1. Introduction
In China, a shortage of protein resources is an important limiting factor to the economic benefit of pig production [
1]. In 2016, China’s soybean imports were 8391 million tons and accounted for more than 26% of the worldwide production, while high-protein (HP) diets led to excretion of excess nitrogen in feces and urine, resulting in low efficiency of nitrogen utilization and environmental pollution. The National Research Council (NRC, 1998) recommends that the requirement of crude protein is 20%, 18%, and 16% for weaned piglets, growing pigs, and finishing pigs, respectively. Previous researches showed that reducing the dietary protein level by less than 4% based on the NRC (1998), supplemented with Lys, Met, Thr, and Trp, did not reduce growth performance of weanling, growing, and finishing pigs [
2,
3,
4,
5]. Thus, reducing dietary protein level is an effective strategy to save the protein resource and decrease the emission of nitrogen in urea and feces without impairing the growth performance in pigs. However, the requirement of crude protein recommended by the latest edition of NRC (2012) is 2–4% lower than that of NRC (1998). Whether the dietary protein level can be further reduced based on NRC (2012) and the responses of growth performance, gut development, and microbiota to low-protein diets are unclear.
The intestinal epithelium morphology is the structural basis for digestive and absorptive functions. Villous height in weaned piglets was not affected by the reduction of dietary crude protein from 21% to 17% when supplemented with Lys, Met, Thr, Trp, Ile, and Val [
6]. Another research supplemented eight essential amino acids in a low-protein diet with crude protein (CP) levels reduced from 23.1% to 18.9% and did not note villous atrophy in piglets after two weeks [
7]; however, further reduction to 17.2% was associated with impaired villous height in both the duodenum and jejunum. Whether long-term protein restriction impairs the villous morphology of pigs remains unknown.
Before dietary protein is absorbed by the villous, it is broken down into peptides and amino acids by proteinase in the gastrointestinal tract (GIT). The production of pepsin from pepsinogen is induced by gastric hydrochloric acid (HCl) regulated by the enteric endocrinal systems [
8]. Somatostatin (SS), which secretes from the D cells of the gastric oxyntic and pyloric mucosa, directly inhibits HCl secretion from parietal cells and indirectly inhibits gastrin secretion from G cells. Gastrin acts as the principal secretagogue of HCl and stimulates both acid secretion and negative feedback inhibition of acid secretion via SS. H
+-K
+-ATPase is expressed in the parietal cells, where it mediates the electro-neutral exchange of intracellular H
+ and extracellular K
+ to achieve acid secretion. However, information about the interaction between dietary protein level and enteric endocrine hormone is limited, especially for pigs fed a long-term low-protein diet.
The pig GIT harbors trillions of commensal bacteria that play a major role in the health of the host. The community and metabolic activities of gut microbiota are affected by dietary protein level composition [
9,
10,
11]. Weaning pigs fed CP levels reduced by 23% to 17% with Lys, Met, Thr, and Trp supplementation had no effect on microbial populations in the ileal and colonic digesta [
12]. However, another study showed that weaning pigs fed CP levels reduced by 20% to 14% with balanced Lys, Met, Thr, and Trp reduced Shannon indices of bacterial diversity and the number of
Firmicutes and
Clostridium Cluster IV in the cecal digesta, decreased concentrations of cecal ammonia, and reduced concentrations of acetate and branch chain fatty acids [
13]. A reduction of dietary CP levels from 22.5% to 17.6% for weaning pigs decreased colonic ammonia, but had no effects on short chain fatty acids [
14]. Considering the various complex metabolites in the hindgut and their potential roles in the host, an understanding of the influence of long-term low-protein diets on gut microbiota and metabolites is needed.
Gut homeostasis is important for pig growth. Thus, the aim of this study was to investigate the effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs, which may provide a theoretical explanation for the change of growth performance under the condition of reducing dietary protein. This study is expected to provide a good foundation for future diet formula in practical use and contributes to saving a large quantity of protein.
2. Materials and Methods
This study was conducted and approved by the Nanjing Agricultural University Animal Care and Use Committee. The license number is SYXK-2017-0027 with a period of validity to 19 June 2022.
2.1. Animals, Experimental Design, and Diet
Eighteen Duroc × Landrace × Yorkshire weaned barrows (35 days of age, average body weight of 9.46 ± 0.61 kg) were randomly assigned using a randomized block design into a normal protein (NP) diet group, a moderately low-protein (MP) diet group, in which protein was reduced by 3% compared to that of the NP group, and a low-protein (LP) diet group, in which protein was reduced by 6% compared to the NP group (
Table 1). Each group consisted of six replicates, and the trial lasted 16 weeks. Experimental diets in the MP and LP groups were supplemented with four essential amino acids (L-lysine, L-methionine, L-threonine, and L-tryptophan) to meet the requirements of weaned, growing, and finishing pigs according to NRC (2012). Pigs were maintained individually in metabolic cages with free access to feed and drinking water.
