Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Soil Sources
2.2. Experimental Design
2.3. Statistical Analysis
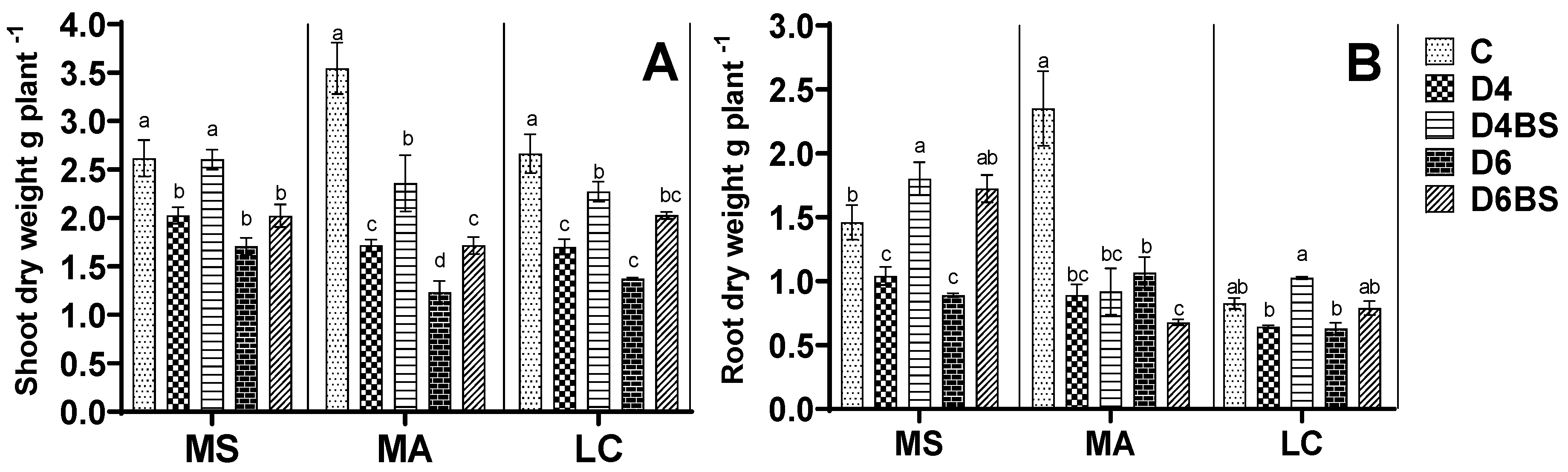
3. Results
3.1. Estimation of Plant Biomass and Growth Rate
3.2. Plant Chlorophyll Fluorescence
3.3. Removal of Diesel
3.4. Soil Nutrients and Soluble Phenols
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | Definitions | References * |
|---|---|---|
| Extracted and technical fluorescence parameters | ||
| Fo | Minimum fluorescence intensity at 50 μs, when all PSII RCs are assumed to be open | [40,41,42] |
| Fm = FP | Maximum fluorescence intensity recorded under saturating illumination at the peak P of OJIP, when all PSII RCs are closed | [40,41,42,43] |
| Fv = Fm − Fo | Maximum variable fluorescence | [40,41,42,43,44] |
| Fv/Fo = kp/kN = (Fm − Fo)/Fo | The maximum ratio of quantum yields of photochemical and non-photochemical energy quenching in PSII RC that is related to the maximal efficiency of OEC on the donor side of PSII (the most sensitive link in the photosynthetic chain of electron transport) | [40,42,45] |
| Vk = (F300μs − Fo)/(Fm − Fo) | Relative variable fluorescence at 300 μs (K-band) | [46,47,48,49] |
| Vj = (F2ms − Fo)/(Fm − Fo) | Relative variable fluorescence at 2 ms | [46,47,48,49] |
| Vk/Vj | Efficiency of electron flow from OEC to PSII RCs, a relative measure of inactivation of OEC | [46,47,48,49] |
| dVG/dto | Expression of excitation energy transfer between RCs | [40,42] |
| (dV/dt)o = Mo ≈ 4(F0.3ms − F0.05ms)/Fv | Initial slope (in ms−1) of the O-J fluorescence rise, which corresponds to the maximal rate of the accumulation of the fraction of closed RCs (expresses the rate of the RCs’ closure) | [37,40,42,44,45] |
| tFm | Time to reach Fm, in ms | [37,38,40,41,43] |
| N | The turnover number that indicates how many times QA has been reduced in the time span from 0 to tFm (number of QA redox turnovers until Fm is reached) | [37,40,41] |
| Area | The total complementary area above the OJIP curve between Fo and Fm and the Fm | [37,38,40,41,42,43,45] |
| Sm = Area/Fv | Normalised area expresses the energy needed to close all RCs during the multiple turnover in the QA reduction (closure of RCs) and is proportional to the pool size of the electron acceptors on the reducing side of PSII and therefore related to the number of electron carriers per electron transport chain | [37,38,40,41,42,43,44] |
| Quantum yields and efficiencies/probabilities | ||
| φPo = Fv/Fm = TRo/ABS = 1 − Fo/Fm | Maximum quantum yield of primary photochemistry reactions in PSII RC | [37,38,40,41,42,43,44,45] |
| ψEo = ETo/TRo = 1 − VJ | Efficiency/probability that PSII trapped electron moves future than QA− (i.e., is transferred from QA− to PQ) | [37,38,41,42,43,44,45] |
| φEo = ETo/ABS = φPo × ψEo | Quantum yield of electron transport (ET) from QA− to PQ | [37,38,41,42,43,44,45] |
| δRo = REo/ETo = (1 − VI)/ψEo | Efficiency/probability with which an electron from the intersystem electron carriers is transferred to reduce end electron acceptors at the PSI acceptor side | [38,42,43,44,45] |
