The Inclusion of Concentrate with Quebracho Is Advisable in Two Forage-Based Diets of Ewes According to the In Vitro Fermentation Parameters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animal and Diets
2.2.1. Feedstuffs and Substrates
2.2.2. Animals and Sampling of Ruminal Digesta
2.2.3. In Vitro Gas Production Technique and Sampling
2.3. Analytical Methods
2.3.1. Chemical Composition
2.3.2. Determination of the Parameters of In Vitro Fermentation
2.4. Calculations and Statistical Analysis
3. Results
3.1. Effect of the Type of Forage on In Vitro Fermentation
3.2. Hay-Based Diets: Effect of the Inclusion of the Control or Quebracho Concentrate
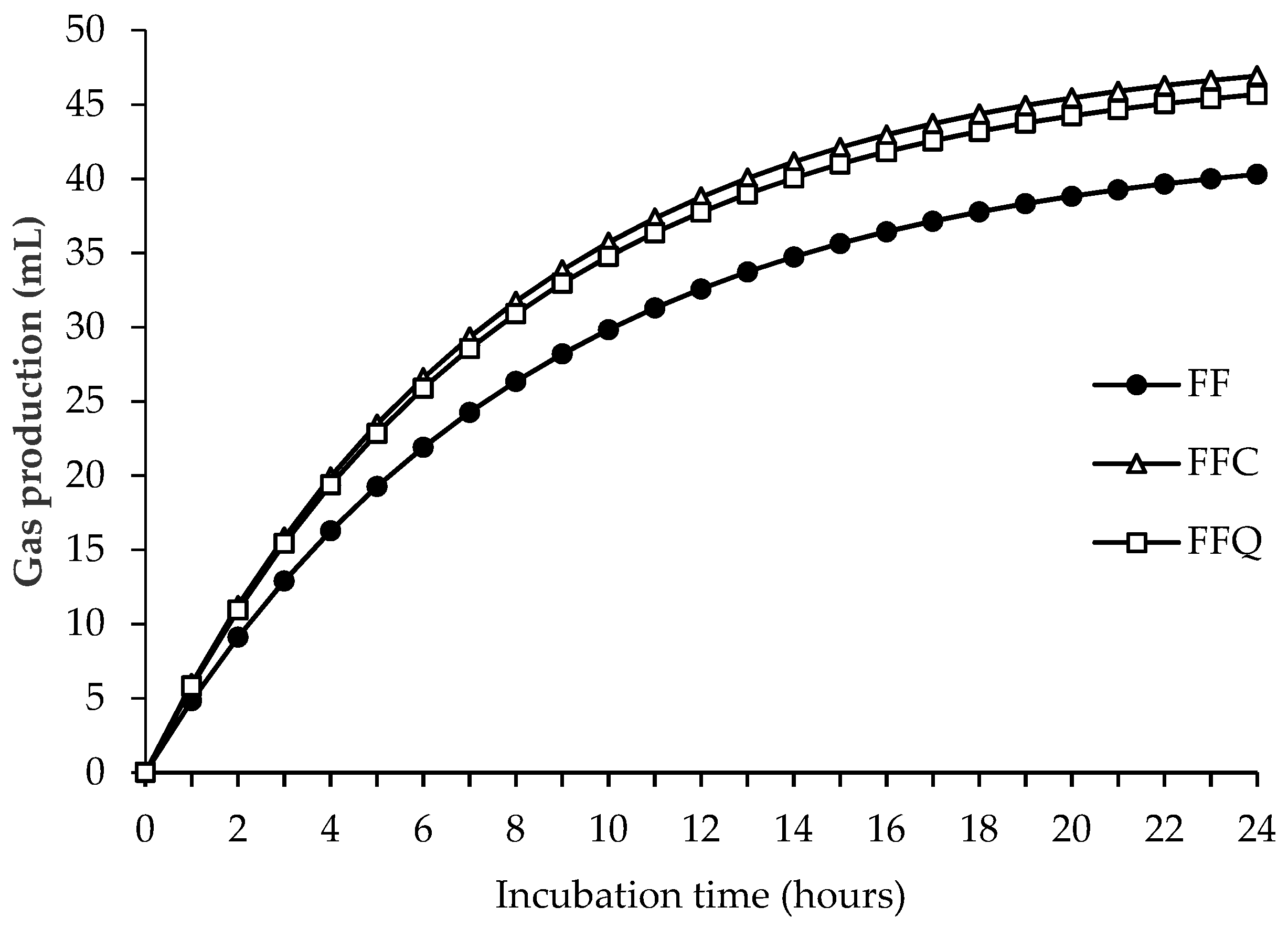
3.3. Fresh-Forage-Based Diet: Effect of the Inclusion of Control or Quebracho Concentrate
4. Discussion
4.1. Effects of the Type of Forage on Fermentation Parameters
4.2. Effect of the Inclusion of Concentrate with or without Quebracho
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Álvarez-Rodríguez, J.; Sanz, A.; Delfa, R.; Revilla, R.; Joy, M. Performance and grazing behaviour of Churra Tensina sheep stocked under different management systems during lactation on Spanish mountain pastures. Livest. Sci. 2007, 107, 152–161. [Google Scholar] [CrossRef]
- Joy, M.; Sanz, A.; Ripoll, G.; Panea, B.; Ripoll-Bosch, R.; Blasco, I.; Alvarez-Rodriguez, J. Does forage type (grazing vs. hay) fed to ewes before and after lambing affect suckling lambs performance, meat quality and consumer purchase intention? Small Rumin. Res. 2012, 104, 1–9. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Marinucci, M.T.; Lupi, P.; Conte, G. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Sanz, A.; Blanco, M.; Joy, M. Efecto del tipo de forraje y la inclusión de quebracho en la dieta de ovejas lactantes sobre sus rendimientos productivos. ITEA Inf. Téc. Econ. Agrar. 2017, 113, 359–375. [Google Scholar]
- Lobón, S.; Sanz, A.; Blanco, M.; Ripoll, G.; Joy, M. The type of forage and condensed tannins in dams’ diet: Influence on meat shelf life of their suckling lambs. Small Rumin. Res. 2017, 154, 115–122. [Google Scholar] [CrossRef]
- Getachew, G.; Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 1998, 72, 261–281. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McAllister, T.A.; McGinn, S.M. Dietary mitigation of enteric methane from cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2009, 4, 1–18. