Epigenetic Effects in HPA Axis Genes Associated with Cortical Thickness, ERP Components and SUD Outcome
Abstract
1. Introduction
2. Methods
2.1. Participants
Follow-Up Samples
2.2. Clinical Assessment
2.3. Childhood Adversity: Life Stressors and Social Resources Inventory (LISRES)
2.4. Event-Related Potentials: Visual Task
2.5. Epigenetic Data: DNA Isolation and Methylation Assays
2.6. Imaging Parameters
FreeSurfer Analysis
3. Results
3.1. Demographic Characteristics
3.2. Cortical Thickness in FreeSurfer Regions and ERP Components
3.3. ERP and Cortical Thickness: Predictors of Substance Use Disorder
3.4. Childhood Adversity, Methylation of the CRHR1 Gene, and Cortical Thickness
3.5. SUD Outcome, NLE, CRHR1 Methylation, and Cortical Thickness
4. Discussion
5. Conclusions, Limitations, and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Global Status Report on Alcohol and Health; World Health Organization: Geneva, Switzerland, 2018.
- Sutton, S.; Barren, M.; Zubin, J.; John, E.R. Evoked potential correlates of stimulus uncertainty. Science 1965, 150, 1187–1188. [Google Scholar] [CrossRef]
- Blackwood, D.H.R.; Whalley, L.J.; Christie, J.E.; Blackburn St, I.M.; Clair, D.M.; McInnes, A. Changes in auditory P3 event-related potentials in schizophrenia and depression. Br. J. Psychiatry 1987, 150, 154–160. [Google Scholar] [CrossRef]
- Bruder, G.E.; Tenke, C.E.; Stewart, J.W.; Towey, J.P.; Leite, P.; Voglmaier, M.; Quitkin, F.M. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology 1995, 32, 373–381. [Google Scholar] [CrossRef]
- Hill, S.Y.; Steinhauer, S.R.; Locke, J. Event-related potentials in alcoholic men, their high-risk male relatives and low-risk male controls. Alcohol. Clin. Exp. Res. 1995, 9, 567–576. [Google Scholar] [CrossRef]
- Begleiter, H.; Porjesz, B.; Bihari, B.; Kissin, B. Event-related brain potentials in boys at risk for alcoholism. Science 1984, 225, 1493–1496. [Google Scholar] [CrossRef]
- Hill, S.Y.; Steinhauer, S.R.; Park, J.; Zubin, J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol. Clin. Exp. Res. 1990, 14, 6–16. [Google Scholar] [CrossRef]
- Carlson, S.R.; Iacono, W.G. Deviant P300 amplitude development in males is associated with paternal externalizing psychopathology. J. Abnorm. Psychol. 2008, 117, 910–923. [Google Scholar] [CrossRef]
- Hill, S.Y.; Shen, S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol. Psychiatry 2002, 51, 621–631. [Google Scholar] [CrossRef]
- Hill, S.Y.; Steinhauer, S.R.; Locke-Wellman, J.; Ulrich, R. Childhood risk factors for young adult substance dependence outcome in offspring from multiplex alcohol dependence families: A prospective study. Biol. Psychiatry 2009, 66, 750–757. [Google Scholar] [CrossRef]
- Iacono, W.G.; Malone, S.M. Developmental endophenotypes: Indexing genetic risk for substance abuse with the P300 brain event-related potential. Child Dev. Perspect. 2011, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Donchin, E. Event-related brain potentials: A tool in the study of human information processing. In Evoked Brain Potentials and Behavior; Begleiter, H., Ed.; Plenum Press: New York, NY, USA, 1979; Volume 2, pp. 13–88. [Google Scholar] [CrossRef]