| φRo = REo/ABS = φPo × (1 − VI) | Quantum yield for reduction of end electron acceptors at the PSI acceptor side | [38,42,43,44] |
| Specific energy fluxes per active (QA reducing) PSII reaction centre (RC) | ||
| ABS/RC =(Mo/VJ)/φPo | Absorption flux of antenna Chls per active PSII RC (also a measure of PSII apparent antenna size) | [37,38,40,41,42,43,44,45] |
| TRo/RC =Mo/VJ | Maximum trapped energy flux leading to QA reduction per active PSII RC | [37,38,41,42,43,44,45] |
| DIo/RC =ABS/RC − TRo/RC | Dissipated energy flux per active PSII RC in processes other than trapping | [37,40,42,44,45] |
| ETo/RC = (Mo/VJ) × ψEo | Electron transport flux further than QA− (i.e., from QA− to PQ) per active PSII RC | [37,38,40,41,42,43,44,45] |
| REo/RC = (Mo/VJ) × (1 − VI) | Electron flux leading to the reduction in the PSI end acceptor per active PSII RC | [38,42,43,44] |
| RC/ABS = φPo × (VJ/Mo) = (ABS/RC)−1 | Density of active RCs on PSII antenna Chl a basis (reciprocal of ABS/RC) | [38,41] |
| Phenomenological energy fluxes per excited cross-section (CSm, subscript m refers to time Fm) of PSII | ||
| ABS/CSm ≈ Fm | Absorbed photon flux of antenna Chls per excited CSm of PSII | [37,41,44] |
| TRo/CSm = φPo × ABS/CSm | Maximum trapped energy flux leading to QA reduction per excited CSm of PSII | [37,41,44] |
| DIo/CSm = ABS/CSm − TRo/CSm | Dissipated energy flux per excited CSm of PSII in processes other than trapping | [37,41] |
| ETo/CSm = φEo × ABS/CSm | Electron transport flux further than QA− (i.e., from QA− to PQ) per excited CSm of PSII | [37,41,44] |
| REo/CSm = φRo × ABS/CSm | Electron transport flux leading to the reduction in the PSI end acceptor per excited CSm of PSII | [44] |
| RC/CSm = φPo × (VJ/Mo) × Fm | Density of active RCs per excited CSm of PSII | [37,41,45] |
| Performance indexes on absorption basis (combination of parameters expressing partial potentials at steps of energy bifurcations of PSII and of specific electron transport reactions) | ||
| PIabs = (RC/ABS) × [φPo/(1 − φPo)] × [ψEo/(1 − ψEo)] | Performance index (potential) for energy conservation from photons absorbed by PSII antenna to the reduction in intersystem electron acceptors | [37,38,40,41,42,43,44,45,46] |
| PItotal = PIabs × [δRo/(1 − δRo)] | Performance index for energy conservation from photons absorbed by PSII antenna to the reduction in PSI end acceptors | [38,40,42,43,44] |
| SFIabs = RC/ABS × φPo × ψEo | Structure–function index, which reflects changes that “favor” photosynthesis | [44] |
| Driving forces on an absorption basis | ||
| DFabs = log(PIabs) | The driving force for the photochemical activity of the processes evaluated by the PIabs | [37,40,44] |
| DFtotal = log(PItotal) | The total driving force for the photochemical activity of the processes evaluated by the PItotal | [37,44] |
References
- Eneh, O.C. A review on petroleum: Source, uses, processing, products and the environment. J. Appl. Sci. 2011, 11, 2084–2091. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An overview of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Cham, Switzerland, 2020; pp. 1–27. [Google Scholar] [CrossRef]