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Owen, E.; Mould, F.L.; Givens, I.; Theodorou, M.K.; France, J.; Davies, D.R.; Dhanoa, M.S. Comparison of bovine rumen liquor and bovine faeces as inoculum for an in vitro gas production technique for evaluating forages. Anim. Feed Sci. Technol. 2001, 89, 33–48. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Agle, M.; Hristov, A.N.; Zaman, S.; Schneider, C.; Ndegwa, P.M.; Vaddella, V.K. Effect of dietary concentrate on rumen fermentation, digestibility, and nitrogen losses in dairy cows. J. Dairy Sci. 2010, 93, 4211–4222. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Getachew, G.; Pittroff, W.; Putnam, D.H.; Dandekar, A.; Goyal, S.; DePeters, E.J. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim. Feed Sci. Technol. 2008, 140, 444–461. [Google Scholar] [CrossRef]
- Martínez, T.F.; McAllister, T.A.; Wang, Y.; Reuter, T. Effects of tannic acid and quebracho tannins on in vitro ruminal fermentation of wheat and corn grain. J. Sci. Food Agric. 2006, 86, 1244–1256. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2013, 93, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. An in vitro study on the ability of polyethylene glycol to inhibit the effect of quebracho tannins and tannic acid on rumen fermentation in sheep, goats, cows, and deer. Aust. J. Agric. Res. 2004, 55, 1125–1132. [Google Scholar] [CrossRef]
- Rufino-Moya, P.J.; Blanco, M.; Bertolín, J.R.; Joy, M. Effect of the method of preservation on the chemical composition and in vitro fermentation characteristics in two legumes rich in condensed tannins. Anim. Feed Sci. Technol. 2019, 251, 12–20. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Horwitz, W. AOAC. Official Methods of Analysis; Association of Official Analytical Chemist: Arlington, VA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOCS. Approved Procedure Am 5-04, Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction. 2005. Available online: http://www.ssco.com.tw/Ankom/PDF_file/Crude%20Fat%20Method.pdf (accessed on 1 May 2019).
- Guglielmelli, A.; Calabrò, S.; Primi, R.; Carone, F.; Cutrignelli, M.I.; Tudisco, R.; Piccolo, G.; Ronchi, B.; Danieli, P.P. In vitro fermentation patterns and methane production of sainfoin (Onobrychis viciifolia Scop.) hay with different condensed tannin contents. Grass Forage Sci. 2011, 66, 488–500. [Google Scholar] [CrossRef]
- Mertens, D.R. Using neutral detergent fiber to formulate dairy rations and estimate the energy content forages. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, Syracuse, NY, USA, 1983; p. 60. Available online: http://agris.fao.org/agris-search/search.do?recordID=US19850069994 (accessed on 16 July 2019).