2.2. Blood Biochemical Parameters and Hormone Analysis
After a 16-week trial, blood samples were collected by jugular venipuncture after fasted for 12 h, followed by 3000× g at 4 °C for 15 min; serum was separated and immediately stored at −20 °C for further biochemical parameter analysis. Serum biochemical parameters, including total protein, blood urea nitrogen, glucose, cholesterol, and triglycerides, were evaluated using commercial kits according to the manufacturers’ instructions (Nanjing Angle Gene Biotechnology, Nanjing, China). Blood hormones of serum were analyzed using commercially-available ELISA kits specific to porcine tissues, according to the manufacturers’ instructions (Nanjing Angle Gene Biotechnology, Nanjing, China).
2.3. Digestive Enzyme and Intestinal Morphology
Pigs fasted overnight were anesthetized with pentobarbital sodium (50 mg/kg body weight). After jugular exsanguination, the abdomen was incised, and the GIT was immediately removed and rinsed with saline (0.9% NaCl). The digesta of the stomach, jejunum, ileum, and colon were separately collected and stored at −20 °C. The intestinal tissues of the duodenum, jejunum, and ileum (~2 cm long) were obtained and stored in a 10% formaldehyde solution. The villous height and crypt depth were determined. Briefly, each tissue sample was used to prepare five slices of three sections (5 µm thick), which were stained in hematoxylin and eosin with intact and well-oriented crypt-villus units selected for intestinal morphology analysis (Scion Image Software, Version 4.02, 2004, Meyer Instruments, Inc., Houston, TX, USA). The activities of gastric pepsin and trypsin in the digesta were analyzed using commercially-available pepsin and trypsin assay kits according to the manufacturer’s instructions (ANG-SH-21041 and ANG-SH-21052, Nanjing Angle Gene Biotechnology, Nanjing, China).
2.4. Determination of Short-Chain Fatty Acids, Ammonia-N, and Biogenic Amines
Colonic luminal contents were collected into separate plastic bags for full mixture. One hundred grams of mixed colonic contents were taken and stored in liquid nitrogen for the analysis of the microbial community. The remaining contents were stored at −20 °C to measure the fermentation metabolites, including SCFAs, ammonia-N (NH3-N), and biogenic amines.
Three grams of each sample were weighed into a 10-mL tube and mixed with 1 mL of 25% (w/v) metaphosphoric acid and 6 mL of water. Samples were then centrifuged at 17,000× g for 10 min, and the supernatant was removed to analyze SCFA concentration using a capillary column gas chromatograph (GC-14B, Shimadzu, Japan; capillary column: 30 m × 0.32 mm × 0.25 mm film thickness).
The concentrations of biogenic amines in the digesta were determined using the method described by [
15]. Briefly, 200 mg of the colonic content was homogenized with 1 mL trichloroacetic acid to precipitate proteins. The mixed solution was centrifuged at 3600 rpm for 10 min, and the supernatant was transferred to a new tube and mixed with an equal volume of n-hexane for fat extraction. This procedure was repeated several times. Then, 20 μL of internal standard, 1.5 mL of saturated sodium bicarbonate solution, 1 mL of dansyl chloride, and 1 mL of NaOH were added to the pretreated sample and vortexed at 60 °C for 45 min. One hundred milliliters of ammonia were added to the mixture to stop the reaction. Finally, the sample was extracted with diethyl ether, dried, and re-dissolved in acetonitrile for injection. The pretreated samples were analyzed by high performance liquid chromatography (HPLC) (Agilent EE00, Palo Alto, CA, USA) equipped with an ultraviolet (UV) detector (Waters 2998, 254 nm, Agilent Technologies, Palo Alto, CA, USA) and a reversed phase column Zorbax Extend-C18 (Agilent Technologies, Palo Alto, CA, USA), 5 mm (250 × 4.6 mm).
For NH
3-N determination, one gram of colonic digesta was transferred into a 1.5-mL Eppendorf tube, and 1 mL of 0.2 M HCl was added to acidify the samples. After homogenization, the mixed solution was centrifuged at 4000 rpm for 10 min; then, a 40-µL supernatant was removed and acidified with 0.96 mL of 0.2 M HCl and stored in a freezer (20 °C) for NH
3-N analysis. NH
3-N concentration was measured using the indophenol method [
16].
