Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Feeding
2.2. Sampling
2.3. Analytical Procedures
2.4. Statistical Analyses
2.5. Ethical Approval
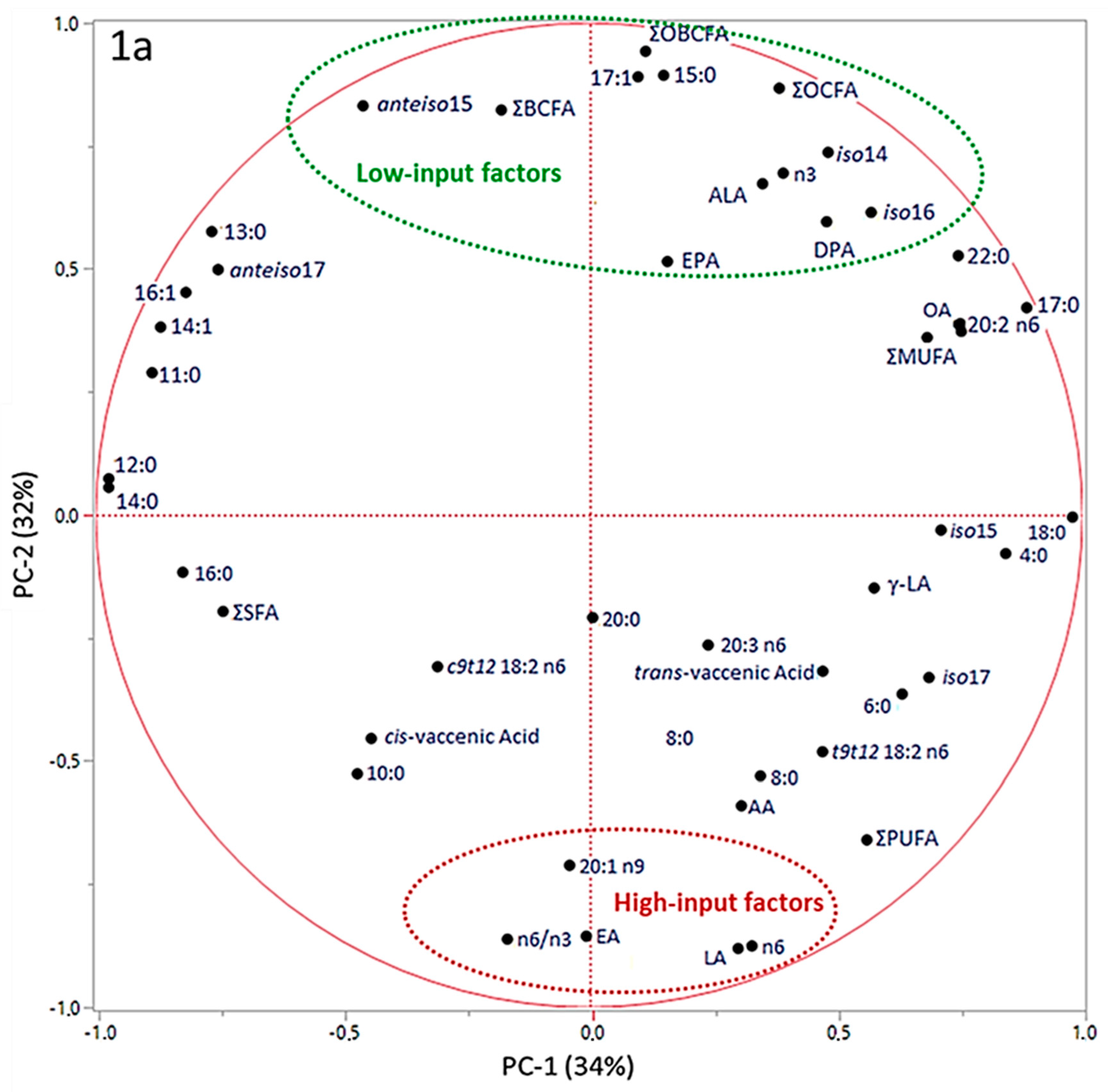
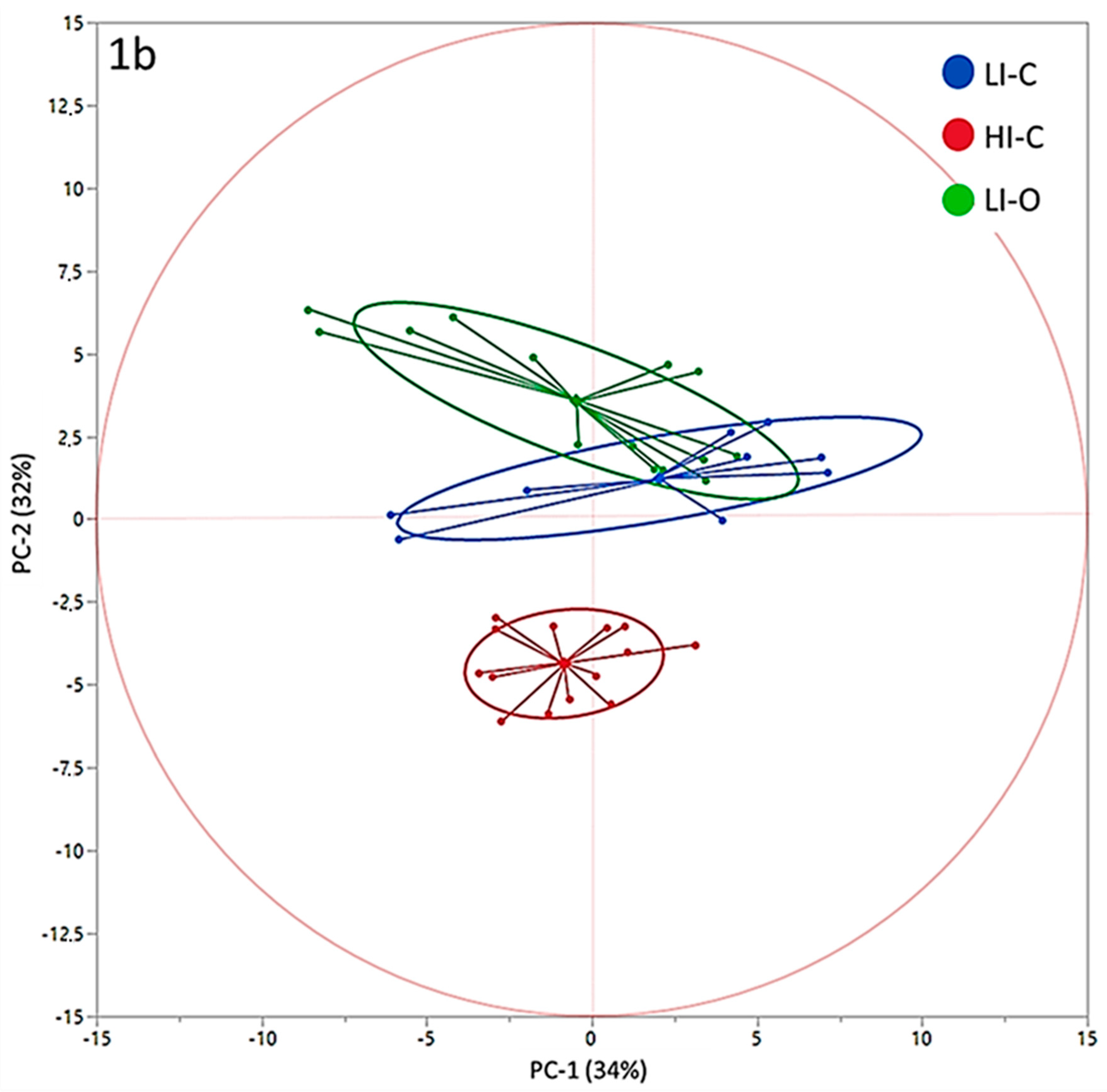
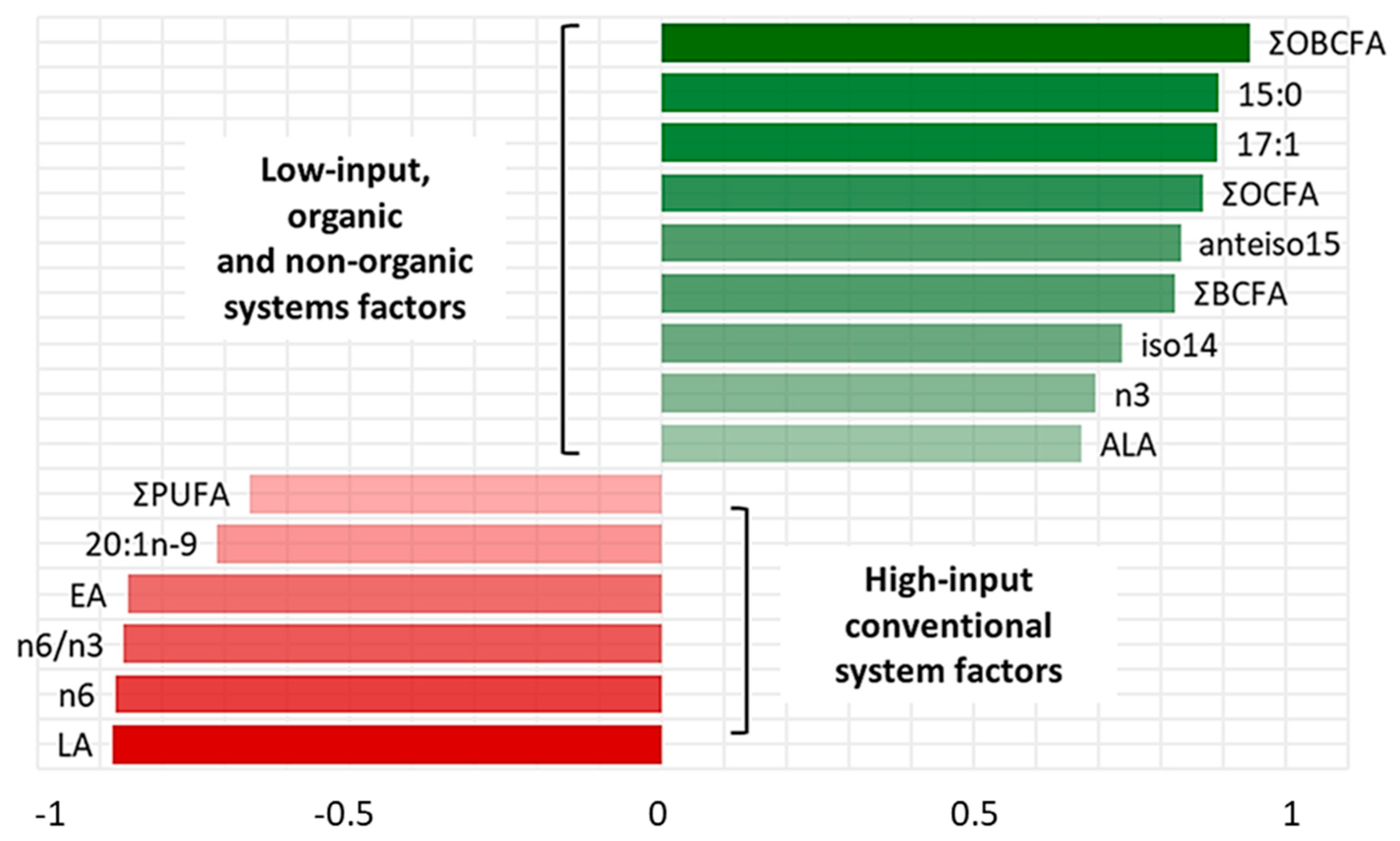
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernard, L.; Bonnet, M.; Delavaud, C.; Delosière, M.; Ferlay, A.; Fougère, H.; Graulet, B. Milk fat globule in ruminant: major and minor compounds, nutritional regulation and differences among species. Eur. J. Lipid Sci. Technol. 2018, 120, 1700039. [Google Scholar] [CrossRef]
- Hanuš, O.; Samkova, E.; Krizova, L.; Hasonova, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability – a review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Fievez, V.; Dohme, F.; Danneels, M.; Raes, K.; Demeyer, D. Fish oils as potent rumen methane inhibitors and associated effects on rumen fermentation in vitro and in vivo. Anim. Feed Sci. Technol. 2003, 104, 41–45. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function-An update. Anim. Feed Sci. Technol. 2002, 172, 51–65. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [PubMed]