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage; FAO/IAEA Working Document IAEA: Vienna, Austria, 2000. [Google Scholar]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Grabber, J.H.; Zeller, W.E.; Mueller-Harvey, I. Acetone enhances the direct analysis of procyanidin- and prodelphinidin-based condensed tannins in lotus species by the butanol-HCl-iron assay. J. Agric. Food Chem. 2013, 61, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.M.; Terrill, T.H.; Muir, J.P. Drying method and origin of standard affect condensed tannin (CT) concentrations in perennial herbaceous legumes using simplified butanol-HCl CT analysis. J. Sci. Food Agric. 2008, 88, 1060–1067. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Cattani, M.; Maccarana, L.; Rossi, G.; Tagliapietra, F.; Schiavon, S.; Bailoni, L. Dose-response and inclusion effects of pure natural extracts and synthetic compounds on in vitro methane production. Anim. Feed Sci. Technol. 2016, 218, 100–109. [Google Scholar] [CrossRef]
- Alvarez Hess, P.S.; Eckard, R.J.; Jacobs, J.L.; Hannah, M.C.; Moate, P.J. Comparison of five methods for the estimation of methane production from vented in vitro systems. J. Sci. Food Agric. 2019, 99, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar]
- Beever, D.; Baker, M.; Crush, J.; Humphreys, L. Ruminant Animal Production from Forages–Present Position and Future Opportunities; Baker, M., Ed.; SIR Publishing: Wellington, New Zealand, 1993. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1838–1844. [Google Scholar] [CrossRef]
- Cushnahan, A.; Gordon, F. The effects of grass preservation on intake, apparent digestibility and rumen degradation characteristics. Anim. Sci. 1995, 60, 429–438. [Google Scholar] [CrossRef]
- Saro, C.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Influence of forage type in the diet of sheep on rumen microbiota and fermentation characteristics. Livest. Sci. 2014, 160, 52–59. [Google Scholar] [CrossRef]
- Ripoll-Bosch, R.; Joy, M.; Sanz, A.; Blasco, I.; Ripoll, G.; Álvarez-Rodríguez, J. Effect of concentrate supplementation and prolificacy on the productive and economic performance of autochthonous sheep breeds fed forage-based diets. Span. J. Agric. Res. 2014, 12, 1099–1104. [Google Scholar] [CrossRef]
- Morand-Fehr, P.; Sauvant, D. Feeding strategies in goats. In Proceedings of the 4th International Conference Goats, Brasilia, Brazil, 8–13 March 1987; Volume 2, pp. 1275–1303. [Google Scholar]
- Hervás, G.; Pérez, V.; Giráldez, F.J.; Mantecón, A.R.; Almar, M.M.; Frutos, P. Intoxication of sheep with quebracho tannin extract. J. Comp. Pathol. 2003, 129, 44–54. [Google Scholar] [CrossRef]
- Serment, A.; Giger-Reverdin, S.; Schmidely, P.; Dhumez, O.; Broudiscou, L.P.; Sauvant, D. In vitro fermentation of total mixed diets differing in concentrate proportion: Relative effects of inocula and substrates. J. Sci. Food Agric. 2016, 96, 160–168. [Google Scholar] [CrossRef]
- Kumar, S.; Dagar, S.S.; Sirohi, S.K.; Upadhyay, R.C.; Puniya, A.K. Microbial profiles, in vitro gas production and dry matter digestibility based on various ratios of roughage to concentrate. Ann. Microbiol. 2013, 63, 541–545. [Google Scholar] [CrossRef]
