Differentiating Individuals with and without Alcohol Use Disorder Using Resting-State fMRI Functional Connectivity of Reward Network, Neuropsychological Performance, and Impulsivity Measures
Abstract
1. Introduction
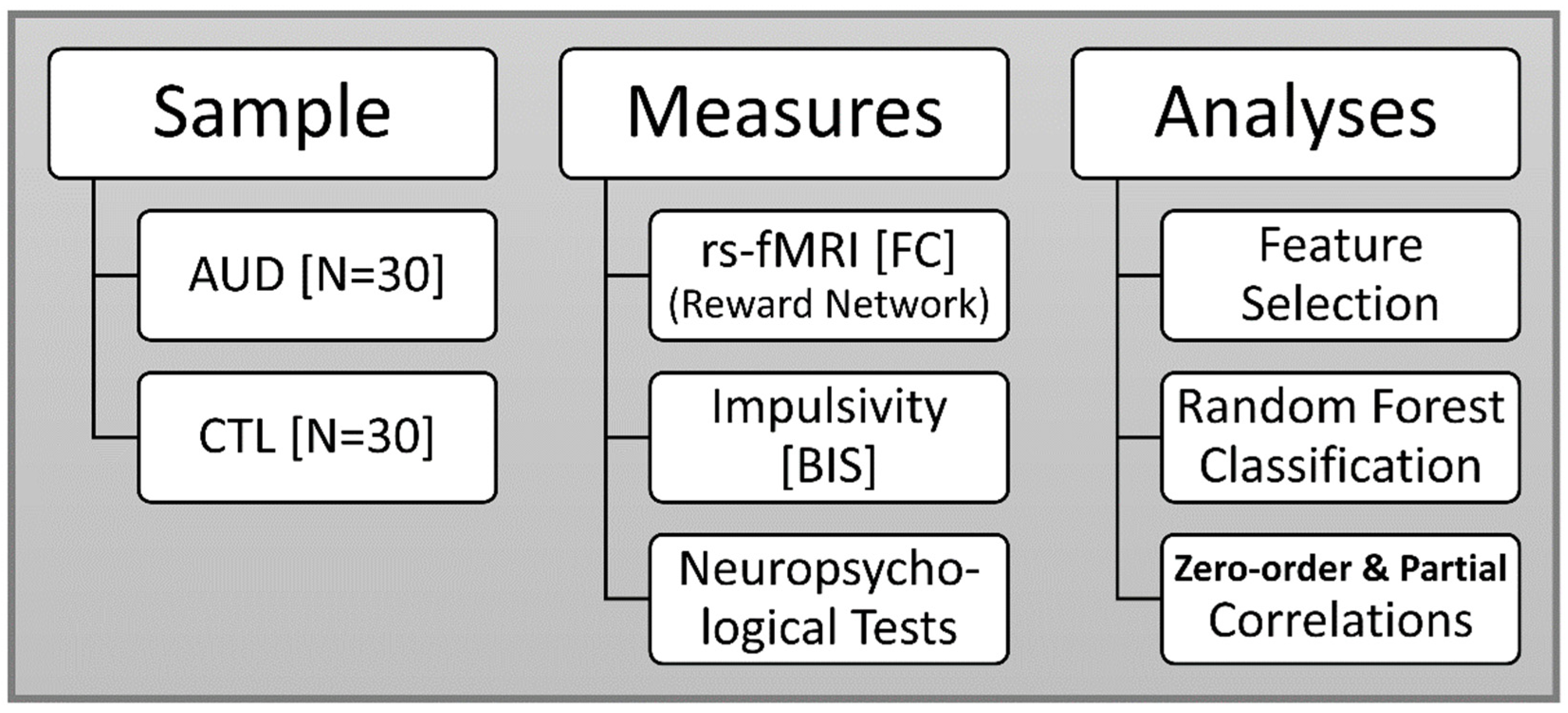
2. Materials and Methods
2.1. Sample
2.2. Neuropsychological Assessment
2.2.1. Tower of London Test (TOLT)
2.2.2. Visual Span Test (VST)
2.3. Impulsivity Scores
2.4. MRI Data Acquisition
2.5. Image Processing
2.6. Reward Network Seed Regions and rsFC Calculations
2.7. Feature Selection of FC Variables
2.8. Random Forest Classification
3. Results
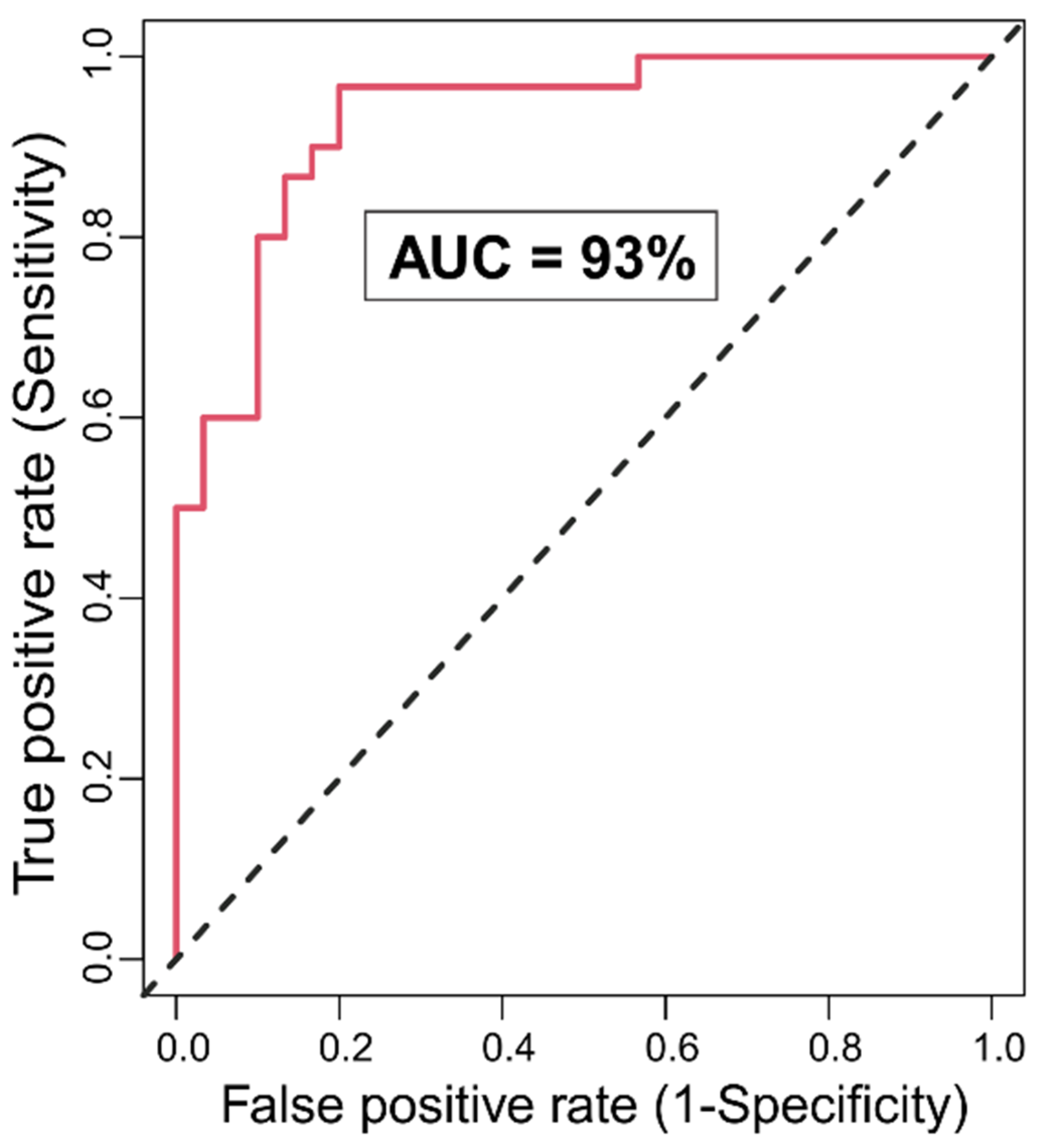
3.1. Random Forests Classification
3.1.1. Classification Accuracy and Top Significant Variables
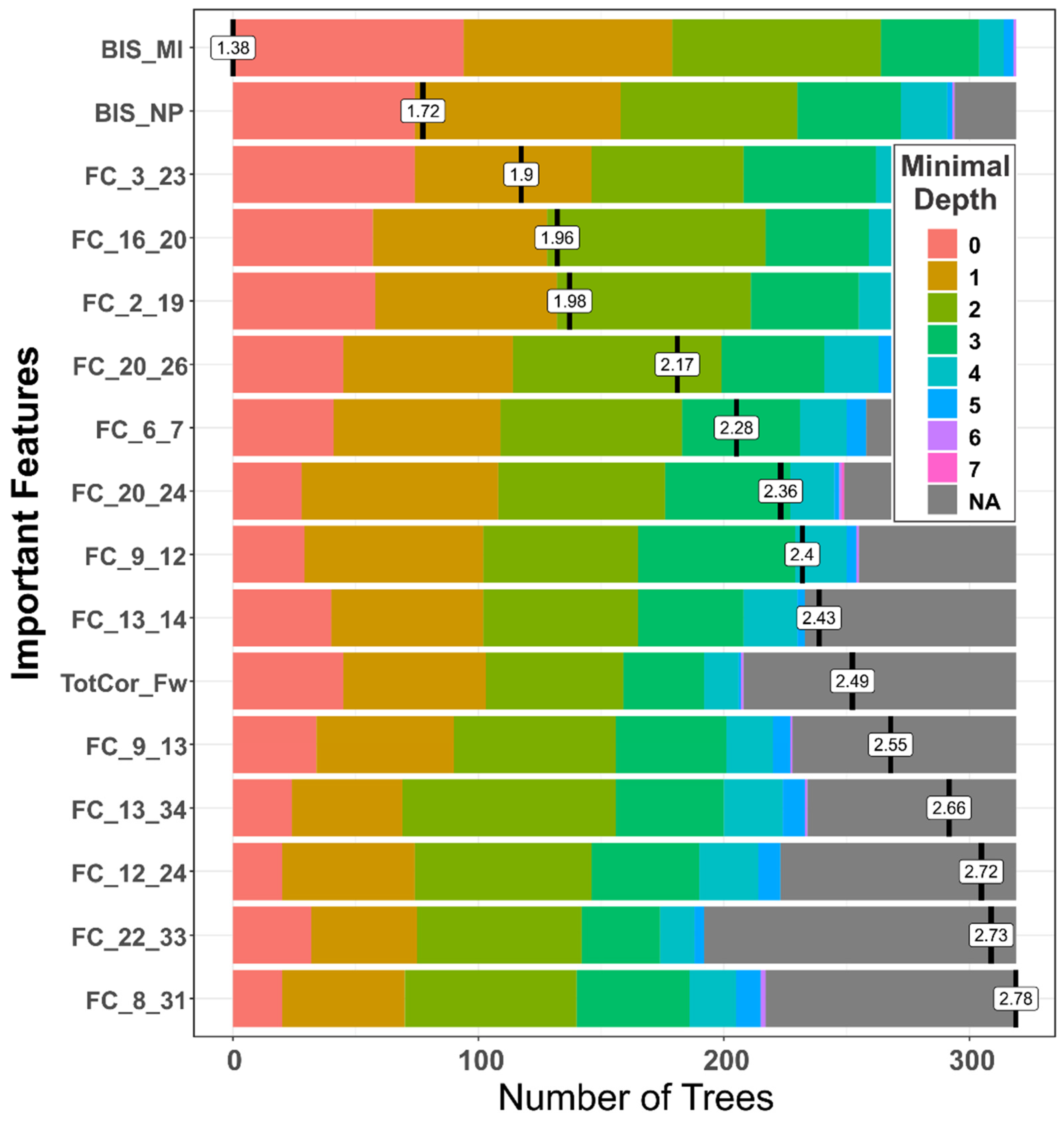
3.1.2. Distribution of Minimal Depth
3.1.3. Multi-Way Importance
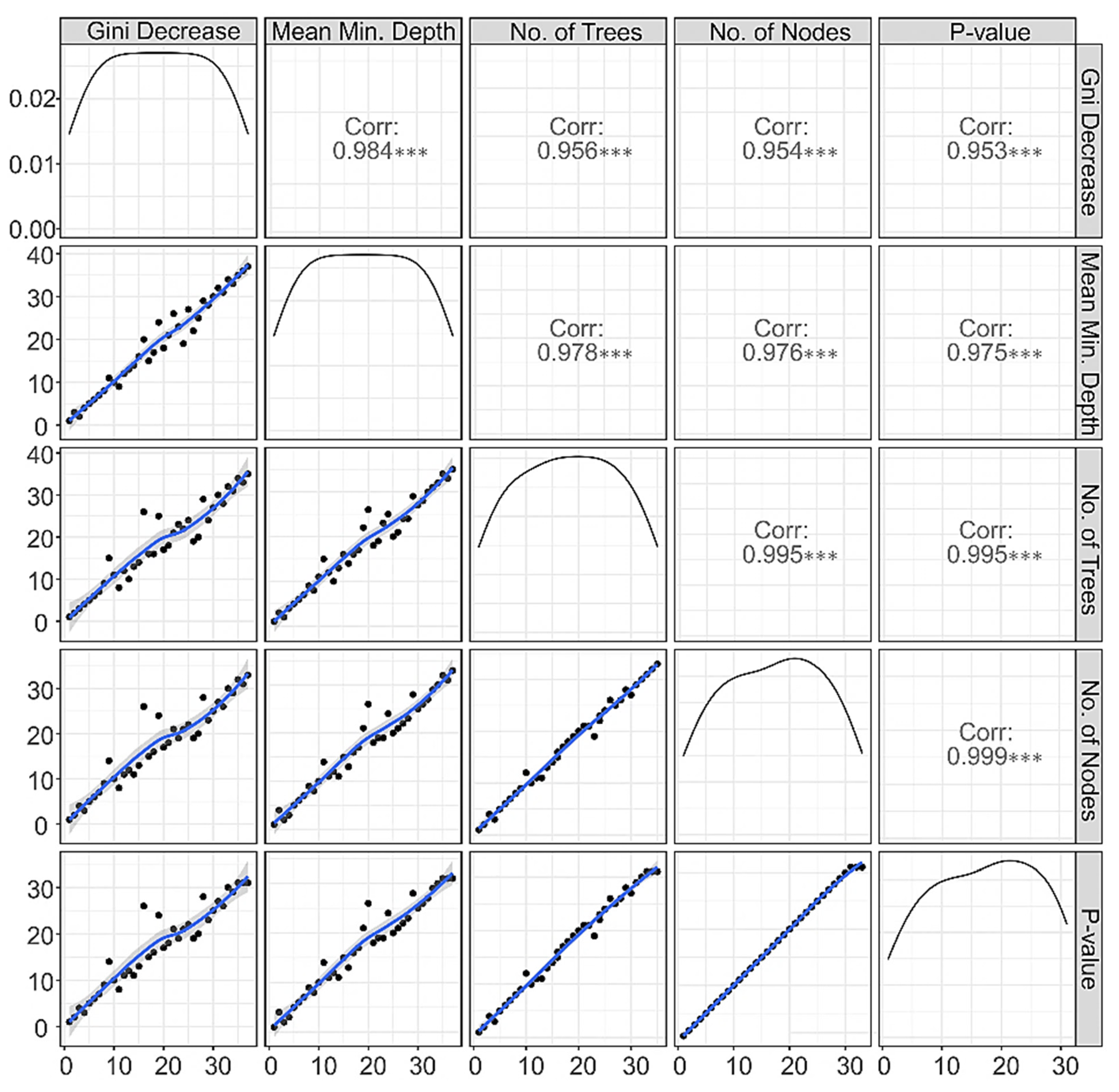
3.1.4. Correlations among Rankings of RF Parameters
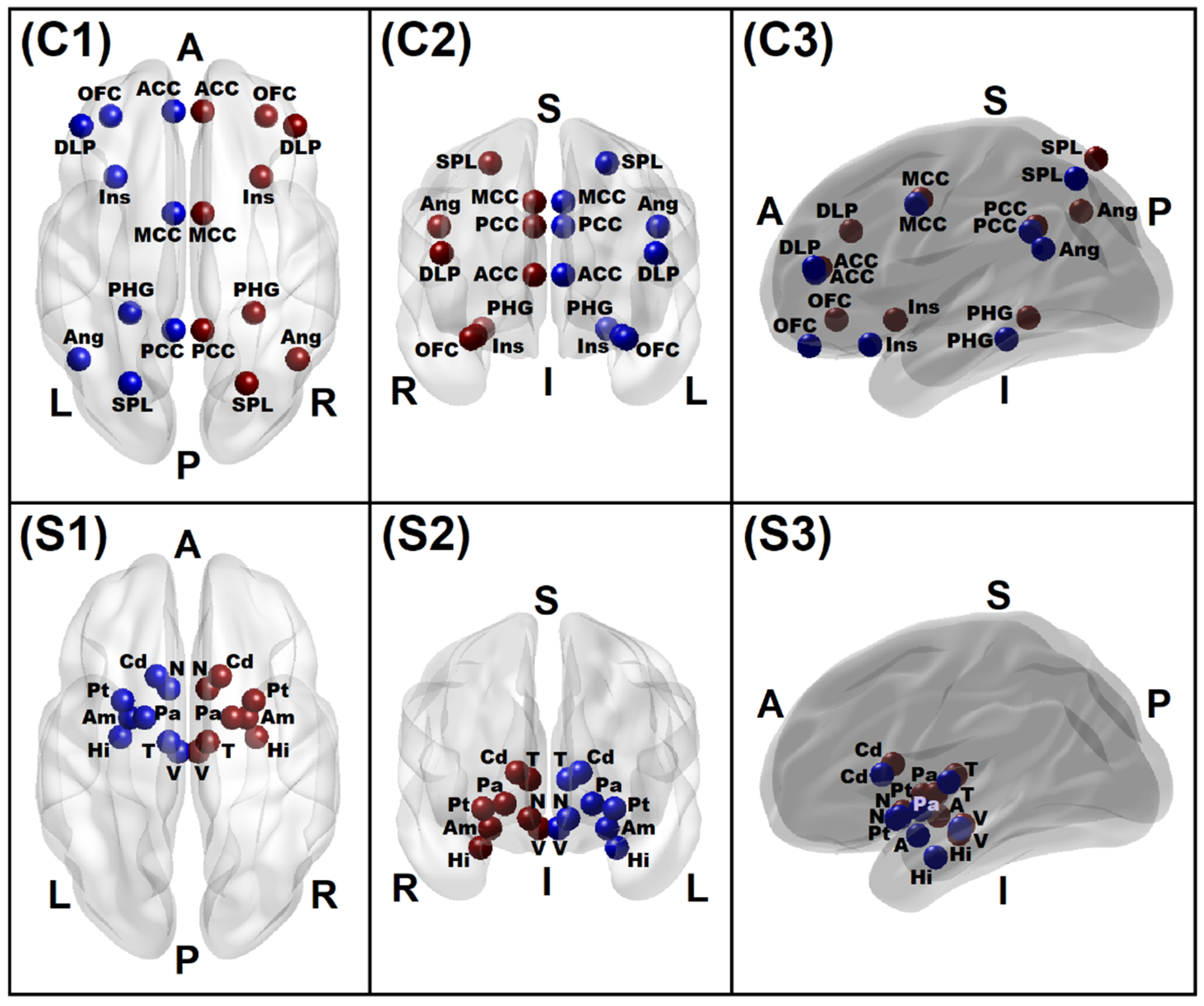
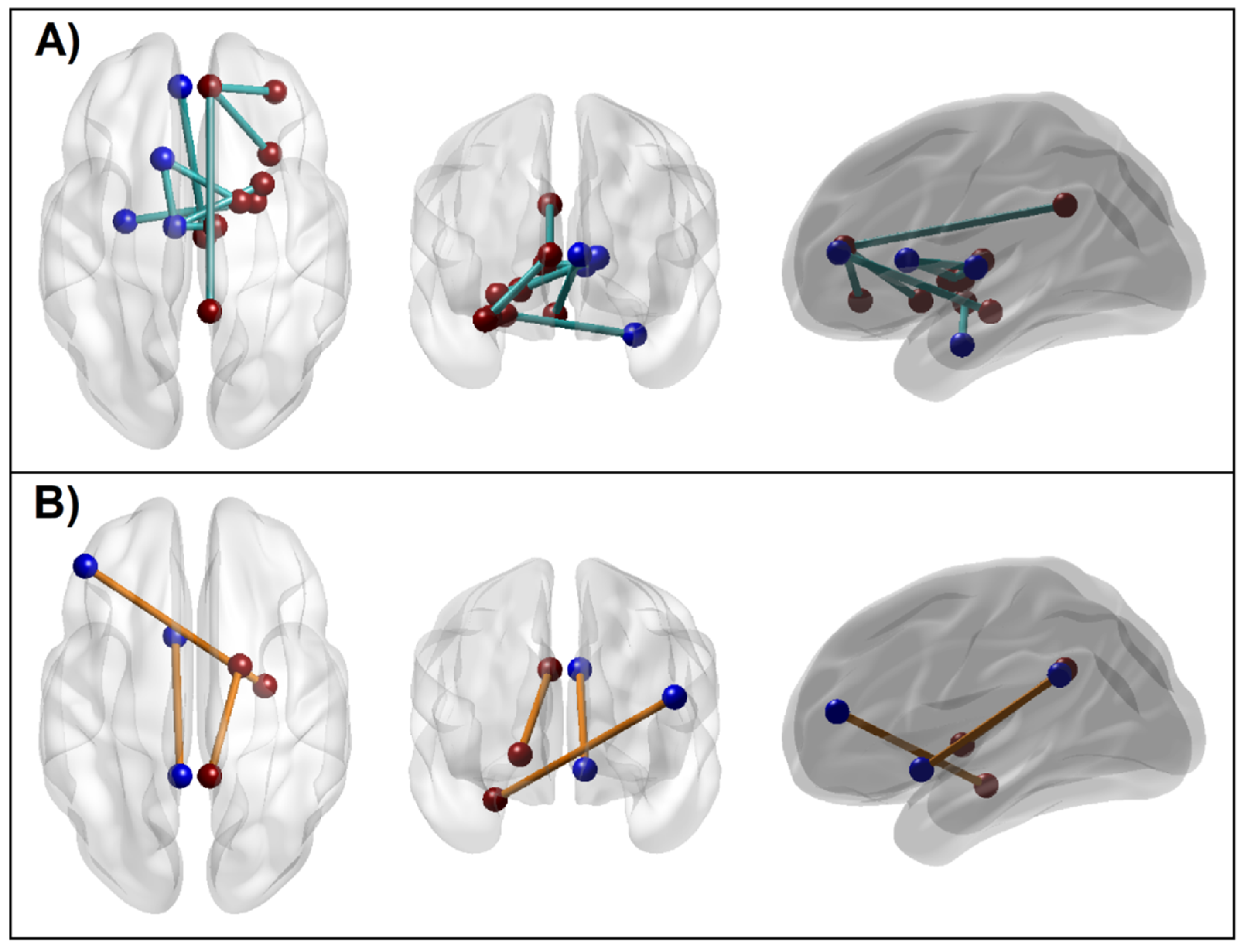
3.1.5. Connectivity Mapping of Significant rsFC Connections
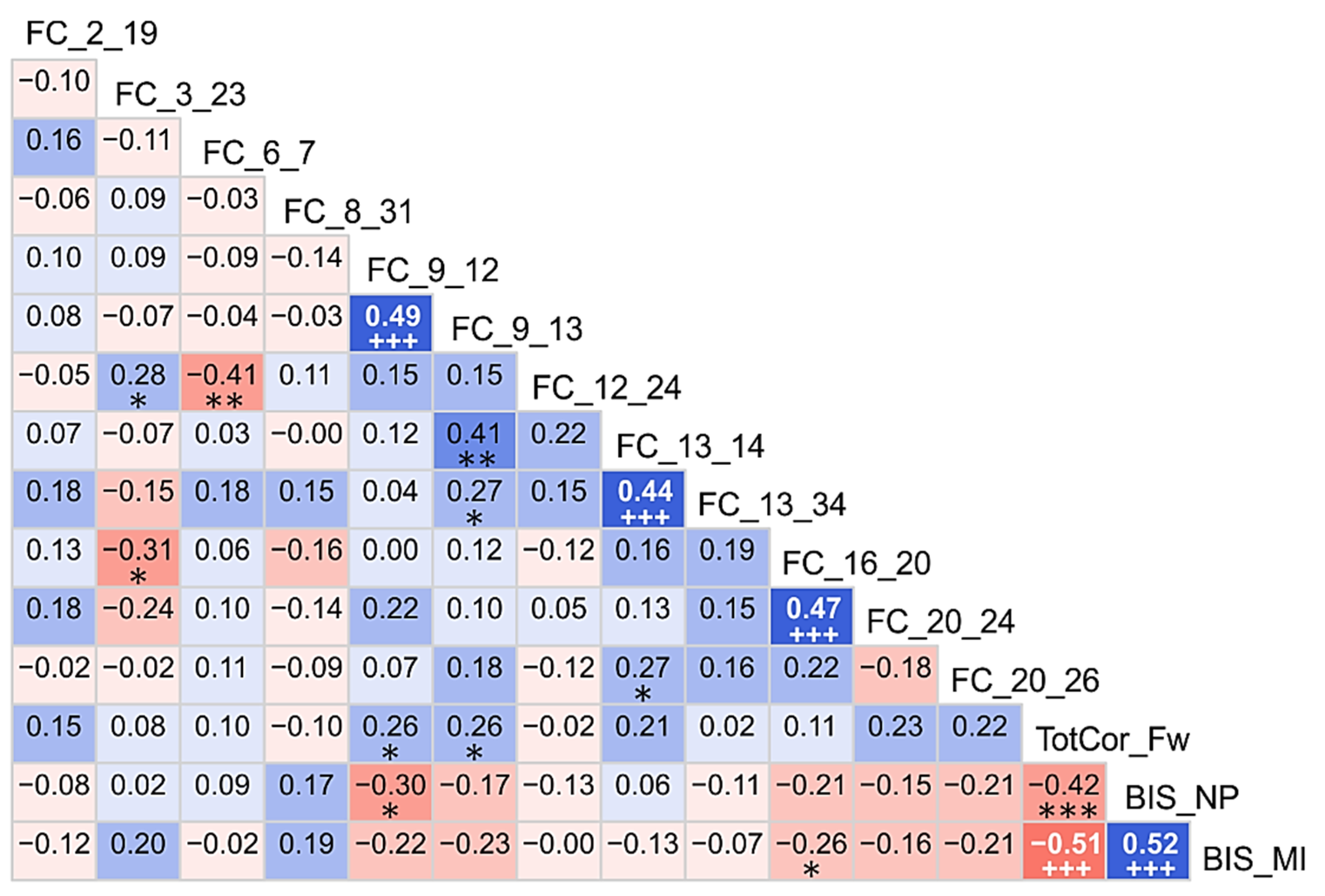
3.2. Correlations among the Top Significant Variables
3.3. Correlations between Significant Variables and Age
3.4. Neuropsychological Scores between the Groups
4. Discussion
4.1. Altered Functional Connectivity across Reward Network in AUD Individuals
4.2. Heightened Impulsivity in AUD Individuals
4.3. Poorer Memory Span in AUD Individuals
4.4. Correlations of Significant Variables among Themselves and with Age
4.5. Limitations of the Current Study
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| F# | Feature | Detail |
|---|---|---|
| 1. | FC_2_19 (R.VTA–L.ACC) | FC between R. Ventral Tegmental Area and L. Anterior Cingulate Cortex |
| 2. | FC_3_23 (L.NAc–L.PCC) | FC between L. Nucleus Accumbens and L. Posterior Cingulate Cortex |
| 3. | FC_6_11 (R.Amg–L.Pal) | FC between R. Amygdala and L. Pallidum |
| 4. | FC_6_7 (R.Amg–L.Hip) | FC between R. Amygdala and L. Hippocampus |
| 5. | FC_8_31 (R.Hip–L.DLP) | FC between R. Hippocampus and L. Dorsolateral Prefrontal Cortex |
| 6. | FC_9_12 (L.Cdt–R.Pal) | FC between L. Caudate and R. Pallidum |
| 7. | FC_9_13 (L.Cdt–L.Tha) | FC between L. Caudate and L. Thalamus |
| 8. | FC_9_18 (L.Cdt–R.PHG) | FC between L. Caudate and R. Parahippocampal Gyrus |
| 9. | FC_9_23 (L.Cdt–L.PCC) | FC between L. Caudate and L. Posterior Cingulate Cortex |
| 10. | FC_9_27 (L.Cdt–L.Ang) | FC between L. Caudate and L. Angular Gyrus |
| 11. | FC_12_24 (R.Pal–R.PCC) | FC between R. Pallidum and R. Posterior Cingulate Cortex |
| 12. | FC_13_14 (L.Tha–R.Tha) | FC between L. Thalamus and R. Thalamus |
| 13. | FC_13_34 (L.Tha–R.Ptm) | FC between L. Thalamus and R. Putamen |
| 14. | FC_16_20 (R.Ins–R.ACC) | FC between R. Insula and R. Anterior Cingulate Cortex |
| 15. | FC_17_31 (L.PHG–L.DLP) | FC between L. Parahippocampal Gyrus and L. Dorsolateral Prefrontal Cortex |
| 16. | FC_20_24 (R.ACC–R.PCC) | FC between R. Anterior Cingulate Cortex and R. Posterior Cingulate Cortex |
| 17. | FC_20_26 (R.ACC–R.OFC) | FC between R. Anterior Cingulate Cortex and R. Orbitofrontal Cortex |
| 18. | FC_20_31 (R.ACC–L.DLP) | FC between R. Anterior Cingulate Cortex and L. Dorsolateral Prefrontal Cortex |
| 19. | FC_21_24 (L.MCC–R.PCC) | FC between L. Middle Cingulate Cortex and R. Posterior Cingulate Cortex |
| 20. | FC_22_33 (R.MCC–L.Ptm) | FC between R. Middle Cingulate Cortex and L. Putamen |
| 21. | FC_28_29 (L.Ang–L.SPL) | FC between L. Angular Gyrus and L. Superior Parietal Lobule |
| 22. | ExcMovMade_All | Overall excess moves beyond the minimum moves required to solve the puzzle |
| 23. | AvgPicTime_All | Overall average pickup time to solve the puzzle |
| 24. | AvgTotTime_All | Overall average total time to solve the puzzle |
| 25. | TotTrlTime_All | Overall total trial time within each puzzle type |
| 26. | AvgTrlTime_All | Overall average trial time across trials per puzzle type |
| 27. | TotCor_Fw | Total number of correctly performed trials in forward sequence |
| 28. | TotCor_Bw | Total number of correctly performed trials in backward sequence |
| 29. | Span_Fw | Maximum span or sequence-length achieved in forward sequence |
| 30. | Span_Bw | Maximum span or sequence-length achieved in backward sequence |
| 31. | TotAvgTime_Fw | Total average time taken across all trials performed in forward sequence |
| 32. | TotAvgTime_Bw | Total average time taken across all trials performed in backward sequence |
| 33. | TotCorAvgTime_Fw | Total correct average time taken across all correct trials in forward sequence |