- Daâssi, D.; Qabil Almaghribi, F. Petroleum-contaminated soil: Environmental occurrence and remediation strategies. 3 Biotech 2022, 12, 139. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Impact of Total Petroleum Hydrocarbons on Human Health. In Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation; Springer: Cham, Switzerland, 2022; pp. 139–165. [Google Scholar] [CrossRef]
- Marinescu, M.; Toti, M.; Tanase, V.; Carabulea, V.; Plopeanu, G.; Calciu, I. An assessment of the effects of crude oil pollution on soil properties. Ann. Food Sci. Technol. 2010, 11, 94–99. [Google Scholar] [CrossRef]
- Adipah, S. Introduction of petroleum hydrocarbons contaminants and its human effects. J. Environ. Sci. Public Health 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated sites in Europe: Review of the current situation based on data collected through a European network. J. Environ. Sci. Public Health 2013, 2013, 158764. [Google Scholar] [CrossRef] [PubMed]
- EPA. Available online: https://www.epa.gov/environmental-topics/land-waste-and-cleanup-topics (accessed on 11 January 2024).
- Zahed, M.A.; Matinvafa, M.A.; Azari, A.; Mohajeri, L. Biosurfactant, a green and effective solution for bioremediation of petroleum hydrocarbons in the aquatic environment. Discov. Water 2022, 2, 5. [Google Scholar] [CrossRef]
- Zuzolo, D.; Guarino, C.; Tartaglia, M.; Sciarrillo, R. Plant-Soil-Microbiota Combination for the Removal of Total Petroleum Hydrocarbons (TPH): An In-Field Experiment. Front. Microbiol. 2021, 11, 621581. [Google Scholar] [CrossRef] [PubMed]
- Ite, A.E.; Harry, T.A.; Obadimu, C.O.; Asuaiko, E.R.; Inim, I.J. Petroleum hydrocarbons contamination of surface water and groundwater in the Niger Delta region of Nigeria. J. Environ. Pollut. Hum. Health 2018, 6, 51–61. [Google Scholar] [CrossRef]
- Dos Santos, E.V.; Bezerra Rocha, J.H.; de Araújo, D.M.; de Moura, D.C.; Martínez-Huitle, C.A. Decontamination of produced water containing petroleum hydrocarbons by electrochemical methods: A minireview. Environ. Sci. Pollut. Res. 2014, 21, 8432–8441. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Poi, G.; Aburto-Medina, A.; Haleyur, N.; Ball, A.S. Bioremediation Approaches for Petroleum Hydrocarbon-Contaminated Environments. In Enhancing Cleanup of Environmental Pollutants; Anjum, N., Gill, S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; Volume 1, pp. 21–41. [Google Scholar] [CrossRef]
- Latif, A.; Abbas, A.; Iqbal, J.; Azeem, M.; Asghar, W.; Ullah, R.; Chen, Z. Remediation of Environmental Contaminants Through Phytotechnology. Water Air Soil Pollut. 2023, 234, 139. [Google Scholar] [CrossRef]
- Pardo, T.; Bernal, M.P.; Clemente, R. Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: I. Effects on trace elements and nutrients solubility and leaching risk. Chemosphere 2014, 107, 121–128. [Google Scholar] [CrossRef]
- Collins, C.D. Implementing Phytoremediation of Petroleum Hydrocarbons. In Phytoremediation; Willey, N., Ed.; Methods in Biotechnology; Humana Press: Totowa, NJ, USA, 2007; Volume 23. [Google Scholar] [CrossRef]