- Pritchard, W.S. Psychophysiology of P300. Psycho. Bull. 1981, 89, 506–540. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Euser, A.S.; Arends, L.R.; Evans, B.E.; Greaves-Lord, K.; Huizink, A.C.; Franken, H.A. The P300 event-related brain potential as a neurobiological endophenotype for substance use disorders: A meta-analytic investigation. Neurosci. Biobehav. Rev. 2012, 36, 572–603. [Google Scholar] [CrossRef]
- Valakos, D.; d’Avossa, G.; Mylonas, D.; Butler, J.; Klein, C.; Smymis, N. P300 response modulation reflects breaches of non-probabilistic expectations. Sci. Rep. 2020, 10, 10254. [Google Scholar] [CrossRef]
- Hill, S.Y.; Jones, B.L.; Holmes, B.; Steinhauer, S.R.; Zezza, N.; Stiffler, S. Cholinergic receptor gene (CHRM2) variation and familial loading for alcohol dependence predict childhood developmental trajectories of P300. Psychiatry Res. 2013, 209, 504–511. [Google Scholar] [CrossRef][Green Version]
- Ehlers, C.L.; Wills, D.N.; Karriker-Jaffe, K.J.; Gilder, D.A.; Phillips, E.; Bernert, R.A. Delta Event-related oscillations are related to a history of extreme binge drinking in adolescence and lifetime suicide risk. Behav. Sci. 2020, 10, 154. [Google Scholar] [CrossRef]
- Kamarajan, C.; Pandey, A.K.; Chorlian, D.B.; Manz, N.; Stimus, A.T.; Anokhin, A.P.; Bauer, L.O.; Kuperman, S.; Kramer, J.; Bucholz, K.K.; et al. Deficient event-related theta oscillations in individuals at risk for alcoholism: A study of reward processing and impulsivity features. PLoS ONE 2015, 10, e0142659. [Google Scholar] [CrossRef]
- Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Jones, K.A.; Padmanabhapillai, A.; Begleiter, H. The utility of neurophysiological markers in the study of alcoholism. Clin. Neurophysiol. 2005, 116, 933–1018. [Google Scholar] [CrossRef]
- Jones, K.A.; Porjesz, B.; Almasy, L.; Bierut, L.J.; Goate, A.; Wang, J.C.; Hinrichs, T.; Kwon, J.; Rice, J.P.; Rohrbaugh, J.; et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: Implications for human brain dynamics and cognition. Int. J. Psychophysiol. 2004, 53, 75–90. [Google Scholar] [CrossRef]
- Rangaswamy, M.; Jones, K.A.; Porjesz, B.; Chorlian, D.B.; Padmanabhapillai, A.; Kamarajan, C.; Kuperman, S.; Rohrbaugh, J.; O’Connor, S.J.; Bauer, L.O.; et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int. J. Psychophysiol. 2007, 63, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.Y.; Steinhauer, S.R. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J. Stud. Alcohol 1993, 54, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, S.R.; Hill, S.Y. Auditory event-related potentials in children at high risk for alcoholism. J. Stud. Alcohol 1993, 54, 408–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hill, S.Y.; Shen, S.; Locke, J.; Steinhauer, S.R.; Konicky, C.; Lowers, L.; Connolly, J. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol. Psychiatry 1999, 46, 970–981. [Google Scholar] [CrossRef]
- Carlson, S.R.; Iacono, W.G. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology 2006, 43, 470–480. [Google Scholar] [CrossRef]
- Carlson, S.R.; McLarmon, M.E.; Iacono, W.G. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. J. Abnorm. Psychol. 2007, 116, 565–577. [Google Scholar] [CrossRef]