2.5. Illumina MiSeq Sequencing
Universal primers targeting V3 to V4 variable regions of the bacterial 16S rRNA gene were applied for PCR amplification and subsequent Illumina MiSeq sequencing to analyze the microbial community. The forward primer was 341F 5′-barcode- CCTAYGGGRBGCASCAG-3′, and the reverse primer was 806R 5′-GGACTACNNGGGTATCTAAT-3′ [
17], for which the barcode was an eight-based sequence unique to each sample. The PCR cycling protocol consisted of an initial denaturation at 95 °C for 3 min, followed by 27 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. A 20-μL mixed reaction was composed of 0.8 μL of 5 μM of each primer, 0.4 μL of Pfu polymerase, 2 μL of 2.5 mM dNTPs, 4 μL of 5-fold Pfu buffer (TransGen Biotech, Shanghai, China), and 10 ng of template DNA. The PCR amplicons were separated by 2% agarose gel electrophoresis and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Shanghai, China). The concentration of PCR products was quantified using QuantiFluor™-ST (Promega, Madison, WI, USA). Then, amplicon pyrosequencing on the IlluminaMiSeq platform was carried out as recommended in the manufacturer’s instructions (Shanghai Technology Majorbio Bio-Pharm Co., Ltd., Shanghai, China).
Universal primers targeting V3 to V4 variable regions of the bacterial 16S rRNA gene were applied for PCR amplification and subsequent Illumina MiSeq sequencing to analyze the microbial community. The forward primer was 341F 5′-barcode- CCTAYGGGRBGCASCAG-3′, and the reverse primer was 806R 5′-GGACTACNNGGGTATCTAAT-3′ [
17], for which the barcode was an eight-based sequence unique to each sample. The PCR cycling protocol consisted of an initial denaturation at 95 °C for 3 min, followed by 27 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. A 20-μL mixed reaction was composed of 0.8 μL of 5 μM of each primer, 0.4 μL of Pfu polymerase, 2 μL of 2.5 mM dNTPs, 4 μL of 5-fold Pfu buffer (TransGen Biotech, Shanghai, China), and 10 ng of template DNA. The PCR amplicons were separated by 2% agarose gel electrophoresis and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Shanghai, China). The concentration of PCR products was quantified using QuantiFluor™-ST (Promega, Madison, WI, USA). Then, amplicon pyrosequencing on the IlluminaMiSeq platform was carried out as recommended in the manufacturer’s instructions (Shanghai Technology Majorbio Bio-Pharm Co., Ltd., Shanghai, China).
2.6. Sequencing Data Analysis
After sequencing, all reads were filtered using QIIME (Version 1.17, Majorbio, Shanghai, China) for quality control. Reads shorter than 50 bp that contained one or more ambiguous bases and two nucleotide mismatches in primer matching were removed. The reads that overlapped longer than 10 bp were assembled. The operational taxonomic units of the effective sequences were carried out based on a 97% similarity cutoff using UPARSE (Version 7.1, Majorbio, Shanghai, China). The chimeric sequences were identified and removed using UCHIME (Majorbio, Shanghai, China). The phylogenetic affiliation of each 16S rRNA gene sequence was analyzed using the RDP Classifier (
http://rdp.cme.msu.edu/) with a standard minimum support threshold of 80% [
18]. The coverage was calculated to evaluate the sampling effort using Good’s method [
19]. The richness estimator (using ACE and Chao indices) and the diversity estimator (using Shannon and Simpson indices) were analyzed using the Mothur program [
20].
2.7. Quantification of Bacterial Populations Using Real-Time PCR
The bacterial groups, including total bacteria and the genes of
Lactobacillus,
Bacteroides,
Ruminococcus,
Clostridium Cluster IV,
Enterobacteriaceae, and
Clostridium Cluster XIV, were quantified using real-time PCR (qPCR) with specific primers (
Supplementary Materials Table S1) and SYBR Green PCR Mastermix (Applied Biosystems, Foster City, CA, USA) in the StepOnePlus real-time PCR system (Life Technologies, Carlsbad, CA, USA), as previously described [
21].
2.8. Statistical Analysis
Statistical analyses were performed using a one-way analysis of variance (ANOVA) with Statistical Software Package (SPSS) 16.0 (IBM, Armonk, NY, USA). The Student–Newman–Keuls multiple range test was employed to compare differences among treatment means. Differences at p < 0.05 were considered significant.