| 34. | TotCorAvgTime_Bw | Total correct average time taken across all correct trials in backward sequence |
| 35. | BIS_AI | Barratt Impulsiveness Scale, Attentional Impulsivity Score |
| 36. | BIS_MI | Barratt Impulsiveness Scale, Motor Impulsivity Score |
| 37. | BIS_NP | Barratt Impulsiveness Scale, Non-planning Impulsivity Score |
References
- Volkow, N.D.; Michaelides, M.; Baler, R. The Neuroscience of Drug Reward and Addiction. Physiol. Rev. 2019, 99, 2115–2140. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Luijten, M.; Schellekens, A.F.; Kuhn, S.; Machielse, M.W.; Sescousse, G. Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 2017, 74, 387–398. [Google Scholar] [CrossRef]
- Heinz, A.; Beck, A.; Grusser, S.M.; Grace, A.A.; Wrase, J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol. 2009, 14, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Liu, X.; Hairston, J.; Schrier, M.; Fan, J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011, 35, 1219–1236. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Telang, F. Addiction: Beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, 15037–15042. [Google Scholar] [CrossRef]
- Porjesz, B.; Begleiter, H.; Bihari, B.; Kissin, B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol 1987, 4, 283–287. [Google Scholar] [CrossRef]
- Bowirrat, A.; Oscar-Berman, M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 132B, 29–37. [Google Scholar] [CrossRef]
- Wrase, J.; Schlagenhauf, F.; Kienast, T.; Wustenberg, T.; Bermpohl, F.; Kahnt, T.; Beck, A.; Strohle, A.; Juckel, G.; Knutson, B.; et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 2007, 35, 787–794. [Google Scholar] [CrossRef]
- Makris, N.; Oscar-Berman, M.; Jaffin, S.K.; Hodge, S.M.; Kennedy, D.N.; Caviness, V.S.; Marinkovic, K.; Breiter, H.C.; Gasic, G.P.; Harris, G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry 2008, 64, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Diekhof, E.K.; Falkai, P.; Gruber, O. Functional neuroimaging of reward processing and decision-making: A review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res. Rev. 2008, 59, 164–184. [Google Scholar] [CrossRef] [PubMed]
- De Greck, M.; Supady, A.; Thiemann, R.; Tempelmann, C.; Bogerts, B.; Forschner, L.; Ploetz, K.V.; Northoff, G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics—A fMRI study. Hum. Brain Mapp. 2009, 30, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C.; Rangaswamy, M.; Manz, N.; Chorlian, D.B.; Pandey, A.K.; Roopesh, B.N.; Porjesz, B. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum. Brain Mapp. 2012, 33, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, C.; Rangaswamy, M.; Tang, Y.; Chorlian, D.B.; Pandey, A.K.; Roopesh, B.N.; Manz, N.; Saunders, R.; Stimus, A.T.; Porjesz, B. Dysfunctional reward processing in male alcoholics: An ERP study during a gambling task. J. Psychiatr. Res. 2010, 44, 576–590. [Google Scholar] [CrossRef]
- Dennis, L.E.; Kohno, M.; McCready, H.D.; Schwartz, D.L.; Schwartz, B.; Lahna, D.; Nagel, B.J.; Mitchell, S.H.; Hoffman, W.F. Neural correlates of reward magnitude and delay during a probabilistic delay discounting task in alcohol use disorder. Psychopharmacology 2020, 237, 263–278. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Sutherland, M.T.; McHugh, M.J.; Pariyadath, V.; Stein, E.A. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 2012, 62, 2281–2295. [Google Scholar] [CrossRef]
- Di Chiara, G.; Bassareo, V.; Fenu, S.; De Luca, M.A.; Spina, L.; Cadoni, C.; Acquas, E.; Carboni, E.; Valentini, V.; Lecca, D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology 2004, 47 (Suppl. S1), 227–241. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox. Res. 2008, 14, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, Z.; Tong, E.; Williams, L.M.; Zaharchuk, G.; Zeineh, M.; Goldstein-Piekarski, A.N.; Ball, T.M.; Liao, C.; Wintermark, M. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. AJNR Am. J. Neuroradiol. 2018, 39, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M. Resting-State Functional Connectivity in the Human Connectome Project: Current Status and Relevance to Understanding Psychopathology. Harv. Rev. Psychiatry 2017, 25, 209–217. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Barch, D.M. Brain network interactions in health and disease. Trends Cogn. Sci. 2013, 17, 603–605. [Google Scholar] [CrossRef][Green Version]
- Mehta, U.M.; Ibrahim, F.A.; Sharma, M.S.; Venkatasubramanian, G.; Thirthalli, J.; Bharath, R.D.; Bolo, N.R.; Gangadhar, B.N.; Keshavan, M.S. Resting-state functional connectivity predictors of treatment response in schizophrenia—A systematic review and meta-analysis. Schizophr. Res. 2021, 237, 153–165. [Google Scholar] [CrossRef]