- Perlman, G.; Markin, A.; Iacono, W.G. P300 amplitude reduction is associated with early-onset and late-onset pathological substance use in a prospectively studied cohort of 14 year-old adolescents. Psychophysiology 2013, 50, 974–982. [Google Scholar] [CrossRef]
- Krueger, R.F.; Hicks, B.M.; Patrick, C.J.; Carlson, S.R.; Iacono, W.G.; McGue, M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J. Abnorm. Psychol. 2002, 111, 411–424. [Google Scholar] [CrossRef]
- Krueger, R.F.; Markon, K.E.; Patrick, C.J.; Iacono, W.G. Externalizing psychopathology in adulthood, a dimensional-spectrum conceptualization and its implications for DSM-V. J. Abnorm. Psychol. 2005, 114, 537–550. [Google Scholar] [CrossRef]
- Patrick, C.J.; Bernat, E.M.; Malone, S.M.; Iacono, W.G.; Krueger, R.F.; McGue, M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology 2006, 43, 84–92. [Google Scholar] [CrossRef]
- Van Der Stelt, O. Visual P3 as a potential vulnerability marker of alcoholism: Evidence from the Amsterdam study of children of alcoholics. Alcohol Alcohol. 1999, 34, 267–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hicks, B.M.; Bernat, E.; Malone, S.M.; Iacono, W.G.; Patrick, C.J.; Krueger, R.F.; McGue, M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology 2007, 44, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Diner, B.C.; Holcomb, P.J.; Dykman, R.A. P300 in major depressive disorder. Psychiatry Res. 1985, 15, 175–184. [Google Scholar] [CrossRef]
- Yanai, I.; Fujikawa, T.; Osada, M.; Yamawaki, S.; Touhouda, Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. J. Affect. Dis. 1997, 46, 263–271. [Google Scholar] [CrossRef]
- Roschke, J.; Wagner, P. A confirmatory study of the mechanisms behind reduced P300 waves in depression. Neuropsychopharmacology 2003, 28, S9–S12. [Google Scholar] [CrossRef]
- O’Connor, S.; Morzorati, S.; Christian, J.; Li, T. Heritable features of the auditory oddball event-related potential peaks, latencies, morphology and topography. Electroencephalogr. Clin. Neurophysiol. 1994, 92, 115–125. [Google Scholar] [CrossRef]
- Katsanis, J.; Iacono, W.G.; McGue, M.K.; Carlson, S.R. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology 1997, 34, 47–58. [Google Scholar] [CrossRef] [PubMed]
- van Beijsterveldt, T.; VanBaal, G.C.M. Twin and family studies of the human electroencephalogram: A review and a meta-analysis. Biol. Psychol. 2002, 61, 111–138. [Google Scholar] [CrossRef]
- Perlman, G.; Johnson, W.; Iacono, W.G. The heritability of P300 amplitude in 18 year-olds is robust to adolescent alcohol use. Psychophysiology 2009, 46, 962–969. [Google Scholar] [CrossRef]
- Hill, S.Y.; Locke, J.; Zezza, N.; Kaplan, B.; Neiswanger, K.; Steinhauer, S.; Wipprecht, G.; Xu, J. Genetic association between reduced P300 amplitude and the DRD2 dopamine receptor A1 allele in children at high risk for alcoholism. Biol. Psychiatry 1998, 43, 40–51. [Google Scholar] [CrossRef]
- Jones, K.A.; Porjesz, B.; Almasy, L.; Bierut, L.; Dick, D.; Goate, A.; Hinrichs, A.; Rice, J.P.; Wang, J.C.; Bauer, L.O.; et al. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav. Genet. 2006, 36, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Porjesz, B.; Rangaswamy, M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Sci. World J. 2007, 7, 131–141. [Google Scholar] [CrossRef]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Miktan, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef]