- Sommart, K.; Parker, D.; Rowlinson, P.; Wanapat, M. Fermentation characteristics and microbial protein synthesis in an in vitro system using cassava, rice straw and dried ruzi grass as substrates. Asian Austral. J. Anim. Sci. 2000, 13, 1084–1093. [Google Scholar] [CrossRef]
- Zicarelli, F.; Calabrò, S.; Cutrignelli, M.I.; Infascelli, F.; Tudisco, R.; Bovera, F.; Piccolo, V. In vitro fermentation characteristics of diets with different forage/concentrate ratios: Comparison of rumen and faecal inocula. J. Sci. Food Agric. 2011, 91, 1213–1221. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Westreicher-Kristen, E.; Henke, A.; Diaby, M.; Susenbeth, A.; Dickhoefer, U. In vitro microbial protein synthesis, ruminal degradation and post-ruminal digestibility of crude protein of dairy rations containing Quebracho tannin extract. J. Anim. Physiol. Anim. Nutr. 2018, 102, e77–e86. [Google Scholar] [CrossRef]
- Pedreira, M.d.S.; Oliveira, S.G.d.; Primavesi, O.; Lima, M.A.d.; Frighetto, R.T.S.; Berchielli, T.T. Methane emissions and estimates of ruminal fermentation parameters in beef cattle fed different dietary concentrate levels. Rev. Bras. Zootecn. 2013, 42, 592–598. [Google Scholar] [CrossRef]
- Van Milgen, J.; Berger, L.L.; Murphy, M.R. An integrated, dynamic model of feed hydration anddigestion, and subsequent bacterial mass accumulation in the rumen. Br. J. Nutr. 1993, 70, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, S.; Ghoorchi, T.; Ramzanpour, S. Use of an in vitro Rumen Gas Production Technique to Evaluate the Nutritive Value of Five Forage to Concentrate Ratios. Iran. J. Appl. Anim. Sci. 2017, 7, 249–259. [Google Scholar]
- Archimède, H.; Sauvant, D.; Hervieu, J.; Ternois, F.; Poncet, C. Effects of the nature of roughage and concentrate and their proportion on ruminal characteristics of non lactating goats, consequences on digestive interactions. Anim. Feed Sci. Technol. 1996, 58, 267–282. [Google Scholar] [CrossRef]
- Wegner, G.; Foster, E. Incorporation of isobutyrate and valerate into cellular plasmalogen by Bacteroides succinogenes. J. Bacteriol. 1963, 85, 53–61. [Google Scholar] [PubMed]

| Parameters | Forage | Concentrate | ||
|---|---|---|---|---|
| Items | Hay | Fresh | Control | Quebracho 1 |
| Dry matter (DM) (g/kg) | 883 | 183 | 887 | 885 |
| Ash (g/kg DM) | 83 | 13 | 72 | 71 |
| CP (g/kg DM) | 75 | 263 | 153 | 155 |
| NDFom (g/kg DM) | 678 | 488 | 270 | 193 |
| ADFom (g/kg DM) | 362 | 202 | 85 | 66 |
| Lignin (sa) (g/kg DM) | 43 | 40 | 22 | 12 |
| Ether extract (g/kg DM) | 18 | 33 | 31 | 30 |
| Nonstructural carbohydrates (g/kg DM) | 146 | 203 | 474 | 551 |
| Metabolizable energy 2,3 | 8.6 | 11.9 | 11.7 | 11.6 |
| Total polyphenols (eq-g tannic acid/kg DM) | 7.3 | 12.7 | 7.2 | 66 |
| Condensed tannins (CT) (eq-g CT/kg DM) 4 | ||||
| Total CT | 1.5 | 1.8 | 10.5 | 76.6 |
| Extractable CT | 0.4 | 0.9 | 7.3 | 72.2 |
| Protein-bound CT | 0.7 | 0.6 | 2 | 2.5 |
| Fiber-bound CT | 0.3 | 0.3 | 1.1 | 2 |
| Parameters | Substrates 1 | (p-Value) | Contrast (p-Value) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | FF | HC | HQ | FFC | FFQ | RMSE 2 | Substrates | Hs vs. FFs 3 | H vs. HC | H vs. HQ | FF vs. FFC | FF vs. FFQ | |
| pH | 6.65 | 6.61 | 6.67 | 6.69 | 6.69 | 6.77 | 0.14 | 0.82 | 0.81 | 0.88 | 0.73 | 0.51 | 0.19 |
| Total gas production (mL/g iDM 4) | 83 | 88 | 99 | 93 | 107 | 102 | 7.3 | 0.01 | 0.04 | 0.023 | 0.13 | 0.007 | 0.04 |