- Zhang, R.; Volkow, N.D. Brain default-mode network dysfunction in addiction. Neuroimage 2019, 200, 313–331. [Google Scholar] [CrossRef]
- Wilcox, C.E.; Abbott, C.C.; Calhoun, V.D. Alterations in resting-state functional connectivity in substance use disorders and treatment implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 79–93. [Google Scholar] [CrossRef]
- Camchong, J.; Stenger, A.; Fein, G. Resting-state synchrony in long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2013, 37, 75–85. [Google Scholar] [CrossRef]
- Camchong, J.; Stenger, A.; Fein, G. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb. Cortex 2013, 23, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; Stenger, V.A.; Fein, G. Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcohol. Clin. Exp. Res. 2013, 37, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Camchong, J.; Stenger, V.A.; Fein, G. Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug Alcohol Depend. 2013, 131, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kong, L.; Chen, L.; Zhang, H.; Zheng, W. Acute effects of alcohol on the human brain: A resting-state FMRI study. Biomed. Res. Int. 2015, 2015, 947529. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ide, J.S.; Chao, H.H.; Zhornitsky, S.; Fischer, K.A.; Wang, W.; Zhang, S.; Li, C.R. Resting state functional connectivity of the amygdala and problem drinking in non-dependent alcohol drinkers. Drug Alcohol Depend. 2018, 185, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cortes, C.R.; Mathur, K.; Tomasi, D.; Momenan, R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: A resting-state study. Addict. Biol. 2017, 22, 206–217. [Google Scholar] [CrossRef]
- Le Berre, A.P.; Fama, R.; Sullivan, E.V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef]
- Fein, G.; Bachman, L.; Fisher, S.; Davenport, L. Cognitive impairments in abstinent alcoholics. West. J. Med. 1990, 152, 531–537. [Google Scholar]
- Shear, P.K.; Jernigan, T.L.; Butters, N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol. Clin. Exp. Res. 1994, 18, 172–176. [Google Scholar] [CrossRef]
- Parsons, O.A. Neuropsychological deficits in alcoholics: Facts and fancies. Alcohol. Clin. Exp. Res. 1977, 1, 51–56. [Google Scholar] [CrossRef]
- Parsons, O.A.; Butters, N.; Nathan, P.E. (Eds.) Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment; The Guilford Press: New York, NY, USA, 1987; p. 414. [Google Scholar]
- Tarter, R.E.; Ryan, C.M. Neuropsychology of alcoholism. Etiology, phenomenology, process, and outcome. Recent Dev. Alcohol. 1983, 1, 449–469. [Google Scholar] [PubMed]
- Oscar-Berman, M. Neuropsychological consequences of long-term chronic alcoholism. Am. Sci. 1980, 68, 410–419. [Google Scholar] [PubMed]
- Oscar-Berman, M. Neuropsychological Vulnerabilities in Chronic Alcoholism. In Review of NIAAA’s Neuroscience and Behavioral Research Portfolio National Institute on Alcohol Abuse and Alcoholism (NIAAA) Research Monograph No 34; Noronha, A., Eckardt, M.J., Warren, K., Eds.; NIAAA: Bethesda, MD, USA, 2000; pp. 437–471. [Google Scholar]
- Day, A.M.; Kahler, C.W.; Ahern, D.C.; Clark, U.S. Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Curr. Drug Abus. Rev. 2015, 8, 26–40. [Google Scholar] [CrossRef]
- Chen, A.C.; Porjesz, B.; Rangaswamy, M.; Kamarajan, C.; Tang, Y.; Jones, K.A.; Chorlian, D.B.; Stimus, A.T.; Begleiter, H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol. Clin. Exp. Res. 2007, 31, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.J.; Luty, J.; Bogdan, N.A.; Sahakian, B.J.; Clark, L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology 2009, 207, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Aragues, M.; Jurado, R.; Quinto, R.; Rubio, G. Laboratory paradigms of impulsivity and alcohol dependence: A review. Eur. Addict. Res. 2011, 17, 64–71. [Google Scholar] [CrossRef]
- Koob, G.F.; Rassnick, S.; Heinrichs, S.; Weiss, F. Alcohol, the reward system and dependence. EXS 1994, 71, 103–114. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Swanson, J.M. Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol. Psychiatry 2004, 9, 557–569. [Google Scholar] [CrossRef]
- Koob, G.F. Dynamics of neuronal circuits in addiction: Reward, antireward, and emotional memory. Pharmacopsychiatry 2009, 42 (Suppl. S1), S32–S41. [Google Scholar] [CrossRef]
- Fede, S.J.; Grodin, E.N.; Dean, S.F.; Diazgranados, N.; Momenan, R. Resting state connectivity best predicts alcohol use severity in moderate to heavy alcohol users. Neuroimage Clin. 2019, 22, 101782. [Google Scholar] [CrossRef]