- Gur, R.E.; Moore, T.M.; Rosen, A.F.G.; Barzilay, R.; Roalf, D.R.; Calkins, M.E.; Ruparel, K.; Scott, J.C.; Almasy, L.; Satterthwaite, T.D.; et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry 2019, 76, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.A.; Viding, E.; Wallace, G.L.; Schaer, M.; De Brito, S.A.; Robustelli, B.; McCrory, E.J. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biol. Psychiatry 2013, 74, 845–852. [Google Scholar] [CrossRef]
- Kelly, P.A.; Viding, E.; Puetz, V.B.; Palmer, A.L.; Samuel, S.; McCrory, E.J. The sexually dimorphic impact of maltreatment on cortical thickness, surface area and gyrification. J. Neural. Transm. 2016, 123, 1069–1083. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Sheridan, M.A.; Winter, W.; Fox, N.A.; Zeanah, C.H.; Nelson, C.A. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to ADHD. Biol. Psychiatry 2014, 76, 629–638. [Google Scholar] [CrossRef]
- Mackes, N.K.; Golm, D.; Sarkar, S.; Kumsta, R.; Rutter, M.; Fairchild, G.; Mehta, M.A.; Sonuga-Barke, E.J.S. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. USA 2020, 117, 641–649. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Weissman, D.; Bitrán, D. Childhood adversity and neural development: A systematic review. Annu. Rev. Dev. Psychol. 2019, 1, 277–312. [Google Scholar] [CrossRef]
- Pannizon, M.S.; Fennena-Notestine, C.; Eyler, L.T.; Prom-Wormley, E.; Neale, M.; Jacobson, K.; Lyons, M.J.; Grant, M.D.; Franz, C.E.; Hong, X.; et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 2009, 19, 2728–2735. [Google Scholar] [CrossRef]
- Henderson, K.E.; Vaidya, J.G.; Kramer, J.R.; Kuperman, S.; Langbehn, D.R.; O’Leary, D.S. Cortical thickness in adolescents with a family history of alcohol use disorder. Alcohol. Clin. Exp. Res. 2018, 42, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.Y. Familial risk for alcohol dependence and brain morphology: The role of cortical thickness across the lifespan. Alcohol. Clin. Exp. Res. 2018, 42, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.C.; Sawchenko, P.E. Do centrally administered neuropeptides access cognate receptors?: An analysis in the central corticotropin releasing factor system. J. Neurosci. 2000, 20, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Segalowitz, S.J.; Santesso, D.L.; Jetha, M.K. Electrophysiological changes during adolescence: A review. Brain Cogn. 2010, 72, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Mathalon, D.H.; Sullivan, E.V.; Rawles, J.M.; Zipursky, R.B.; Lim, K.O. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 1994, 51, 874–887. [Google Scholar] [CrossRef]
- Giedd, J.N.; Rapoport, J.L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron 2010, 67, 728–734. [Google Scholar] [CrossRef]
- Blakemore, S.J. Imaging brain development: The adolescent brain. Neuroimage 2012, 61, 397–406. [Google Scholar] [CrossRef]