- Mackie, R.I.; White, B.A.; Bryant, M.P. Lipid metabolism in anaerobic ecosystems. Crit. Rev. Microbiol. 1991, 17, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Falchero, L.; Lombardi, G.; Gorlier, A.; Lonati, M.; Odoardi, M.; Cavallero, A. Variation in fatty acid composition of milk and cheese from cows grazed on two alpine pastures. Dairy Sci. Technol. 2010, 90, 657–672. [Google Scholar] [CrossRef]
- Povolo, M.; Pelizzola, V.; Lombardi, G.; Tava, A.; Contarini, G. Hydrocarbon and fatty acid composition of cheese as affected by the pasture vegetation type. J. Agric. Food Chem. 2012, 60, 299–308. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, S.; Chen, X.; Chen, H.; Huang, M.; Zheng, J. Induction of apoptotic cell death and in vivo growth inhibition of human cancer cells by a saturated branched-chain fatty acid, 13-methyltetradecanoic acid. Cancer Res. 2000, 60, 505–509. [Google Scholar]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Ran-Ressler, R.R.; Bae, S.; Lawrence, P.; Wang, D.H.; Thomas Brenna, J. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014, 112, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Martínez Marín, A.L.; Gómez-Cortés, P.; Gómez Castro, A.G.; Juárez, M.; Pérez