- Zhu, X.; Du, X.; Kerich, M.; Lohoff, F.W.; Momenan, R. Random forest based classification of alcohol dependence patients and healthy controls using resting state MRI. Neurosci. Lett. 2018, 676, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Ardekani, B.A.; Kamarajan, C.; Zhang, J.; Chorlian, D.B.; Byrne, K.N.; Pandey, G.; Meyers, J.L.; Kinreich, S.; Stimus, A.; et al. Lower Prefrontal and Hippocampal Volume and Diffusion Tensor Imaging Differences Reflect Structural and Functional Abnormalities in Abstinent Individuals with Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2018, 42, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, K.K.; Cadoret, R.; Cloninger, C.R.; Dinwiddie, S.H.; Hesselbrock, V.M.; Nurnberger, J.I., Jr.; Reich, T.; Schmidt, I.; Schuckit, M.A. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J. Stud. Alcohol. 1994, 55, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Davis, H.P.; Keller, F. Colorado Assessment Tests (CATs), Version 1.2; Springs: Colorado, CO, USA, 2002. [Google Scholar]
- Shallice, T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982, 298, 199–209. [Google Scholar] [CrossRef]
- Berch, D.B.; Krikorian, R.; Huha, E.M. The Corsi block-tapping task: Methodological and theoretical considerations. Brain Cogn. 1998, 38, 317–338. [Google Scholar] [CrossRef]
- Milner, B. Interhemispheric differences in the localization of psychological processes in man. Br. Med. Bull. 1971, 27, 272–277. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Stanford, M.S.; Mathias, C.W.; Dougherty, D.M.; Lake, S.L.; Anderson, N.E.; Patton, J.H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers. Individ. Differ. 2009, 47, 385–395. [Google Scholar] [CrossRef]
- Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Kinreich, S.; Pandey, G.; Chorlian, D.B.; Meyers, J.L.; Zhang, J.; Bermudez, E.; Stimus, A.T.; et al. Random Forest Classification of Alcohol Use Disorder Using fMRI Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Brain. Sci. 2020, 10, 115. [Google Scholar] [CrossRef]
- Ardekani, B.A.; Braun, M.; Hutton, B.F.; Kanno, I.; Iida, H. A fully automatic multimodality image registration algorithm. J. Comput. Assist. Tomogr. 1995, 19, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Ardekani, B.A.; Bappal, A.; D’Angelo, D.; Ashtari, M.; Lencz, T.; Szeszko, P.R.; Butler, P.D.; Javitt, D.C.; Lim, K.O.; Hrabe, J.; et al. Brain morphometry using diffusion-weighted magnetic resonance imaging: Application to schizophrenia. Neuroreport 2005, 16, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009, 46, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Davis, B.; Jomier, M.; Gerig, G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage 2004, 23 (Suppl. S1), S151–S160. [Google Scholar] [CrossRef] [PubMed]
- Koay, C.G.; Carew, J.D.; Alexander, A.L.; Basser, P.J.; Meyerand, M.E. Investigation of anomalous estimates of tensor-derived quantities in diffusion tensor imaging. Magn. Reson. Med. 2006, 55, 930–936. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Functional connectivity of substantia nigra and ventral tegmental area: Maturation during adolescence and effects of ADHD. Cereb. Cortex 2014, 24, 935–944. [Google Scholar] [CrossRef]
- Flannery, J.S.; Riedel, M.C.; Bottenhorn, K.L.; Poudel, R.; Salo, T.; Hill-Bowen, L.D.; Laird, A.R.; Sutherland, M.T. Meta-analytic clustering dissociates brain activity and behavior profiles across reward processing paradigms. Cogn. Affect Behav. Neurosci. 2020, 20, 215–235. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Yang, G.; Gerig, G. ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 3342–3345. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Dansereau, C.; Benhajali, Y.; Risterucci, C.; Pich, E.M.; Orban, P.; Arnold, D.; Bellec, P. Statistical power and prediction accuracy in multisite resting-state fMRI connectivity. Neuroimage 2017, 149, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Wang, Y.; Nguyen, H.N. Random forest classifier combined with feature selection for breast cancer diagnosis and prognostic. J. Biomed. Sci. Eng. 2013, 6, 551–560. [Google Scholar] [CrossRef]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Cai, J.; Luo, J.W.; Wang, S.L.; Yang, S. Feature selection in machine learning: A new perspective. Neurocomputing 2018, 300, 70–79. [Google Scholar] [CrossRef]
- Kamala, R.F.; Thangaiah, P.R.J. A Novel Two-Stage Selection of Feature Subsets in Machine Learning. Eng. Technol. Appl. Sci. Res. 2019, 9, 4169–4175. [Google Scholar] [CrossRef]