- Norbom, L.B.; Ferschmann, L.; Parker, N.; Agartz, I.; Andreassen, O.A.; Pau, T.; Westlye, L.T.; Tannes, C.K. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro- and microstructural MRI findings. Prog. Neurobiol. 2021, 204, 102109. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Stability of brain potentials, mental abilities, and cortical thickness. Neuroreport 2007, 18, 725–728. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B.; Fischl, B.; Reinvang, I. Cognitive function P3a/P3b brain potentials, and cortical aging. Hum. Brain Mapp. 2007, 28, 1098–1116. [Google Scholar] [CrossRef]
- Pergher, V.; Tournoy, J.; Schoenmakers, B.; Van Hulle, M.M. P300, gray matter volume and individual characteristics correlates in healthy elderly. Front. Aging Neurosci. 2019, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Overbye, K.; Hester, R.J.; Walhovd, K.B.; Fjell, A.M.; Tannes, C.K. Development of the P300 from childhood to adulthood: A multimodal EEG and MRI study. Brain Struct. Funct. 2018, 223, 4337–4349. [Google Scholar] [CrossRef]
- Chambers, W.J.; Puig-Antich, J.; Hirsch, M.; Paez, P.; Ambrosini, P.J.; Tabrizi, M.A.; Davies, M. The assessment of affective disorders in children and adolescents by semistructured interview: Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch. Gen. Psychiatry 1985, 42, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.Y.; Shen, S.; Locke-Wellman, J.; Matthews, A.G.; McDermott, M. Psychopathology in offspring from multiplex alcohol dependence families: A prospective study during childhood and adolescence. Psychiatry Res. 2008, 160, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Janca, A.; Robins, L.N.; Cottler, L.B.; Early, T.S. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI Field Trials—Wave II at the St Louis site. Br. J. Psychiatry 1992, 160, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.H.; Fenn, C.B.; Billings, A.G. Life stressors and social resources: An integrated assessment approach. Soc. Sci. Med. 1998, 27, 999–1002. [Google Scholar] [CrossRef]
- Iacono, W.G.; Malone, S.M.; McGue, M. Substance use disorders, externalizing psychopathology, and P300 event related potential amplitude. Int. J. Psychophysiol. 2003, 48, 147–178. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly accurate inverse consistent registration: A robust approach. Neuroimage 2010, 53, 1181–1196. [Google Scholar] [CrossRef]
- Segonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef]
- Fischl, B.; Sereno, M.I.; Dale, A.M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Segonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.J.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.; Makris, N.; Segonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004, 23 (Suppl. 1), S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; van der Kouwe, A.; Destrieux, C.; Halgren, E.; Segonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef]
- Rosas, H.D.; Liu, A.K.; Hersch, S.; Glessner, M.; Ferrante, R.J.; Salat, D.H.; van der Kouwe, A.; Jenkins, B.G.; Dale, A.M.; Fischl, B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 2002, 58, 695–701. [Google Scholar] [CrossRef]
- Kuperberg, G.R.; Broome, M.R.; McGuire, P.K.; David, A.S.; Eddy, M.; Ozawa, F.; Goff, D.; West, W.C.; Williams, S.C.; van der Kouwe, A.J.; et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch. Gen. Psychiatry 2003, 60, 878–888. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef]
