5 | 1.4 | 35.5 | 20 | 0.05 |
| Ilexa | 8.0 | 307 | 44.5 | 110.9 | 1.2 | 3.5 | 1.7 | 17.8 | 1.1 | 46.8 | 6 | 0.15 |
| Kovzha | 7.9 | 26 | 0.4 | 2.3 | n.d. | 18.1 | 0.8 | 1.3 | 0.5 | 3.9 | 13 | 0.19 |
| Pionerka | 7.5 | 54 | 0.5 | 31.0 | 1.9 | 3.9 | 4.0 | 2.5 | 1.8 | 6.3 | 7 | 0.02 |
| Vuoksa | 7.5 | 57 | 35.2 | 3.3 | 2.3 | 4.6 | 3.7 | 2.7 | 1.9 | 6.7 | 15 | 0.03 |
| Lemb | 7.8 | 33 | 31.7 | 0.6 | 0.2 | 1.0 | 1.5 | 1.4 | 0.5 | 16.6 | 22 | 0.43 |
| Lundozhma | 7.1 | 39 | 36.7 | 3.9 | 0.3 | 0.7 | 4.5 | 2.4 | 0.4 | 14.9 | 27 | 0.57 |
| Ukhta | 7.7 | 27 | 18.9 | 0.4 | 0.1 | 0.6 | 1.4 | 1.5 | 0.5 | 2.5 | 8 | 0.15 |
| Cherpayoki | 6.9 | 33 | 42.2 | 23.7 | 0.1 | 0.4 | 4.0 | 2.1 | 25.5 | 3.2 | 12 | 0.94 |
| Olanga | 7.5 | 47 | 31.2 | 0.7 | 0.8 | 1.9 | 1.2 | 1.9 | 0.9 | 6.6 | 6 | 0.03 |
| Object Name | E254/E436 | E470/E655 | S275–295 | S350–400 | SR | SUVA254 |
|---|---|---|---|---|---|---|
| Senga | 20.0 | 10.3 | 0.016 | 0.019 | 0.84 | 4.9 |
| Mezha | 15.8 | 8.3 | 0.014 | 0.017 | 0.84 | 4.2 |
| Ivnyashka | 9.2 | 3.3 | 0.013 | 0.013 | 0.97 | 2.7 |
| Mga | 22.5 | 4.0 | 0.017 | 0.019 | 0.89 | 2.7 |
| Koy | 19.7 | 20.0 | 0.016 | 0.018 | 0.92 | 3.3 |
| Ilexa | 34.8 | 3.0 | 0.019 | 0.02 | 0.93 | 2.3 |
| Kovzha | 19.3 | 7.5 | 0.015 | 0.018 | 0.85 | 3.3 |
| Pionerka | 22.0 | 2.0 | 0.019 | 0.015 | 1.21 | 1.8 |
| Vuoksa | 11.9 | 2.8 | 0.015 | 0.015 | 0.98 | 2.5 |
| Lemb | 14.3 | 20.0 | 0.013 | 0.016 | 0.80 | 2.1 |
| Lundozhma | 12.6 | 11.8 | 0.013 | 0.016 | 0.81 | 3.3 |
| Ukhta | 49.5 | 3.0 | 0.019 | 0.023 | 0.83 | 2.5 |
| Cherpayoki | 14.2 | 9.5 | 0.013 | 0.017 | 0.73 | 3.6 |
| Olanga | 20.1 | 5.0 | 0.015 | 0.018 | 0.84 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleshina, A.; Rusakova, M.-A.; Drozdova, O.Y.; Pokrovsky, O.S.; Lapitskiy, S.A. Dissolved Iron and Organic Matter in Boreal Rivers across a South–North Transect. Environments 2024, 11, 65. https://doi.org/10.3390/environments11040065
Aleshina A, Rusakova M-A, Drozdova OY, Pokrovsky OS, Lapitskiy SA. Dissolved Iron and Organic Matter in Boreal Rivers across a South–North Transect. Environments. 2024; 11(4):65. https://doi.org/10.3390/environments11040065
Chicago/Turabian StyleAleshina, Alisa, Maria-Anna Rusakova, Olga Y. Drozdova, Oleg S. Pokrovsky, and Sergey A. Lapitskiy. 2024. "Dissolved Iron and Organic Matter in Boreal Rivers across a South–North Transect" Environments 11, no. 4: 65. https://doi.org/10.3390/environments11040065
APA StyleAleshina, A., Rusakova, M.-A., Drozdova, O. Y., Pokrovsky, O. S., & Lapitskiy, S. A. (2024). Dissolved Iron and Organic Matter in Boreal Rivers across a South–North Transect. Environments, 11(4), 65. https://doi.org/10.3390/environments11040065