- Raj, S.; Singh, S.; Kumar, A.; Sarkar, S.; Pradhan, C. Feature Selection and Random Forest Classification for Breast Cancer Disease. In Data Analytics in Bioinformatics; Wiley: Hoboken, NJ, USA, 2021; pp. 191–210. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Simon, N.; Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef]
- Tibshirani, R.; Bien, J.; Friedman, J.; Hastie, T.; Simon, N.; Taylor, J.; Tibshirani, R.J. Strong rules for discarding predictors in lasso-type problems. J. R. Stat. Soc. Ser. B Stat. Methodol. 2012, 74, 245–266. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Rohini, R. LASSO: A Feature Selection Technique in Predictive Modeling for Machine Learning. In Proceedings of the IEEE International Conference on Advances in Computer Applications (ICACA), Coimbatore, India, 24 October 2016; pp. 18–20. [Google Scholar]
- Fonti, V.; Belitser, E. Feature Selection Using LASSO. 2017. Available online: https://beta.vu.nl/nl/Images/werkstuk-fonti_tcm235-836234.pdf (accessed on 1 June 2019).
- Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Chorlian, D.B.; Kinreich, S.; Pandey, G.; Meyers, J.L.; Zhang, J.; Kuang, W.; Stimus, A.T.; et al. Random Forest Classification of Alcohol Use Disorder Using EEG Source Functional Connectivity, Neuropsychological Functioning, and Impulsivity Measures. Behav. Sci. 2020, 10, 62. [Google Scholar] [CrossRef]
- Breiman, L.; Cutler, A. Random Forest. Available online: https://www.stat.berkeley.edu/~breiman/RandomForests/cc_home.htm#ooberr (accessed on 1 June 2019).
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. London B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. Neurocircuitry of Addiction. In Neuropsychopharmacology—The fifth Generation of Progress; Davis, K.L., Charney, D., Coyle, J.T., Nemeroff, C., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2002; Volume 17, pp. 1357–1366. [Google Scholar]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Resad, S. Resting State Functional Connectivity in Addiction: Drug Abuse and Reward Dysregulation; Boston University: Boston, MA, USA, 2017. [Google Scholar]
- Chanraud, S.; Pitel, A.L.; Pfefferbaum, A.; Sullivan, E.V. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb. Cortex 2011, 21, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Muller-Oehring, E.M.; Jung, Y.C.; Pfefferbaum, A.; Sullivan, E.V.; Schulte, T. The Resting Brain of Alcoholics. Cereb. Cortex 2015, 25, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.J.; Ma, L.; Bjork, J.M.; Hammond, C.J.; Zhou, Y.; Snyder, A.; Moeller, F.G. Altered effective connectivity of the reward network during an incentive-processing task in adults with alcohol use disorder. Alcohol. Clin. Exp. Res. 2021, 45, 1563–1577. [Google Scholar] [CrossRef]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. Neuroimage 2016, 132, 390–397. [Google Scholar] [CrossRef]
- Camchong, J.; Macdonald, A.W., III; Mueller, B.A.; Nelson, B.; Specker, S.; Slaymaker, V.; Lim, K.O. Changes in resting functional connectivity during abstinence in stimulant use disorder: A preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 2014, 139, 145–151. [Google Scholar] [CrossRef]
- Blum, K.; Braverman, E.R.; Holder, J.M.; Lubar, J.F.; Monastra, V.J.; Miller, D.; Lubar, J.O.; Chen, T.J.; Comings, D.E. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 2000, 32 (Suppl. 1–5), 1–112. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Baler, R.; Telang, F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 2009, 56 (Suppl. S1), 3–8. [Google Scholar] [CrossRef]
- Tops, M.; Quirin, M.; Boksem, M.A.S.; Koole, S.L. Large-scale neural networks and the lateralization of motivation and emotion. Int. J. Psychophysiol. 2017, 119, 41–49. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Gordon, H.W. Laterality of Brain Activation for Risk Factors of Addiction. Curr. Drug Abus. Rev. 2016, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Choi, J.S.; Kim, H.; Sohn, B.K.; Jung, H.Y.; Lee, J.Y.; Kim, D.J.; Park, S.W.; Kim, Y.K. Altered hippocampal volume and functional connectivity in males with Internet gaming disorder comparing to those with alcohol use disorder. Sci. Rep. 2017, 7, 5744. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Tindell, A.J.; Aldridge, J.W.; Berridge, K.C. Ventral pallidum roles in reward and motivation. Behav. Brain Res. 2009, 196, 155–167. [Google Scholar] [CrossRef]
- Forbes, E.E.; Rodriguez, E.E.; Musselman, S.; Narendran, R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS ONE 2014, 9, e94640. [Google Scholar] [CrossRef]
- Ruiz, M.A.; Skeem, J.L.; Poythress, N.G.; Douglas, K.S.; Lilienfeld, S.O. Structure and correlates of the Barratt Impulsiveness Scale (BIS-11) in offenders: Implications for psychopathy and externalizing pathology. Int. J. Forensic Ment. Health 2010, 9, 237–244. [Google Scholar] [CrossRef]