- Iacoboni, M.; Molnar-Szakacs, I.; Gallese, V.; Buccino, G.; Mazziota, J.C. Grasping the intentions of others with one’s own mirror neuron system. PLOS Biol. 2005, 3, 0520–0535.e79. [Google Scholar] [CrossRef]
- Leslie, K.R.; Johnson-Frey, S.H.; Grafton, S.T. Functional imaging of face and hand imitation: Towards a motor theory of empathy. Neuroimage 2004, 21, 601–607. [Google Scholar] [CrossRef]
- Curley, W.B.; Newman, E.; Thompson, W.K.; Brown, T.T.; Hagler, D.J.; Akshoomoff, N.; Reuter, C.; Dale, A.M.; Jerigan, T.L. Cortical morphology of the pars opercularis and its relationship to motor-inhibitory performance in a longitudinal, developing cohort. Brain Struc. Funct. 2018, 223, 211–220. [Google Scholar] [CrossRef]
- Nogueira, R.; Abolafia, J.M.; Drugowitsch, J.; Balaguer-Ballester, E.B.; Snchez-Vives, M.V.; Morena-Bote, R. Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat. Commun. 2017, 8, 148223. [Google Scholar] [CrossRef] [PubMed]
- Rudebeck, P.H.; Saunders, R.C.; Lundren, D.A.; Murray, E.A. Specialized representations of value in the orbital and ventrolateral prefrontal cortex: Desirability versus availability of outcomes. Neuron 2017, 95, 120601220. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Ramo-Fernandez, L.; Boeck, C.; Koenig, A.M.; Schury, K.; Binder, E.B.; Gundel, H.; Fegert, J.M.; Karabatslakis, A.; Kolassa, I.-T. The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: An intergenerational approach. Sci. Rep. 2019, 9, 497–508. [Google Scholar] [CrossRef]
- Schartner, C.; Ziegler, C.; Schiele, M.A.; Kollert, L.; Weber, H.; Zwanzer, P.; Arolt, V.; Pauli, P.; Deckert, J.; Reif, A.; et al. CRHR1 promoter hypomethylation: An epigenetic readout of panic disorder? Eur. Neuropsychopharmacol. 2017, 27, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.B.; Zandi, P.P.; Potash, J.B.; Nestadt, G.; Wand, G.S. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology 2013, 227, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Summer, J.A.; McLaughlin, K.A.; Walsh, K.; Sheridan, M.A.; Koenen, K.C. CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology 2014, 43, 71–80. [Google Scholar] [CrossRef]
- Glaser, Y.G.; Zubieta, J.-K.; Hsu, D.T.; Villafuerte, S.; Mickey, B.J.; Trucco, E.M.; Burmeister, M.; Zucker, R.A.; Heitzig, M.M. Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. J. Neurosci. 2014, 34, 4099–4107. [Google Scholar] [CrossRef][Green Version]
- Vetkas, A.; Prans, E.; Koks, S.; Ratsep, T.; Asser, T. Aneurysmal subarachnoid haemorrhage: Effect of CRHR1 genotype on fatigue and depression. BMC Neurol. 2020, 20, 142. [Google Scholar] [CrossRef]
- Pape, J.C.; Carillo-Roa, T.; Rothbaum, B.O.; Nemeroff, C.B.; Czmara, D.; Zannas, A.S.; Iosifescu, D.; Matthew, S.J.; Neylan, T.C.; Mayberg, H.S.; et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin. Epigenetics 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Govorko, O.; Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol. Psychiatry 2012, 72, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gangisetty, O.; Chaudhary, S.; Palagani, A.; Sarkar, D.K. Transgenerational inheritance of fetal alcohol effects on proopiomelancortin gene expression and methylation, cortisol response to stress, and anxiety-like behaviors in offspring for three generations in rats: Evidence for male germline transmission. PLoS ONE 2022, 17, e0263340. [Google Scholar] [CrossRef] [PubMed]