Alba, L.M.; Pérez Hernández, M.; de la Fuente, M.A. Animal performance and milk fatty acid profile of dairy goats fed diets with different unsaturated plant oils. J. Dairy Sci. 2011, 94, 5359–5368. [Google Scholar] [CrossRef]
- Mele, M.; Serra, A.; Buccioni, A.; Conte, G.; Pollicardo, A.; Secchiari, P. Effect of soybean oil supplementation on milk fatty acid composition from Saanen goats fed diets with different forage: concentrate ratios. Ital. J. Anim. Sci. 2008, 7, 297–311. [Google Scholar] [CrossRef]
- Serment, A.; Schmidely, P.; Giger-Reverdin, S.; Chapoutot, P.; Sauvant, D. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats. J. Dairy Sci. 2011, 94, 3960–3972. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.K.; Lee, K.J.; Son, N.Y.; An, M.J.; Lee, J.H.; An, Y.J.; Kim, S.H. Discrimination of organic milk by stable isotope ratio, vitamin E, and fatty acid profiling combined with multivariate analysis: A case study of monthly and seasonal variation in Korea for 2016-2017. Food Chem. 2018, 261, 112–123. [Google Scholar] [CrossRef]
- Collomb, M.; Bisig, W.; Butikofer, U.; Sieber, R.; Bregy, M.; Etter, L. Fatty acid composition of mountain milk from Switzerland: comparison of organic and integrated farming system. Int. Dairy J. 2008, 18, 976–982. [Google Scholar] [CrossRef]
- Malissiova, E.; Tzora, A.; Katsioulis, A.; Hatzinikou, M.; Tsakalof, A.; Arvanotoyannis, I.S.; Govaris, A.; Hadjichristodoulou, C. Relationship between production conditions and milk gross composition in ewe’s and goat’s organic and conventional farms in central Greece. Dairy Sci. Technol. 2015, 95, 437–450. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited review: Organic and conventionally produced milk – an evaluation of factors influencing milk composition. J. Dairy Sci. 2015, 92, 721–746. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Kotrotsios, V.; Hadjigeorgiou, I.; Zervas, G. Differences in sheep and goats milk fatty acid profile between conventional and organic farming systems. J. Dairy Res. 2010, 77343–77349. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: seasonal variations. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/484 of the European Parliament and of the Council of 30 May 2018 on organic production and labelling of organic products and repealing Council Regulation (EC) No 834/2007. OJ L 150, 14.6. 2018; 1–92.