- Carlson, S.R.; Katsanis, J.; Iacono, W.G.; Mertz, A.K. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology 1999, 36, 583–590. [Google Scholar] [CrossRef]
- Hill, S.Y.; Lowers, L.; Locke, J.; Snidman, N.; Kagan, J. Behavioral inhibition in children from families at high risk for developing alcoholism. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 410–417. [Google Scholar] [CrossRef]
- Eiden, R.D.; Edwards, E.P.; Leonard, K.E. A conceptual model for the development of externalizing behavior problems among kindergarten children of alcoholic families: Role of parenting and children’s self-regulation. Dev. Psychol. 2007, 43, 1187–1201. [Google Scholar] [CrossRef]
- Dick, D.M.; Aliev, F.; Wang, J.C.; Grucza, R.A.; Schuckit, M.; Kuperman, S.; Kramer, J.; Hinrichs, A.; Bertelsen, S.; Budde, J.P.; et al. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch. Gen. Psychiatry 2008, 65, 310–318. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Dong, Y.; Ma, M.; Ma, Y.; Dong, Y.; Niu, Y.; Jiang, Y.; Wang, H.; Wang, Z.; et al. Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. PLoS ONE 2016, 11, e0161956. [Google Scholar] [CrossRef]
- Gropper, S.; Spengler, S.; Stuke, H.; Gawron, C.K.; Parnack, J.; Gutwinski, S.; Wiers, C.E.; Bermpohl, F. Behavioral impulsivity mediates the relationship between decreased frontal gray matter volume and harmful alcohol drinking: A voxel-based morphometry study. J. Psychiatr. Res. 2016, 83, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lejuez, C.W.; Magidson, J.F.; Mitchell, S.H.; Sinha, R.; Stevens, M.C.; de Wit, H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol. Clin. Exp. Res. 2010, 34, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A.; Dzemidzic, M.; Eiler, W.J.; Coskunpinar, A.; Karyadi, K.; Kareken, D.A. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcohol. Clin. Exp. Res. 2014, 38, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, S.M.; Kim, Y.J.; Kim, D.J.; Choi, S.W.; Kwon, J.S.; Choi, J.S. Resting-state EEG activity related to impulsivity in gambling disorder. J. Behav. Addict. 2017, 6, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Diaz, A.; Mendoza-Quinones, R.; Melie-Garcia, L.; Martinez-Montes, E.; Sanabria-Diaz, G.; Romero-Quintana, Y.; Salazar-Guerra, I.; Carballoso-Acosta, M.; Caballero-Moreno, A. Functional Connectivity and Quantitative EEG in Women with Alcohol Use Disorders: A Resting-State Study. Brain Topogr. 2016, 29, 368–381. [Google Scholar] [CrossRef]
- Um, M.; Hershberger, A.R.; Whitt, Z.T.; Cyders, M.A. Recommendations for applying a multi-dimensional model of impulsive personality to diagnosis and treatment. Bord. Pers. Disord. Emot. Dysregul. 2018, 5, 6. [Google Scholar] [CrossRef]
- Hershberger, A.R.; Um, M.; Cyders, M.A. The relationship between the UPPS-P impulsive personality traits and substance use psychotherapy outcomes: A meta-analysis. Drug Alcohol Depend. 2017, 178, 408–416. [Google Scholar] [CrossRef]
- Oscar-Berman, M.; Ellis, R.J. Cognitive deficits related to memory impairments in alcoholism. Recent Dev. Alcohol. 1987, 5, 59–80. [Google Scholar] [CrossRef]
- Ober, B.A.; Stillman, R.C. Memory in chronic alcoholics: Effects of inconsistent versus consistent information. Addict. Behav. 1988, 13, 11–15. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Desmond, J.E.; Galloway, C.; Menon, V.; Glover, G.H.; Sullivan, E.V. Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. Neuroimage 2001, 14, 7–20. [Google Scholar] [CrossRef]
- Pitel, A.L.; Beaunieux, H.; Witkowski, T.; Vabret, F.; Guillery-Girard, B.; Quinette, P.; Desgranges, B.; Eustache, F. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol. Clin. Exp. Res. 2007, 31, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Kotz, S.A.; Anwander, A.; Axer, H.; Knosche, T.R. Beyond cytoarchitectonics: The internal and external connectivity structure of the caudate nucleus. PLoS ONE 2013, 8, e70141. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Fox, P.M.; Uecker, A.; Friehs, G.; Young, K.A.; et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 2012, 60, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Washington, S.D.; VanMeter, J.W. Anterior-Posterior Connectivity within the Default Mode Network Increases During Maturation. Int. J. Med. Biol. Front. 2015, 21, 207–218. [Google Scholar]
- Qadir, H.; Krimmel, S.R.; Mu, C.; Poulopoulos, A.; Seminowicz, D.A.; Mathur, B.N. Structural Connectivity of the Anterior Cingulate Cortex, Claustrum, and the Anterior Insula of the Mouse. Front. Neuroanat. 2018, 12, 100. [Google Scholar] [CrossRef]