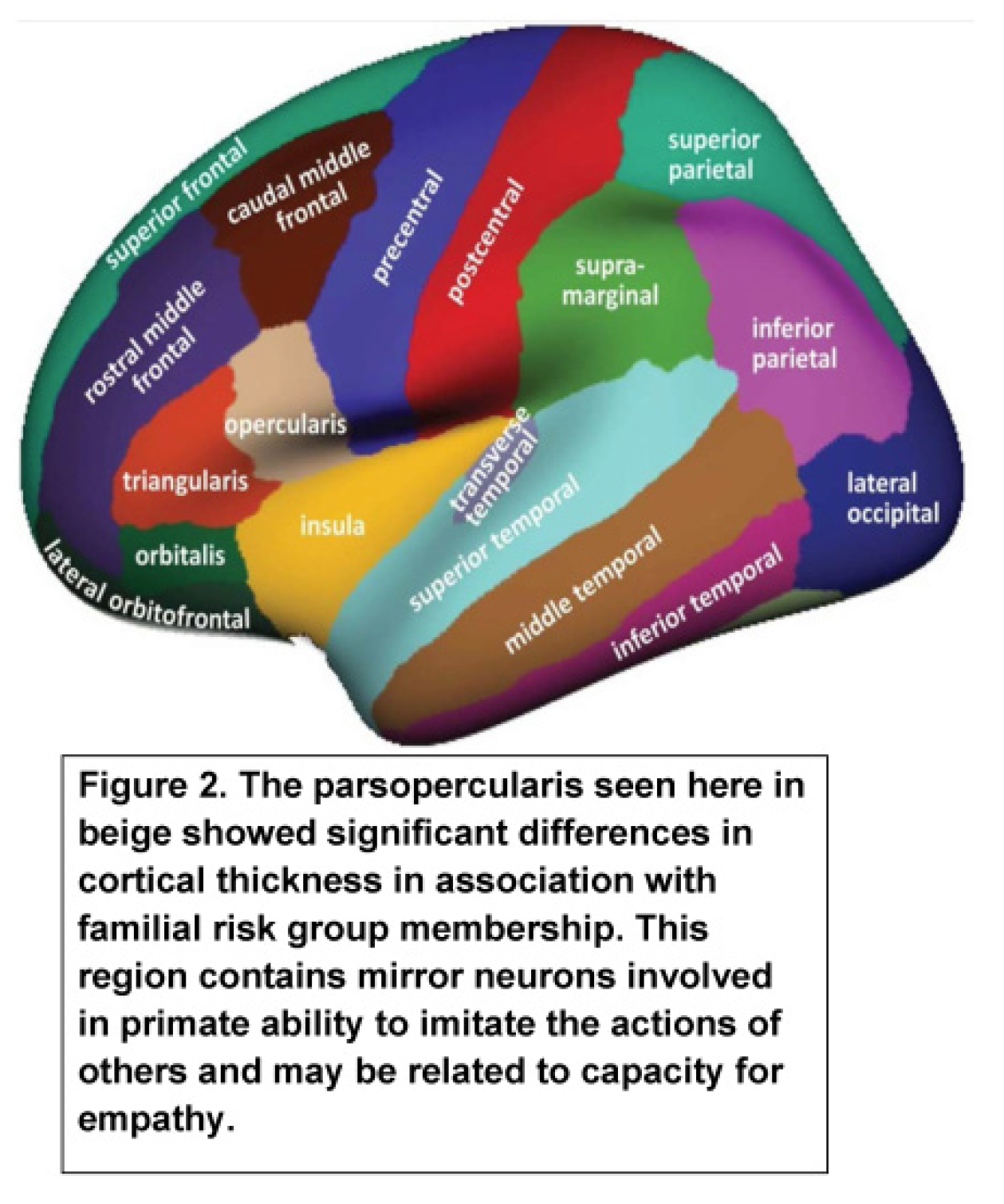
| High-Risk (N=118) 54 Male and 64 Female | Low-Risk (N=99) 59 Males and 40 Females | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | df | p | |
| Age at MRI Scan | 26.18 | 4.47 | 24.13 | 5.15 | 3.15 | 1 | 0.002 |
| Age at Nearest ERP | 25.17 | 4.52 | 23.22 | 5.35 | 2.90 | 1/214 | 0.005 |
| Age at First ERP | 11.29 | 2.71 | 11.15 | 2.43 | 0.37 | 1/197 | NS |
| Age at Last Follow up | 26.90 | 4.68 | 24.98 | 5.06 | 2.91 | 1/215 | 0.004 |
| BMI (at scan age) | 27.89 | 6.27 | 27.41 | 6.15 | 0.56 | 1 | NS |
| SES | 39.92 | 10.55 | 45.70 | 8.87 | 4.32 | 1/215 | 0.019 |
| PPVT (@118 ± 2.6 Years) | 105.57 | 15.79 | 112.28 | 16.95 | 2.86 | 1/194 | 0.005 |
| Number (%) Right-Handed | 113 (95.8%) | 90 (91%) | X2 = 2.10 | 1/215 | NS | ||
| Alcohol or Drug Abuse/Dependence (Lifetime) | 67 | 17 | X2 = 35.60 | 1 | <0.0001 | ||
| Alcohol or Drug Abuse/Dependence < Scan | 64 | 15 | X2 = 35.52 | 1 | <0.0001 | ||
| Left Hemisphere | N1 Cz | N2 Cz | P2 Cz | P3 Pz | N1 Latency | N1 Latency | N2 Latency | P2 Latency | P3 Latency |
|---|---|---|---|---|---|---|---|---|---|
| Frontopole | 0.008 | ||||||||
| Lateral occipital | 0.008 | ||||||||
| Medial orbitofrontal | 0.008 | ||||||||
| Lateral orbitofrontal | 0.002 | ||||||||
| Precuneous | 0.002 | ||||||||
| Rostral Mid Frontal | 0.005 | ||||||||
| Sup Frontal | 0.009 | ||||||||
| Sup parietal | 0.004 | ||||||||
| Temporalpole | 0.003 |
| Right Hemisphere | N1 Cz | N2 Cz | P2 Cz | P3 Pz | N1 Latency | N1 Latency | N2 Latency | P2 Latency | P3 Latency |
|---|---|---|---|---|---|---|---|---|---|
| Inferior Parietal | 0.004 | ||||||||
| Isthmus Cingulate | 0.006 | ||||||||
| Parsopercularis | <0.0001 | ||||||||
| Postcentral | 0.009 | 0.004 | |||||||
| Sup Frontal | 0.009 |
| High Risk | Low risk | ||||
|---|---|---|---|---|---|
| Variable 1 | Variable 2 | R | P Value | R | P Value |
| Lh Frontopole | P3 amp@Pz | 0.037 | NS | 0.329 | 0.001 |
| Lh Lateral Occipital | P2 amp @ Cz | 0.199 | 0.032 | 0.159 | NS |
| Lh Lateraloribitofrontal | P3 amp@Pz | 0.275 | 0.003 | 0.385 | 0.001 |
| Lh Precuneous b | N1 amp @ Cz | 0.000 | NS | -0.373 | <0.001 |
| Lh Rostral Midfrontal | P3 amp @Pz | 0.045 | NS | 0.318 | 0.001 |
| Lh Superior Frontal | P3 amp @Pz | 0.102 | NS | 0.243 | 0.015 |
| Lh Superior Parietal C | N1 amp @ Cz | -0.062 | NS | -0.311 | 0.002 |
| Lh Medial Orbital | P3 latency | 0.149 | NS | 0.185 | 0.067 |
| Lh Tempotalpole d | P2 amp @ Cz | 0.109 | NS | 0.320 | 0.001 |
| Rh Inferior Parietal | P3 latency | 0.207 | 0.025 | 0.154 | NS |
| Rh Isthmus Cingulate | N1 amp @ Cz | -0.182 | 0.049 | -0.171 | NS |
| Rh Parsopercularis | P3 amp @ Pz | 0.179 | 0.053 | 0.304 | 0.002 |
| Rh Postcentral e | P2 amp @ Cz | 0.218 | 0.018 | 0.129 | NS |
| Rh Postcentral f | P3 latency | 0.162 | 0.080 | 0.230 | 0.022 |
| Rh superior Frontal | P3 amp@Pz | 0.129 | NS | 0.225 | 0.025 |
| B | SE | Wald | df | p | Exp(B) | |
|---|---|---|---|---|---|---|
| RH Parsopercularis Thickness | -1.275 | 0.766 | 2.76 | 1 | 0.096 | 0.280 |
| N2_Cz_Closest to Scan | 0.077 | 0.024 | 9.95 | 1 | 0.002 | 1.080 |
| P2_Cz_Closest to Scan | -0.060 | 0.027 | 5.15 | 1 | 0.023 | 0.941 |