- Pulina, G.; Avondo, M.; Molle, G.; Dias Francesconi, H.E.; Atzori, A.S.; Cannas, A. Invited review: Models for estimating feed intake in small ruminants. R. Bras. Zootec. 2013, 42, 675–690. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. OJ L 54, 26.2. 2009; 1–130.
- ISO 9622 | IDF 141:2013: Milk and liquid milk products — Guidelines for the application of mid-infrared spectrometry. ISO and IDF 2013.
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Christie, W.W. Preparation of derivatives of fatty acids. In Lipid Analysis. Isolation, Separation, Identification and Structural Analysis of Lipids, Third Edition; Christie, W.W., Ed.; The Oily Press: Bridgwater, UK, 2003; pp. 205–215. ISBN 0-9531949-5-7. [Google Scholar]
- Ulberth, F.; Gabernig, R.G.; Schrammel, F. Flame-ionization detector response to methyl, ethyl, propyl, and butyl esters of fatty acids. J. Am. Oil Chem. Soc. 1999, 76, 263–266. [Google Scholar] [CrossRef]
- Bernard, L.; Rouel, J.; Leroux, C.; Ferlay, A.; Faulconnier, Y.; Legrand, P.; Chilliard, Y. Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed vegetable lipids. J. Dairy Sci. 2005, 88, 1478–1489. [Google Scholar] [CrossRef]
- Alonso, L.; Fontecha, J.; Lozada, L.; Fraga, M.J.; Juàrez, M. Fatty acid composition of caprine milk: major, branched chain, and trans fatty acids. J. Dairy Sci. 1999, 82, 878–884. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Livest. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Rouel, J.; Lamberet, G. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. J. Dairy Sci. 2003, 86, 1751–1770. [Google Scholar] [CrossRef]
- Glasser, F.; Ferlay, A.; Chilliard, Y. Oilseed Lipid Supplements and Fatty Acid Composition of Cow Milk: A Meta-Analysis. J. Dairy Sci. 2008, 91, 4687–4703. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- LeDoux, M.; Rouzeau, A.; Bas, P.; Sauvant, D. Occurrence of trans-C18:1 fatty acid isomers in goat milk: effect of two dietary regimens. J. Dairy Sci. 2002, 85, 190–197. [Google Scholar] [CrossRef]
- Hanlein, G.F.W. Goat milk in human nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Bickerstaffe, R.; Noakes, D.E.; Annison, E.F. Quantitative aspects of fatty acid biohydrogenation, absorption and transfer into milk fat in the lactating goat, with special reference to the cis- and trans-isomers of octadecenoate and linoleate. Biochem. J. 1972, 130, 607–617. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, M.; Cutrignelli, I.; Calabrò, S.; Bovera, F.; Tudisco, R.; Piccolo, V.; Infascelli, F. Influence of pasture on fatty acid profile of goat milk. J. Anim. Physiol. Anim. Nutr. 2008, 92, 405–410. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef]
- Elgersma, A.; Tammingab, S.; Ellenca, G. Modifying milk composition through forage. Anim. Feed Sci. Technol. 2006, 131, 207–225. [Google Scholar] [CrossRef]