- Bhuyan, B.; Kotoky, R.; Pandey, P. Impacts of rhizoremediation and biostimulation on soil microbial community, for enhanced degradation of petroleum hydrocarbons in crude oil-contaminated agricultural soils. Environ. Sci. Pollut. Res. Int. 2023, 30, 94649–94668. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Franzetti, A.; Gandolfi, I.; Raimondi, C.; Bestetti, G.; Banat, I.M.; Smyth, T.J.; Papacchini, M.; Cavallo, M.; Fracchia, L. Environmental fate, toxicity, characteristics and potential applications of novel bioemulsifiers produced by Variovorax paradoxus 7bCT5. Bioresour. Technol. 2012, 108, 245–251. [Google Scholar] [CrossRef]
- Kapadia, S.G.; Yagnik, B.N. Current trend and potential for microbial biosurfactants. Asian J. Exp. Biol. Sci. 2013, 4, 1–8. [Google Scholar]
- Mulligan, C.N. Sustainable Remediation of Contaminated Soil Using Biosurfactants. Front. Bioeng. Biotechnol. 2021, 9, 635196. [Google Scholar] [CrossRef]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the Mechanisms of Positive Microbial Interactions That Benefit Lactic Acid Bacteria Co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential Agents for Controlling Cellular Communication, Motility, and Antagonism. Front. Mol. Biosci. 2021, 11, 727070. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ramírez, M.; Silva-Jiménez, H.; Banat, I.M.; Díaz De Rienzo, M.A. Surfactants: Physicochemical interactions with biological macromolecules. Biotechnol. Lett. 2021, 43, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Hajabbasi, M.A. Importance of soil physical characteristics for petroleum hydrocarbons phytoremediation: A review. Afric. J. Environ. Scien. Tech. 2016, 10, 394–405. [Google Scholar] [CrossRef]
- Osam, M.U.; Wegwu, M.O.; Ayalogu, E.O. Soil pH, moisture content and some macro non-metallic elements in crude oil contaminated soils remediated by some wild-type legumes. Inter. J. Eng. Sci. Invent. 2013, 2, 54–59. [Google Scholar]
- Gilan, R.S.; Parvizi, Y.; Pazira, E.; Rejali, F. Bioremediation of petroleum-contaminated soil in arid region using different arid-tolerant tree, shrub, and grass plant species with bacteria. Int. J. Environ. Sci. Technol. 2022, 19, 11879–11890. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Nath, R.; Sengupta, K.; Nalia, A.; Banerjee, S.; Chandran, M.A.S.; Hossain, A. The response of lentil (Lens culinaris Medik.) to soil moisture and heat stress under different dates of sowing and foliar application of micronutrients. Front. Plant Sci. 2021, 12, 679469. [Google Scholar] [CrossRef] [PubMed]
- Zabala, J.M.; Marinoni, L.; Giavedoni, J.A.; Schrauf, G.E. Breeding strategies in Melilotus albus Desr., a salt-tolerant forage legume. Euphytica 2018, 214, 22. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Kaur, J.; Shukla, A.K.; Singh, J.; Singh, P. Cadmium phytoremediation potential of Brassica genotypes grown in Cd spiked Loamy sand soils: Accumulation and tolerance. Chemosphere 2022, 302, 134842. [Google Scholar] [CrossRef]
- ISO 16703:2004; ISO 2004 Soil Quality—Determination of Content of in the Range C10—C40 by Gas Chromatography. International Organization for Standardization: Geneva, Switzerland, 2004.