- Day, A.M.; Metrik, J.; Spillane, N.S.; Kahler, C.W. Working memory and impulsivity predict marijuana-related problems among frequent users. Drug Alcohol Depend. 2013, 131, 171–174. [Google Scholar] [CrossRef]
- Kjome, K.L.; Lane, S.D.; Schmitz, J.M.; Green, C.; Ma, L.; Prasla, I.; Swann, A.C.; Moeller, F.G. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 2010, 178, 299–304. [Google Scholar] [CrossRef]
- Enticott, P.G.; Ogloff, J.R.P.; Bradshaw, J.L. Associations between laboratory measures of executive inhibitory control and self-reported impulsivity. Pers. Individ. Differ. 2006, 41, 285–294. [Google Scholar] [CrossRef]
- Evren, C.; Durkaya, M.; Evren, B.; Dalbudak, E.; Cetin, R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug Alcohol Rev. 2012, 31, 81–90. [Google Scholar] [CrossRef]

| Variable | AUD | CTL | ||||
|---|---|---|---|---|---|---|
| N * | Mean | SD | N * | Mean | SD | |
| Age (in years) | 30 | 41.42 | 7.31 | 30 | 27.44 | 4.74 |
| Education (in Years) | 30 | 11.93 | 2.35 | 30 | 15.77 | 1.87 |
| Age of onset (regular alcohol use) | 30 | 15.77 | 2.58 | 12 | 20.50 | 3.80 |
| Alcohol: Drinks/day (heavy alcohol use period) | 30 | 12.08 | 10.02 | 12 | 2.88 | 1.93 |
| Alcohol: Days/month (heavy alcohol use period) | 30 | 20.30 | 9.01 | 12 | 3.35 | 3.64 |
| Alcohol: Drinks (last 6 months) | 30 | 2.68 | 6.61 | 18 | 2.61 | 1.98 |
| Alcohol: Days (last 6 months) | 30 | 3.97 | 8.02 | 18 | 2.94 | 3.62 |
| Length of Abstinence (in months) | 30 | 22.43 | 28.16 | 18 | 1.9 | 4.99 |
| Tobacco: Times/day (last 6 months) | 20 | 9.90 | 5.80 | 6 | 2.33 | 1.63 |
| Tobacco: Days/month (last 6 months) | 20 | 28.35 | 4.83 | 6 | 14.17 | 13.82 |
| Marijuana: Times in last 6 months | 10 | 98.80 | 91.38 | 4 | 18.75 | 27.61 |
| ROI | Brain Region | Notation | Location | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 1 | L. Ventral Tegmental Area | L.VTA | Subcortical | −4 | −16 | −14 |
| 2 | R. Ventral Tegmental Area | R.VTA | Subcortical | 4 | −16 | −14 |
| 3 | L. Nucleus Accumbens | L.NAc | Subcortical | −8 | 10 | −10 |
| 4 | R. Nucleus Accumbens | R.NAc | Subcortical | 8 | 10 | −10 |
| 5 | L. Amygdala | L.Amg | Subcortical | −24 | −2 | −14 |
| 6 | R. Amygdala | R.Amg | Subcortical | 24 | −2 | −14 |
| 7 | L. Hippocampus | L.Hip | Subcortical | −28 | −10 | −22 |
| 8 | R. Hippocampus | R.Hip | Subcortical | 28 | −10 | −22 |
| 9 | L. Caudate | L.Cdt | Subcortical | −13 | 15 | 9 |
| 10 | R. Caudate | R.Cdt | Subcortical | 13 | 15 | 9 |
| 11 | L. Pallidum | L.Pal | Subcortical | −18 | −2 | −4 |
| 12 | R. Pallidum | R.Pal | Subcortical | 18 | −2 | −4 |
| 13 | L. Thalamus | L.Tha | Subcortical | −8 | −12 | 6 |
| 14 | R. Thalamus | R.Tha | Subcortical | 8 | −12 | 6 |
| 15 | L. Insula (anterior) | L.Ins | Subcortical | −30 | 17 | −15 |
| 16 | R. Insula (anterior) | R.Ins | Subcortical | 30 | 17 | −15 |
| 17 | L. Parahippocampal Gyrus | L.PHG | Cortical | −24 | −39 | −12 |
| 18 | R. Parahippocampal Gyrus | R.PHG | Cortical | 27 | −39 | −12 |
| 19 | L. Anterior Cingulate Cortex | L.ACC | Cortical | −6 | 44 | 10 |
| 20 | R. Anterior Cingulate Cortex | R.ACC | Cortical | 6 | 44 | 10 |
| 21 | L. Mid-Cingulate Cortex | L.MCC | Cortical | −6 | 2 | 40 |
| 22 | R. Mid-Cingulate Cortex | R.MCC | Cortical | 6 | 2 | 40 |
| 23 | L. Posterior Cingulate Cortex | L.PCC | Cortical | −6 | −46 | 30 |
| 24 | R. Posterior Cingulate Cortex | R.PCC | Cortical | 6 | −46 | 30 |
| 25 | L. Orbitofrontal Cortex | L.OFC | Cortical | −32 | 42 | −16 |
| 26 | R. Orbitofrontal Cortex | R.OFC | Cortical | 32 | 42 | −16 |
| 27 | L. Angular Gyrus | L.Ang | Cortical | −45 | −58 | 30 |
| 28 | R. Angular Gyrus | L.Ang | Cortical | 45 | −58 | 30 |
| 29 | L. Superior Parietal Lobule | L.SPL | Cortical | −24 | −68 | 56 |
| 30 | R. Superior Parietal Lobule | R.SPL | Cortical | 24 | −68 | 56 |
| 31 | L. Dorsolateral PFC | L.DLP | Cortical | −44 | 38 | 19 |
| 32 | R. Dorsolateral PFC | R.DLP | Cortical | 44 | 38 | 19 |
| 33 | L. Putamen | L.Ptm | Subcortical | −27 | 5 | −6 |
| 34 | R. Putamen | R.Ptm | Subcortical | 27 | 5 | −6 |
| Feature | Mean Minimum Depth | No. of Nodes | Accuracy Decrease | Gini Decrease | No. of Trees | Time a Root | p-Value | Direction |
|---|---|---|---|---|---|---|---|---|
| BIS Motor Impulsivity | 1.3824 | 348 | 0.0170 | 2.0202 | 319 | 94 | 1.87E-19 | A > C |
| FC_3_23 (L.NAc–L.PCC) | 1.9354 | 330 | 0.0130 | 1.6903 | 295 | 74 | 1.82E-15 | A > C |
| FC_16_20 (R.Ins–R.ACC) | 2.0062 | 326 | 0.0149 | 1.5132 | 291 | 57 | 1.23E-14 | C > A |
| BIS Non-planning | 1.7619 | 319 | 0.0187 | 1.6830 | 294 | 74 | 3.05E-13 | A > C |
| FC_2_19 (R.VTA–L.ACC) | 2.0561 | 313 | 0.0040 | 1.4706 | 274 | 58 | 4.23E-12 | C > A |