- Loor, J.; Ueda, K.; Ferlay, A.; Chilliard, Y.; Doreau, M.J. Biohydrogenation, Duodenal Flow, and Intestinal Digestibility of Trans Fatty Acids and Conjugated Linoleic Acids in Response to Dietary Forage:Concentrate Ratio and Linseed Oil in Dairy Cows. J. Dairy Sci. 2004, 87, 2472–2485. [Google Scholar] [CrossRef]
- Kalsehur, K.F.; Teter, B.B.; Piperova, L.S.; Erdman, R.A. Effect of Dietary Forage Concentration and Buffer Additionon Duodenal Flow of Trans-C18:1 Fatty Acids and Milk Fat Production in Dairy Cows. J. Dairy Sci. 1997, 80, 2104–2114. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Klevenhusen, F.; Soliva, C.R.; Kreuzer, M.; Leiber, F. Transfer of linoleic and linolenic acid from feed to milk in cows fed isoenergetic diets differing in proportion and origin of concentrates and roughages. J. Dairy Res. 2010, 77, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A. Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Shingfield, K.J.; Rouel, J.; Ferlay, A.; Chilliard, Y. Effect of plant oils in the diet on performance and milk fatty acid composition in goats fed diets based on grass hay or maize silage. Br. J. Nutr. 2009, 101, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ricordeau, G. Etat des recherches sur le lait de chèvre en France. Lait 1993, 73, 443–453. [Google Scholar] [CrossRef]
- Wolk, A.; Vessby, B.; Ljung, H.; Barrefors, P. Evaluation of a biological marker of dairy fat intake. Am. J. Clin. Nutr. 1998, 68, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Smedman, A.E.; Gustafsson, I.B.; Berglund, L.G.; Vessby, B.O. Pentadecanoic acid in serum as a marker for intake of milk fat: relations between intake of milk fat and metabolic risk factors. Am. J. Clin. Nutr. 1999, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Jansson, J.H.; Bergkund, L.; Boman, K.; Ahrén, B.; Weinehall, L.; Lindahl, B.; Hallmans, G.; Vessby, B. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br. J. Nutr. 2004, 91, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Sejrsen, K.; Andersen, H.R.; Lund, P.; Straarup, E.M. Effect of silage type and energy concentration on conjugated linoleic acid (CLA) in milk fat from dairy cows. J. Anim. Feed Sci. 2004, 13, 697–700. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Salo-Vaananen, P.; Pahkala, E.; Toivonen, V.; Jaakkola, S.; Piironen, V.; Huhtanen, P. Effect of forage conservation method, concentrate level and propylene glycol on the fatty acid composition and vitamin content of cows’ milk. J. Dairy Res. 2005, 72, 1–13. [Google Scholar] [CrossRef]
- Markiewicz-Kęszycka, M.; Czyżak-Runowska, G.; Lipińska, P.; Wójtowski, A. Fatty acid profile of milk - A review. Bull. Vet. Inst. Pulawy 2013, 57, 135–139. [Google Scholar] [CrossRef]