| Total gas production (mL/g dOM 5) | 181 | 150 | 191 | 172 | 175 | 163 | 14 | 0.04 | 0.013 | 0.42 | 0.43 | 0.04 | 0.27 |
| Potential gas production (A) (mL) | 61.9 | 42.7 | 47.8 | 42.1 | 49.1 | 47.8 | 10.4 | 0.28 | 0.42 | 0.12 | 0.04 | 0.47 | 0.56 |
| Rate of gas production (c) (h−1) | 0.06 | 0.12 | 0.11 | 0.14 | 0.13 | 0.13 | 0.03 | 0.04 | 0.06 | 0.06 | 0.005 | 0.84 | 0.82 |
| Total CH4 production (mL/g iDM) | 44 | 48 | 49 | 48 | 51 | 49 | 1.4 | 0.001 | 0.012 | <0.001 | 0.004 | 0.02 | 0.42 |
| Total CH4 production (mL/g dOM) | 92.0 | 81.6 | 95.9 | 89.1 | 82.8 | 77.6 | 4.9 | 0.005 | <0.001 | 0.004 | 0.087 | 0.04 | 0.004 |
| CH4/gas (mL/L) | 300 | 321 | 310 | 311 | 297 | 298 | 12.9 | 0.25 | 0.807 | 0.38 | 0.36 | 0.04 | 0.06 |
| IVOMD (g/kg) | 498 | 668 | 554 | 582 | 703 | 723 | 37.7 | <0.001 | <0.001 | 0.097 | 0.02 | 0.28 | 0.099 |
| NH3-N (mg/L) | 105 | 134 | 87 | 91 | 118 | 103 | 16 | 0.03 | 0.007 | 0.2 | 0.31 | 0.23 | 0.03 |
| Total VFA (mmol/L) | 58.5 | 62.7 | 63.5 | 61 | 61.3 | 59 | 5.49 | 0.85 | 0.99 | 0.29 | 0.59 | 0.75 | 0.42 |
| Acetic acid (C2) (mmol/mol) | 684 | 678 | 697 | 693 | 682 | 679 | 11.34 | 0.31 | 0.06 | 0.18 | 0.33 | 0.7 | 0.94 |
| Propionic acid (C3) (mmol/mol) | 159 | 142 | 142 | 145 | 138 | 143 | 5.23 | 0.007 | 0.01 | 0.002 | 0.008 | 0.41 | 0.85 |
| Butyric acid (mmol/mol) | 98 | 109 | 109 | 111 | 118 | 119 | 5.93 | 0.014 | 0.007 | 0.04 | 0.03 | 0.098 | 0.06 |
| Valeric acid (mmol/mol) | 14.9 | 17.6 | 14.1 | 13.5 | 16.7 | 15.4 | 1.05 | 0.004 | <0.001 | 0.35 | 0.11 | 0.33 | 0.02 |
| Isobutyric acid (mmol/mol) | 15.4 | 18 | 13.7 | 13.8 | 16 | 15.8 | 0.98 | 0.002 | <0.001 | 0.06 | 0.06 | 0.03 | 0.02 |
| Isovaleric acid (mmol/mol) | 28.9 | 35.2 | 24.1 | 23.9 | 29.4 | 28 | 1.94 | <0.001 | <0.001 | 0.011 | 0.009 | 0.003 | 0.001 |
| C2:C3 ratio (mol/mol) | 4.33 | 4.79 | 4.94 | 4.8 | 4.93 | 4.76 | 0.23 | 0.059 | 0.22 | 0.006 | 0.03 | 0.47 | 0.86 |
| CH4/VFAtotal (mL/mmol) | 5.90 | 5.87 | 6.02 | 6.08 | 6.44 | 6.40 | 0.52 | 0.64 | 0.36 | 0.78 | 0.68 | 0.20 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino-Moya, P.J.; Blanco, M.; Lobón, S.; Bertolín, J.R.; Armengol, R.; Joy, M. The Inclusion of Concentrate with Quebracho Is Advisable in Two Forage-Based Diets of Ewes According to the In Vitro Fermentation Parameters. Animals 2019, 9, 451. https://doi.org/10.3390/ani9070451
Rufino-Moya PJ, Blanco M, Lobón S, Bertolín JR, Armengol R, Joy M. The Inclusion of Concentrate with Quebracho Is Advisable in Two Forage-Based Diets of Ewes According to the In Vitro Fermentation Parameters. Animals. 2019; 9(7):451. https://doi.org/10.3390/ani9070451
Chicago/Turabian StyleRufino-Moya, Pablo Jose, Mireia Blanco, Sandra Lobón, Juan Ramon Bertolín, Ramón Armengol, and Margalida Joy. 2019. "The Inclusion of Concentrate with Quebracho Is Advisable in Two Forage-Based Diets of Ewes According to the In Vitro Fermentation Parameters" Animals 9, no. 7: 451. https://doi.org/10.3390/ani9070451
APA StyleRufino-Moya, P. J., Blanco, M., Lobón, S., Bertolín, J. R., Armengol, R., & Joy, M. (2019). The Inclusion of Concentrate with Quebracho Is Advisable in Two Forage-Based Diets of Ewes According to the In Vitro Fermentation Parameters. Animals, 9(7), 451. https://doi.org/10.3390/ani9070451