- Hood-Nowotny, R.; Umana, N.H.N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative Methods for Measuring Inorganic, Organic, and Total Dissolved Nitrogen in Soil. Soil Sci. Soc. Am. J. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Cheema, S.A.; Khan, M.I.; Tang, X.; Zhang, C.; Shen, C.; Malik, Z.; Chen, Y. Enhancement of phenanthrene and pyrene degradation in rhizosphere of tall fescue (Festuca arundinacea). J. Hazard. Mater. 2009, 166, 1226–1231. [Google Scholar] [CrossRef]
- Fernández, J.A.; Niell, F.X.; Lucena, J. A rapid and sensitive automated determination of phosphate in natural waters 1. Limnol. Oceanogr. 1985, 30, 227–230. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Samborska, I.A.; Kalaji, H.M.; Sieczko, L.; Borucki, W.; Mazur, R.; Kouzmanova, M.; Goltsev, V. Can just one-second measurement of chlorophyll a fluorescence be used to predict sulphur deficiency in radish (Raphanus sativus L. sativus) plants? Curr. Plant Biol. 2019, 19, 100096. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, H.; Raj, S.; Soni, V. Chlorophyll a fluorescence kinetics of mung bean (Vigna radiata L.) grown under artificial continuous light. Biochem. Biophys. Rep. 2020, 24, 100813. [Google Scholar] [CrossRef]
- Esmaeilizadeh, M.; Malekzadeh Shamsabad, M.R.; Roosta, H.R.; Dąbrowski, P.; Rapacz, M.; Zieliński, A.; Kalaji, H.M. Manipulation of light spectrum can improve the performance of photosynthetic apparatus of strawberry plants growing under salt and alkalinity stress. PLoS ONE 2021, 16, e0261585. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Cetner, M.D. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Kalachanis, D.; Manetas, Y. Analysis of fast chlorophyll fluorescence rise (O-K-J-I-P) curves in green fruits indicates electron flow limitations at the donor side of PSII and the acceptor sides of both photosystems. Physiol. Plant. 2010, 139, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Kotakis, C.; Kyzeridou, A.; Manetas, Y. Photosynthetic electron flow during leaf senescence: Evidence for a preferential maintenance of photosystem I activity and increased cyclic electron flow. Photosynthetica 2014, 52, 413–420. [Google Scholar] [CrossRef]
- Swoczyna, T.; Łata, B.; Stasiak, A.; Stefaniak, J.; Latocha, P. JIP-test in assessing sensitivity to nitrogen deficiency in two cultivars of Actinidia arguta (Siebold et Zucc.) planch. ex miq. Photosynthetica 2019, 57, 646–658. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental stress—What can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef] [PubMed]
- Riskuwa-Shehu, M.L.; Ijah, U.J.J.; Manga, S.B.; Bilbis, L.S. Evaluation of the use of legumes for biodegradation of petroleum hydrocarbons in soil. Int. J. Environ. Sci. Technol. 2017, 14, 2205–2214. [Google Scholar] [CrossRef]
- Hatami, E.; Abbaspour, A.; Dorostkar, V. Phytoremediation of a petroleum-polluted soil by native plant species in Lorestan Province, Iran. Environ. Sci. Pollut. Res. 2019, 26, 24323–24330. [Google Scholar] [CrossRef] [PubMed]
- Korneykova, M.V.; Myazin, V.A.; Fokina, N.V.; Chaporgina, A.A. Bioremediation of soil of the kola peninsula (Murmansk region) contaminated with diesel fuel. Geogr. Environ. Sustain. 2021, 14, 171–176. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of soil contaminated with diesel fuel and biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef] [PubMed]
- Burdová, H.; Nebeská, D.; Al Souki, K.S.; Pilnaj, D.; Kwoczynski, Z.; Kříženecká, S.; Trögl, J. Miscanthus × giganteus stress tolerance and phytoremediation capacities in highly diesel contaminated soils. J. Environ. Manag. 2023, 344, 118475. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Effect of Diesel Fuel on Growth of Selected Plant Species. Environ. Geochem. Health 1999, 21, 353–357. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. The Effect of Diesel Fuel on Common Vetch (Vicia sativa L.) Plants. Environ. Geochem. Health 2023, 25, 123–130. [Google Scholar] [CrossRef]