| N2 Latency Closest to Scan | 0.008 | 0.003 | 6.16 | 1 | 0.013 | 1.008 |
| N1 Cz Earliest | -0.091 | 0.032 | 8.03 | 1 | 0.005 | 0.913 |
| N2 Latency Earliest | 0.007 | 0.003 | 5.59 | 1 | 0.018 | 1.007 |
| P3 Latency Earliest | -0.003 | 0.002 | 3.92 | 1 | 0.048 | 0.997 |
| High Risk | Low-Risk | t | df | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | MEAN | SD | N | MEAN | SD | ||||
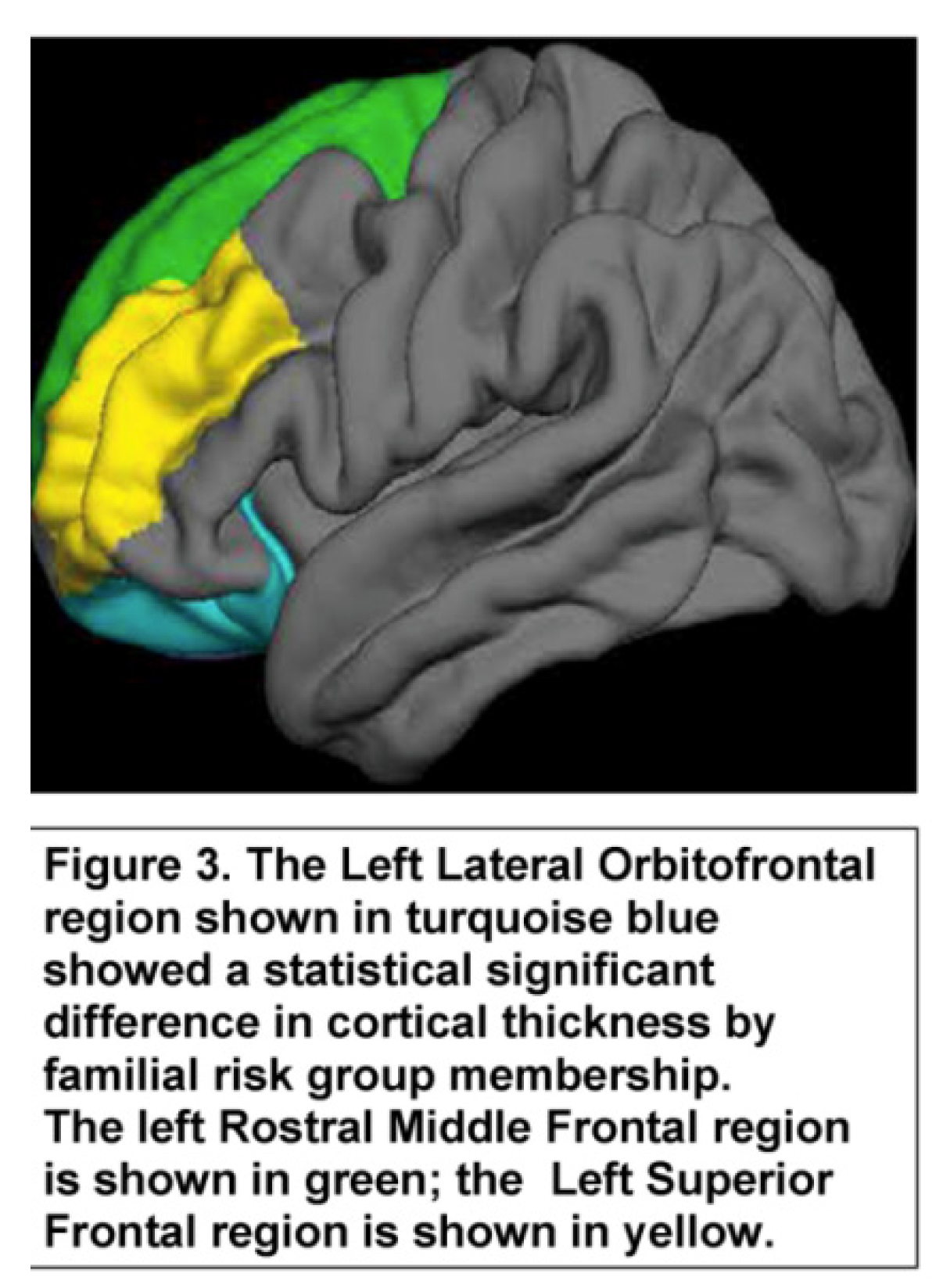
| Lh lateral orbitofrontal | 118 | 2.63 | 0.16 | 99 | 2.69 | 0.17 | -2.74 | 215 | 0.007 |
| Rh parsopercularis | 118 | 2.54 | 0.16 | 99 | 2.60 | 0.16 | -2.64 | 216 | 0.009 |
| CRHR1 Methylation a | 105 | 0.93 | 0.17 | 71 | 0.86 | 0.21 | 2.32 | 126.4 | 0.022 |
| POMC | 99 | 0.273 | 0.05 | 71 | -0.263 | 0.05 | 1.25 | 168 | NS |
| POMC Males | 47 | 0.280 | 0.03 | 45 | 0.254 | 0.06 | 2.75 | 90 | 0.007 |
| POMC Females | 52 | 0.265 | 0.05 | 26 | 0.278 | 0.04 | -1.11 | 76 | NS |
| NLE Close to Sample Collection b | 106 | 50.4 | 10.6 | 72 | 44.7 | 7.5 | 4.17 | 175.8 | <0.001 |
| NLE Before Sample Collection c | 42 | 54.6 | 11.4 | 47 | 46.8 | 7.8 | 3.7 | 71.9 | <0.001 |
| B | SE | Wald | df | p | Exp(B) | |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| CRHR1 Methylation | 19.682 | 10.983 | 3.21 | 1 | 0.073 | 3.53 × 108 |
| LISRES NLE Closest to DNA Collection | 0.54 | 0.011 | 23.46 | 1 | <0.001 | 1.06 |
| Rh Parsopercularis | 6.77 | 3.84 | 3.10 | 1 | 0.078 | 8.69 × 102 |
| CRHR1 Methylation ∗ RhParsopercularis | -8.31 | 4.34 | 3.66 | 1 | 0.056 | <0.001 |
| Model 2 | ||||||
| LISRES NLE Closest to DNA Collection | 0.053 | 0.011 | 24.24 | 1 | <0.001 | 1.05 |
| CRHR1 Methylation ∗ Lh Lateral orbitofrontal | -0.43 | 0.257 | 2.86 | 1 | 0.091 | 0.647 |
| Model 3 | ||||||
| POMC METHYLATION | 26.89 | 10.38 | 6.71 | 1 | 0.01 | 4.78 × 1011 |
| LISRES NLE Closest to DNA Collection | 9/51 | 0.011 | 20.66 | 1 | <0.001 | 1.05 |
| Rh Parsopercularis | 2.28 | 1.16 | 3.84 | 1 | 0.05 | 9.78 |
| SEX | 5.05 | 1.65 | 9.36 | 1 | 0.002 | 155.59 |
| POMC ∗ LISRES NLE Closest to DNA Collextion ∗ SEX ∗ Rh Parsopercularis | -7.73 | 2.42 | 10.23 | 1 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, S.Y.; Wellman, J.L.; Zezza, N.; Steinhauer, S.R.; Sharma, V.; Holmes, B. Epigenetic Effects in HPA Axis Genes Associated with Cortical Thickness, ERP Components and SUD Outcome. Behav. Sci. 2022, 12, 347. https://doi.org/10.3390/bs12100347
Hill SY, Wellman JL, Zezza N, Steinhauer SR, Sharma V, Holmes B. Epigenetic Effects in HPA Axis Genes Associated with Cortical Thickness, ERP Components and SUD Outcome. Behavioral Sciences. 2022; 12(10):347. https://doi.org/10.3390/bs12100347
Chicago/Turabian StyleHill, Shirley Y., Jeannette L. Wellman, Nicholas Zezza, Stuart R. Steinhauer, Vinod Sharma, and Brian Holmes. 2022. "Epigenetic Effects in HPA Axis Genes Associated with Cortical Thickness, ERP Components and SUD Outcome" Behavioral Sciences 12, no. 10: 347. https://doi.org/10.3390/bs12100347
APA StyleHill, S. Y., Wellman, J. L., Zezza, N., Steinhauer, S. R., Sharma, V., & Holmes, B. (2022). Epigenetic Effects in HPA Axis Genes Associated with Cortical Thickness, ERP Components and SUD Outcome. Behavioral Sciences, 12(10), 347. https://doi.org/10.3390/bs12100347