| FC_20_26 (R.ACC–R.OFC) | 2.2513 | 299 | 0.0101 | 1.3203 | 272 | 45 | 1.24E-09 | C > A |
| FC_6_7 (R.Amg–L.Hip) | 2.3798 | 275 | 0.0063 | 1.1669 | 258 | 41 | 4.40E-06 | C > A |
| FC_9_12 (L.Cdt–R.Pal) | 2.5018 | 268 | 0.0039 | 0.9725 | 255 | 29 | 3.26E-05 | C > A |
| FC_20_24 (R.ACC–R.PCC) | 2.4732 | 266 | 0.0054 | 1.0002 | 249 | 28 | 5.58E-05 | C > A |
| FC_13_14 (L.Tha–R.Tha) | 2.5681 | 252 | 0.0023 | 0.9843 | 233 | 40 | 0.0016 | C > A |
| FC_12_24 (R.Pal–R.PCC) | 2.8735 | 249 | 0.0022 | 0.8844 | 223 | 20 | 0.0030 | A > C |
| FC_9_13 (L.Cdt–L.Tha) | 2.7035 | 249 | 0.0056 | 0.9541 | 228 | 34 | 0.0030 | C > A |
| FC_13_34 (L.Tha–R.Ptm) | 2.7980 | 247 | 0.0053 | 0.9212 | 234 | 24 | 0.0044 | C > A |
| FC_8_31 (R.Hip–L.DLP) | 2.9451 | 238 | 0.0047 | 0.8020 | 217 | 20 | 0.0220 | A > C |
| Feature | AUD (N = 30) | CTL (N = 30) | § ALL (N = 60) | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| FC_2_19 (R.VTA–L.ACC) | −0.08 | 0.6744 | 0.22 | 0.2449 | 0.03 | 0.8131 |
| FC_3_23 (L.NAc–L.PCC) | 0.16 | 0.3949 | −0.21 | 0.2693 | 0.02 | 0.8956 |
| FC_6_7 (R.Amg–L.Hip) | −0.02 | 0.8993 | 0.38 | 0.0374 *○ | 0.11 | 0.4225 |
| FC_8_31 (R.Hip–L.DLP) | 0.16 | 0.3977 | 0.27 | 0.1489 | 0.20 | 0.1276 |
| FC_9_12 (L.Cdt–R.Pal) | 0.16 | 0.3964 | −0.01 | 0.9583 | 0.09 | 0.5079 |
| FC_9_13 (L.Cdt–L.Tha) | −0.15 | 0.4430 | 0.05 | 0.7812 | −0.06 | 0.6349 |
| FC_12_24 (R.Pal–R.PCC) | 0.01 | 0.9579 | −0.09 | 0.6402 | −0.03 | 0.8298 |
| FC_13_14 (L.Tha–R.Tha) | −0.26 | 0.1616 | −0.02 | 0.9168 | −0.15 | 0.2588 |
| FC_13_34 (L.Tha–R.Ptm) | 0.07 | 0.7127 | 0.12 | 0.5196 | 0.09 | 0.5002 |
| FC_16_20 (R.Ins–R.ACC) | −0.19 | 0.3149 | 0.09 | 0.6203 | −0.07 | 0.6062 |
| FC_20_24 (R.ACC–R.PCC) | −0.05 | 0.7849 | 0.01 | 0.9574 | −0.03 | 0.8195 |
| FC_20_26 (R.ACC–R.OFC) | 0.19 | 0.3182 | 0.11 | 0.5460 | 0.16 | 0.2110 |
| BIS_NP (Non-planning) | 0.03 | 0.8936 | 0.21 | 0.2644 | 0.09 | 0.4815 |
| BIS_MI (Motor Impulsivity) | 0.23 | 0.2121 | 0.12 | 0.5432 | 0.20 | 0.1268 |
| AUD | CTL | F | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| ExcMovMade_All | 15.04 | 17.02 | 7.83 | 6.66 | 4.43 | 0.0402 * |
| AvgPicTime_All | 3.09 | 1.09 | 2.81 | 0.96 | 1.02 | 0.3167 |
| AvgTotTime_All | 5.16 | 1.58 | 4.72 | 1.64 | 1.01 | 0.3199 |
| TotTrlTime_All | 482.60 | 178.18 | 404.24 | 139.05 | 3.29 | 0.0755 |
| AvgTrlTime_All | 22.98 | 8.48 | 19.25 | 6.62 | 3.29 | 0.0755 |
| TotCor_Fw | 7.00 | 2.58 | 10.21 | 2.78 | 19.06 | 0.0001 ++ |
| TotCor_Bw | 6.31 | 3.02 | 8.31 | 1.87 | 8.95 | 0.0042 * |
| Span_Fw | 5.44 | 1.33 | 6.83 | 1.36 | 14.25 | 0.0004 ++ |
| Span_Bw | 4.65 | 1.44 | 5.52 | 0.95 | 7.02 | 0.0106 * |
| TotAvgTime_Fw | 26.72 | 9.13 | 28.31 | 10.53 | 0.35 | 0.5591 |
| TotAvgTime_Bw | 17.72 | 9.39 | 17.79 | 10.01 | 0.00 | 0.9791 |
| TotCorAvgTime_Fw | 38.15 | 12.39 | 32.48 | 8.07 | 4.06 | 0.0490 * |
| TotCorAvgTime_Bw | 28.93 | 13.92 | 27.16 | 10.66 | 0.28 | 0.5963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarajan, C.; Ardekani, B.A.; Pandey, A.K.; Kinreich, S.; Pandey, G.; Chorlian, D.B.; Meyers, J.L.; Zhang, J.; Bermudez, E.; Kuang, W.; et al. Differentiating Individuals with and without Alcohol Use Disorder Using Resting-State fMRI Functional Connectivity of Reward Network, Neuropsychological Performance, and Impulsivity Measures. Behav. Sci. 2022, 12, 128. https://doi.org/10.3390/bs12050128
Kamarajan C, Ardekani BA, Pandey AK, Kinreich S, Pandey G, Chorlian DB, Meyers JL, Zhang J, Bermudez E, Kuang W, et al. Differentiating Individuals with and without Alcohol Use Disorder Using Resting-State fMRI Functional Connectivity of Reward Network, Neuropsychological Performance, and Impulsivity Measures. Behavioral Sciences. 2022; 12(5):128. https://doi.org/10.3390/bs12050128
Chicago/Turabian StyleKamarajan, Chella, Babak A. Ardekani, Ashwini K. Pandey, Sivan Kinreich, Gayathri Pandey, David B. Chorlian, Jacquelyn L. Meyers, Jian Zhang, Elaine Bermudez, Weipeng Kuang, and et al. 2022. "Differentiating Individuals with and without Alcohol Use Disorder Using Resting-State fMRI Functional Connectivity of Reward Network, Neuropsychological Performance, and Impulsivity Measures" Behavioral Sciences 12, no. 5: 128. https://doi.org/10.3390/bs12050128
APA StyleKamarajan, C., Ardekani, B. A., Pandey, A. K., Kinreich, S., Pandey, G., Chorlian, D. B., Meyers, J. L., Zhang, J., Bermudez, E., Kuang, W., Stimus, A. T., & Porjesz, B. (2022). Differentiating Individuals with and without Alcohol Use Disorder Using Resting-State fMRI Functional Connectivity of Reward Network, Neuropsychological Performance, and Impulsivity Measures. Behavioral Sciences, 12(5), 128. https://doi.org/10.3390/bs12050128