- Rafique, H.M.; Khan, M.Y.; Asghar, H.N.; Ahmad Zahir, Z.; Nadeem, S.M.; Sohaib, M.; Al-Barakah, F.N. Converging alfalfa (Medicago sativa L.) and petroleum hydrocarbon acclimated ACC-deaminase containing bacteria for phytoremediation of petroleum hydrocarbon contaminated soil. Int. J. Phytoremediation 2023, 25, 717–727. [Google Scholar] [CrossRef]
- Pawluśkiewicz, B.; Gnatowski, T.; Janicka, M. The influence of soil contamination with diesel oil on germination dynamics and seedling development of selected species of the Fabaceae family. J. Ecol. Eng. 2020, 21, 210–218. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Wijatkowski, A.; Mikiciuk, M. Influence of diesel and biodiesel fuel-contaminated soil on microorganisms, growth and development of plants. Plant Soil Environ. 2015, 61, 189–194. [Google Scholar] [CrossRef]
- Barrutia, O.; Garbisu, C.; Epelde, L.; Sampedro, M.C.; Goicolea, M.A.; Becerril, J.M. Plant tolerance to diesel minimizes its impact on soil microbial characteristics during rhizoremediation of diesel-contaminated soils. Sci. Total Environ. 2011, 409, 4087–4093. [Google Scholar] [CrossRef]
- Han, T.; Zhao, Z.; Bartlam, M.; Wang, Y. Combination of biochar amendment and phytoremediation for hydrocarbon removal in petroleum-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 21219–21228. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The co-application of biochar with bioremediation for the removal of petroleum hydrocarbons from contaminated soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Chen, S.; Zhou, W. Enhanced phytoremediation of petroleum-contaminated soil by biochar and urea. J. Hazard. Mater. 2023, 453, 131404. [Google Scholar] [CrossRef] [PubMed]
- Deebika, P.; Merline Sheela, A.; Ilamathi, R. Biochar and compost-based phytoremediation of crude oil contaminated soil. Indian J. Sci. Technol. 2021, 14, 220–228. [Google Scholar] [CrossRef]
- Veerapagu, M.; Jeya, K.R.; Sankaranarayanan, A. Role of plant growth-promoting microorganisms in phytoremediation efficiency. In Plant-Microbe Interaction-Recent Advances in Molecular and Biochemical Approaches; Academic Press: Cambridge, MA, USA, 2023; pp. 45–61. [Google Scholar] [CrossRef]
- Ambust, S.; Das, A.J.; Kumar, R. Bioremediation of petroleum contaminated soil through biosurfactant and Pseudomonas sp. SA3 amended design treatments. Curr. Res. Microb. Sci. 2021, 2, 100031. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.F.; Patowary, R.; Sarma, H. Biosurfactant-assisted phytoremediation for a sustainable future. In Assisted Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 399–414. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Rosatelli, A.; Prasad, S.; Gomez, F.H.; Vaccari, M. Ex-situ bioremediation of petroleum hydrocarbon contaminated soil using mixed stimulants: Response and dynamics of bacterial community and phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108814. [Google Scholar] [CrossRef]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, J.; Arguelles-Arias, A.; Dhondt-Cordelier, S.; Cordelier, S.; Pršić, J.; Hoff, G.; Dorey, S. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020, 8, 1014. [Google Scholar] [CrossRef]
- Memarian, R.; Ramamurthy, A.S. Effects of surfactants on rhizodegradation of oil in a contaminated soil. J. Environ. Sci. Health A J. 2012, 47, 1486–1490. [Google Scholar] [CrossRef]
- Silva, R.D.C.F.S.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Applications of Biosurfactants in the Petroleum Industry and the Remediation of Oil Spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef]
- Ptaszek, N.; Pacwa-Płociniczak, M.; Noszczyńska, M.; Płociniczak, T. Comparative study on multiway enhanced bio-and phytoremediation of aged petroleum-contaminated soil. Agronomy 2020, 10, 947. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Hasan, H.A.; Idris, M.; Abdullah, S.R.S.; Anuar, N. Potential application of a biosurfactant in phytoremediation technology for treatment of gasoline-contaminated soil. Ecol. Eng. 2015, 84, 113–120. [Google Scholar] [CrossRef]