| Item | Farm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LI-O Low Input, Organic | LI-C Low Input, Conventional | HI-C High Input, Conventional | ||||||||
| C1 | AH 2 | PGH 3 | P4 | C 1 | AH 2 | PGH 3 | C 1 | AH 2 | RH 5 | |
| supplied (g/day) | 800 | 1000 | 500 | Ad libitum | 1000 | 500 | Ad libitum | 1200 | 500 | Ad libitum |
| g/Kg DM | 297.1 | 362.1 | 183.1 | - | 423 | 215.6 | - | 488.1 | 207.7 | - |
| F/C ratio | 70/30 | 60/40 | 50/50 | |||||||
| Milk yield(g/day) | 2.55 ± 0.5 | 2.50 ± 0.6 | 2.60 ± 0.5 | |||||||
| Fat (%) | 3.2 ± 0.7 | 3.3 ± 0.5 | 3.6 ± 0.55 | |||||||
| Protein (%) | 3.2 ± 0.3 a | 3.3 ± 0.3 a | 3.7 ± 0.4 b | |||||||
| Lactose (%) | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.6 ± 0.15 | |||||||
| Fatty Acid | Low Input-Organic (n = 14 *) | Low Input -Conventional (n = 9 *) | High Input-Conventional (n = 14 *) | |||
|---|---|---|---|---|---|---|
| Saturated Fatty Acids | ||||||
| 4:0, Butirric Acid | 2.0 | ±0.30 | 1.9 | ±0.29 | 1.9 | ±0.17 |
| 6:0, Caproic Acid | 2.0 | ±0.28 | 1.9 | ±0.17 | 2.1 | ±0.14 |
| 8:0, Caprylic Acid | 2.7 | ±0.36 | 2.6 | ±0.19 | 2.9 | ±0.16 |
| 10:0, Capric Acid | 10.0 a | ±0.77 | 9.3 a | ±1.39 | 10.8 b | ±0.52 |
| 12:0, Lauric Acid | 5.7 | ±1.33 | 5.2 | ±1.74 | 5.6 | ±0.66 |
| 14:0, Myristic Acid | 11.7 | ±1.81 | 10.8 | ±1.85 | 11.8 | ±0.56 |
| 16:0, Palmitic Acid | 26.3 b | ±2.05 | 23.2 a | ±1.97 | 26.9 b | ±1.07 |
| 18:0, Stearic Acid | 8.5 | ±2.66 | 10.6 | ±3.42 | 8.3 | ±1.38 |
| 20:0, Eicosanoic Acid + | 0.82 a | ±0.14 | 0.98 b | ±0.23 | 0.86 ab | ±0.10 |
| 22:0, Docosanoic Acid | 0.09 b | ±0.02 | 0.10 b | ±0.03 | 0.06 a | ±0.01 |
| Σ Saturated Fatty Acids (SFA) | 69.8 b | ±1.91 | 66.7 a | ±3.50 | 71.22 b | ±1.17 |
| Odd and Branched-Chain Fatty Acids | ||||||
| 11:0, Undecanoic Acid | 0.13 | ±0.04 | 0.10 | ±0.06 | 0.10 | ±0.02 |
| 13:0, Tridecanoic Acid | 0.13 b | ±0.02 | 0.11 a | ±0.03 | 0.10 a | ±0.01 |
| iso 14:0 | 0.16 c | ±0.02 | 0.13 b | ±0.03 | 0.10 a | ±0.01 |
| iso 15 | 0.23 | ±0.02 | 0.24 | ±0.05 | 0.25 | ±0.03 |
| 15:0 | 1.14 c | ±0.09 | 1.07 b | ±0.08 | 0.91 a | ±0.05 |
| anteiso 15:0 | 0.67 b | ±0.09 | 0.63 b | ±0.03 | 0.54 a | ±0.05 |
| iso 16:0 | 0.34 c | ±0.05 | 0.29 b | ±0.08 | 0.25 a | ±0.02 |
| iso 17:0 | 0.35 | ±0.03 | 0.37 | ±0.07 | 0.39 | ±0.03 |
| 17:0 | 0.70 b | ±0.11 | 0.75 b | ±0.18 | 0.57 a | ±0.06 |
| anteiso 17:0 | 0.82 | ±0.21 | 0.76 | ±0.08 | 0.72 | ±0.08 |
| 17:1n-8 | 0.33 b | ±0.04 | 0.32 b | ±0.05 | 0.22 a | ±0.02 |
| Σ Branched Chain Fatty Acids (BCFA) | 2.6 b | ±0.23 | 2.4 b | ±0.18 | 2.2 a | ±0.13 |
| Σ Odd Chain Fatty Acids (OCFA) | 2.1 b | ±0.12 | 2.0 b | ±0.15 | 1.7 a | ±0.08 |
| Σ Odd and Branched Chain Fatty Acids (OBCFA) | 4.7 c | ±0.24 | 4.4 b | ±0.32 | 3.9 a | ±0.17 |
| Monounsaturated Fatty Acids | ||||||
| 14:1, Miristoleic Acid | 0.28 | ±0.21 | 0.22 | ±0.14 | 0.20 | ±0.07 |