- Wang, J.; Bao, H.; Pan, G.; Zhang, H.; Li, J.; Li, J.; Wu, F. Combined application of rhamnolipid and agricultural wastes enhances PAHs degradation via increasing their bioavailability and changing microbial community in contaminated soil. J. Environ. Manag. 2021, 294, 112998. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Lv, Y.; Liu, C.; Li, S.; Yin, Z.; Yu, Y.; Zhu, L. Performance evaluation of rhamnolipids addition for the biodegradation and bioutilization of petroleum pollutants during the composting of organic wastes with waste heavy oil. iScience 2022, 25, 104403. [Google Scholar] [CrossRef]
- Sima, N.A.K.; Ebadi, A.; Reiahisamani, N.; Rasekh, B. Bio-based remediation of petroleum-contaminated saline soils: Challenges, the current state-of-the-art and future prospects. J. Environ. Manag. 2019, 250, 109476. [Google Scholar] [CrossRef]
- Gao, Q.T.; Tam, N.F.Y. Growth, photosynthesis and antioxidant responses of two microalgal species, Chlorella vulgaris and Selenastrum capricornutum, to nonylphenol stress. Chemosphere 2011, 82, 346–354. [Google Scholar] [CrossRef]
- Dikšaitytė, A.; Kniuipytė, I.; Žaltauskaitė, J.; Abdel-Maksoud, M.A.; Asard, H.; AbdElgawad, H. Enhanced Cd phytoextraction by rapeseed under future climate as a consequence of higher sensitivity of HMA genes and better photosynthetic performance. Sci. Total Environ. 2024, 908, 168164. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chilean J. Agricu. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef]
- Gupta, R. Tissue specific disruption of photosynthetic electron transport rate in pigeonpea (Cajanus cajan L.) under elevated temperature. Plant Signal. Behav. 2019, 14, 1601952. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Wang, J.; Wu, X.; Ma, S.; Xu, Z.; Zhou, T.; Xu, N.; Tang, X.; An, B. Increased CO2 concentrations increasing water use efficiency and improvement PSII function of mulberry seedling leaves under drought stress. J. Plant Interact. 2019, 14, 213–223. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Xu, Z.; Lv, X.; Wang, Y. A self-photoprotection mechanism helps Stipa baicalensis adapt to future climate change. Sci. Rep. 2016, 6, 25839. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Janeeshma, E.; Kalaji, H.M.; Puthur, J.T. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol. Plant. 2021, 43, 12. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kato, H.; Shinzaki, Y.; Horiguchi, S.; Shikanai, T.; Hase, T.; Endo, T.; Nishioka, M.; Makino, A.; Tomizawa, K.I.; et al. Ferredoxin limits cyclic electron flow around PSI (CEF-PSI) in higher plants—Stimulation of CEF-PSI enhances non-photochemical quenching of chl fluorescence in transplastomic tobacco. Plant. Cell Physiol. 2006, 47, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- Rumeau, D.; Peltier, G.; Cournac, L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant. Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–560. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Casano, L.M.; Zapata, J.M.; Guéra, A.; Del Campo, E.M.; Schmitz-Linneweber, C.; Maier, R.M.; Sabater, B. Role of thylakoid Ndh complex and peroxidase in the protection against photo-oxidative stress: Fluorescence and enzyme activities in wild-type and ndhF-deficient tobacco. Physiol. Plant. 2004, 122, 443–452. [Google Scholar] [CrossRef]
- Bukhov, N.; Carpentier, R. Alternative photosystem I-driven electron transport routes: Mechanisms and functions. Photosynth. Res. 2004, 82, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef]
- Lacalle, R.G.; Gómez-Sagasti, M.T.; Artetxe, U.; Garbisu, C.; Becerril, J.M. Brassica napus has a key role in the recovery of the health of soils contaminated with metals and diesel by rhizoremediation. Sci. Total Environ. 2018, 618, 347–356. [Google Scholar] [CrossRef]
- Eze, M.O.; George, S.C.; Hose, G.C. Dose-response analysis of diesel fuel phytotoxicity on selected plant species. Chemosphere 2021, 263, 128382. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, R.; Lin, X.G.; Liu, W.W.; Chen, R.R.; Li, X.Z. Interactive effect of biosurfactant and microorganism to enhance phytoremediation for removal of aged polycyclic aromatic hydrocarbons from contaminated soils. J. Health Sci. 2010, 56, 257–266. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Dubey, R.C. Biosurfactants: Role in Plant Growth Promotion and Disease Management. In Multifunctional Microbial Biosurfactants; Springer: Cham, Switzerland, 2023; pp. 213–229. [Google Scholar] [CrossRef]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. Assisted phytoremediation for restoring soil fertility in contaminated and degraded land. Ital. J. Agron. 2018, 13 (Suppl. S1), 34–44. [Google Scholar]
- Banov, M.; Rousseva, S.; Pavlov, P. Sustainable Management and Restoration of the Fertility of Damaged and Contaminated Lands and Soils. In Soil Health Restoration and Management; Meena, R., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Lavania, M.; Lal, B. Biosurfactant: A next-generation tool for sustainable remediation of organic pollutants. Front. Microbiol. 2022, 12, 821531. [Google Scholar] [CrossRef]
- Mir, M.I.; Quadriya, H.; Kumar, B.K.; Adeeb, S.; Ali, M.; Khan, M.Y.; Hameeda, B. 14 Biosurfactants of Nitrogen Fixers and their Potential Applications. In Microbial Surfactants: Volume 2: Applications in Food and Agriculture; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Hall, J.; Soole, K.; Bentham, R. Hydrocarbon phytoremediation in the family Fabacea—A review. Int. J. Phytoremediation. 2011, 13, 317–332. [Google Scholar] [CrossRef]
- Raza, A.; Zahra, N.; Hafeez, M.B.; Ahmad, M.; Iqbal, S.; Shaukat, K.; Ahmad, G. Nitrogen Fixation of Legumes: Biology and Physiology. In The Plant Family Fabaceae; Hasanuzzaman, M., Araújo, S., Gill, S., Eds.; Springer: Singapore, 2020; pp. 43–74. [Google Scholar] [CrossRef]
- Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10, 1047279. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of Phenolic Compounds in Plant-Defensive Mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic compounds in the plant development and defense: An overview. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: Rijeka, Croatia, 2020; pp. 125–140. [Google Scholar] [CrossRef]





| Treatment | ||||
|---|---|---|---|---|
| Plant Species | D4 | D4BS | D6 | D6BS |
| MS | 0.782 ± 0.065 b | 1.008 ± 0.093 a | 0.654 ± 0.022 b | 0.776 ± 0.043 b |
| MA | 0.487 ± 0.020 a | 0.603 ± 0.061 a | 0.354 ± 0.052 a | 0.493 ± 0.061 a |
| LC | 0.644 ± 0.046 b | 0.867 ± 0.097 a | 0.521 ± 0.040 b | 0.771 ± 0.074 a |
| Treatment | |||||
|---|---|---|---|---|---|
| Plant Species | C | D4 | D4BS | D6 | D6BS |
| MS | 5.260 a | 5.096 a | 5.796 a | 5.119 a | 5.636 a |
| MA | 6.150 a | 3.757 b | 4.187 b | 3.689 b | 4.414 b |
| LC | 4.043 a | 3.513 b | 3.886 ab | 3.721 ab | 3.931 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meištininkas, R.; Vaškevičienė, I.; Dikšaitytė, A.; Pedišius, N.; Žaltauskaitė, J. Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species. Environments 2024, 11, 64. https://doi.org/10.3390/environments11040064
Meištininkas R, Vaškevičienė I, Dikšaitytė A, Pedišius N, Žaltauskaitė J. Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species. Environments. 2024; 11(4):64. https://doi.org/10.3390/environments11040064
Chicago/Turabian StyleMeištininkas, Rimas, Irena Vaškevičienė, Austra Dikšaitytė, Nerijus Pedišius, and Jūratė Žaltauskaitė. 2024. "Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species" Environments 11, no. 4: 64. https://doi.org/10.3390/environments11040064
APA StyleMeištininkas, R., Vaškevičienė, I., Dikšaitytė, A., Pedišius, N., & Žaltauskaitė, J. (2024). Biosurfactant-Assisted Phytoremediation of Diesel-Contaminated Soil by Three Different Legume Species. Environments, 11(4), 64. https://doi.org/10.3390/environments11040064