| 16:1 c7+9, Palmitoleic Acid | 0.79 | ±0.31 | 0.64 | ±0.19 | 0.61 | ±0.13 |
| 18:1 t9, Elaidic Acid (EA) | 0.29 a | ±0.05 | 0.41 b | ±0.09 | 0.48c | ±0.06 |
| 18:1 t11, trans-Vaccenic Acid | 1.0 | ±0.38 | 1.4 | ±0.22 | 1.2 | ±0.41 |
| 18:1 c9, Oleic Acid (OA) | 18.9 a | ±1.60 | 21.1 b | ±3.42 | 17.4 a | ±0.80 |
| 18:1 c11, cis-Vaccenic Acid | 0.17 a | ±0.08 | 0.25 b | ±0.09 | 0.26 b | ±0.05 |
| 20:1n-9, Gondoic Acid | 0.04 a | ±0.01 | 0.04 a | ±0.01 | 0.06 b | ±0.01 |
| Σ Monounsaturated Fatty Acids (MUFA) | 21.9 a | ±1.58 | 24.4 b | ±3.13 | 20.5 a | ±0.97 |
| Polyunsaturated Fatty Acids | ||||||
| 18:2n-6 t9t12 | 0.20 a | ±0.06 | 0.26 b | ±0.04 | 0.24 ab | ±0.04 |
| 18:2n-6 c9t12 | 0.11 a | ±0.01 | 0.17 b | ±0.07 | 0.14 ab | ±0.04 |
| 18:2 c9c12, Linoleic Acid | 2.0 a | ±0.27 | 2.6 b | ±0.29 | 3.1 c | ±0.22 |
| 18:3n-6, γ-Linolenic Acid (GLA) | 0.03 | ±0.01 | 0.04 | ±0.02 | 0.04 | ±0.00 |
| 18:3n-3 c9c12c15, α-Linolenic Acid (ALA) | 0.72 b | ±0.11 | 0.81 b | ±0.10 | 0.41 a | ±0.11 |
| 20:2n-6, Eicosadienoic Acid | 0.07 b | ±0.01 | 0.07 b | ±0.02 | 0.06 a | ±0.01 |
| 20:3n-6, Dihomo-γ-Linolenic Acid (DGLA) | 0.03 a | ±0.01 | 0.03 a | ±0.01 | 0.04 b | ±0.01 |
| 20:4n-6, Arachidonic Acid (AA) | 0.15 a | ±0.01 | 0.17 b | ±0.02 | 0.18 b | ±0.02 |
| 20:5n-3, Eicosapentaenoic Acid (EPA) | 0.10 b | ±0.03 | 0.10 b | ±0.02 | 0.06 a | ±0.03 |
| 22:5n-3, Docosapentaenoic Acid (DPA) | 0.18 b | ±0.04 | 0.17 b | ±0.03 | 0.11 a | ±0.03 |
| n3 | 1.0 a | ±0.14 | 1.1 a | ±0.12 | 0.6 b | ±0.15 |
| n6 | 2.6 a | ±0.35 | 3.4 b | ±0.27 | 3.8 c | ±0.27 |
| n6/n3 | 2.6 a | ±0.27 | 3.1 a | ±0.35 | 6.5 b | ±1.45 |
| Σ Polyunsaturated Fatty Acids (PUFA) | 3.6 a | ±0.46 | 4.4 b | ±0.31 | 4.4 b | ±0.31 |
| Principal Component | Farm | ||||||
|---|---|---|---|---|---|---|---|
| Low Input-Organic | Low Input-Conventional | High Input-Conventional | |||||
| Mean | SEM | Mean | SEM | Mean | SEM | P | |
| PC-1 | −0.47 | 1.05 | 2.04 | 1.31 | −0.84 | 1.06 | 0.2123 |
| PC-2 | 3.57 a | 0.40 | 1.23 b | 0.50 | −4.36 c | 0.40 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, A.; Vasconi, M.; Moretti, V.M.; Bellagamba, F. Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems. Animals 2019, 9, 452. https://doi.org/10.3390/ani9070452
Lopez A, Vasconi M, Moretti VM, Bellagamba F. Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems. Animals. 2019; 9(7):452. https://doi.org/10.3390/ani9070452
Chicago/Turabian StyleLopez, Annalaura, Mauro Vasconi, Vittorio Maria Moretti, and Federica Bellagamba. 2019. "Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems" Animals 9, no. 7: 452. https://doi.org/10.3390/ani9070452
APA StyleLopez, A., Vasconi, M., Moretti, V. M., & Bellagamba, F. (2019). Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems. Animals, 9(7), 452. https://doi.org/10.3390/ani9070452