5. Conclusions
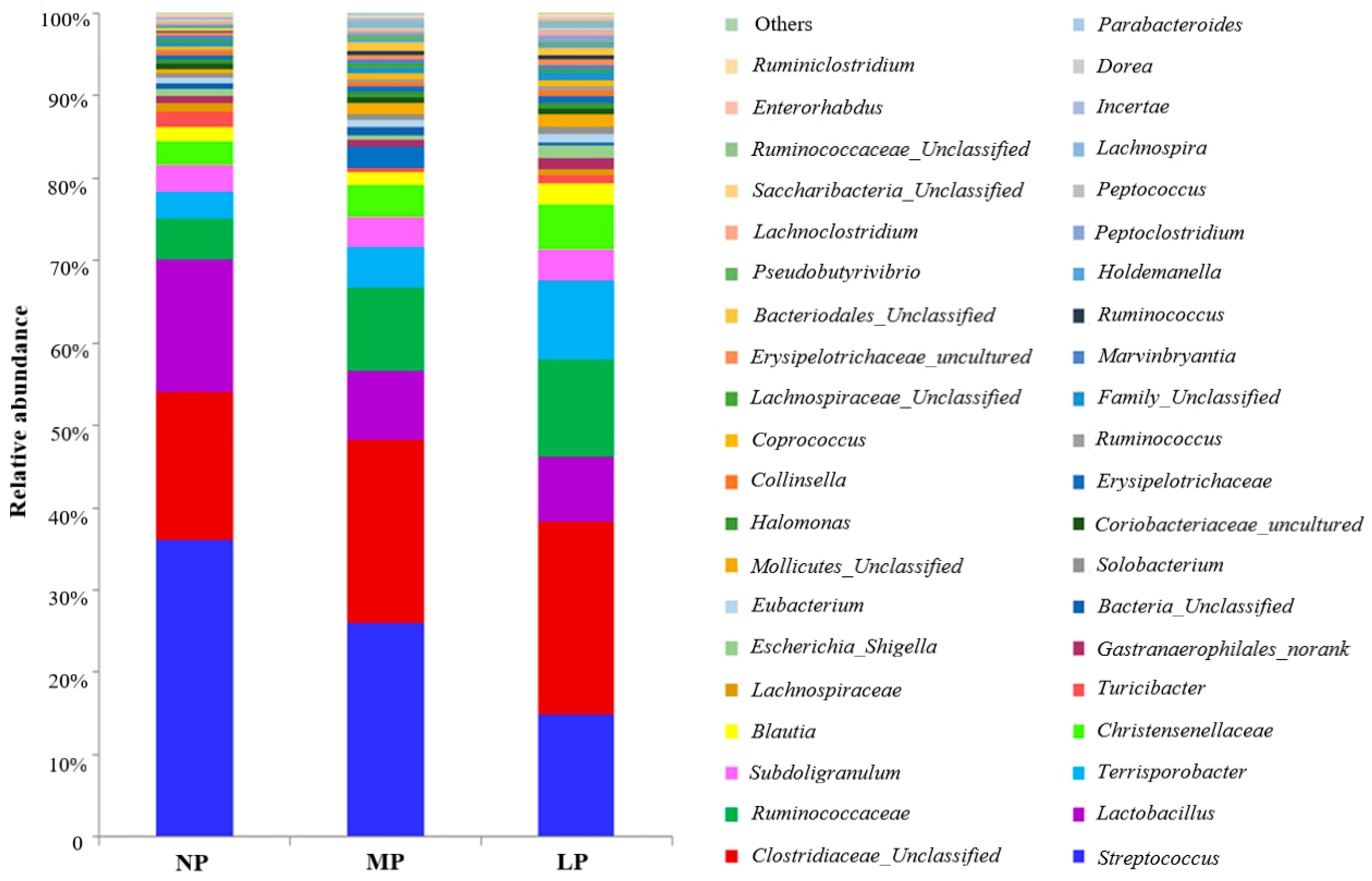
In summary, when presented with moderate dietary protein restriction, pigs are able to adjust their absorption and consumption of nutrients to maintain growth performance. However, extremely low-protein diets suppress appetite and reduce energy expenditure, although glucose homeostasis remains stable. Protein-restriction diets affect colonic microbial composition at the genus level, while bacteria diversity showed no significant difference. The production of microbial fermentation was decreased by extremely low-protein diets. However, the large number of pigs and the optimal length of different feeding periods should be considered when the effect of low-protein diets on the growth performance, gut development, and microbiota is investigated in future studies. Moreover, the production scale should also be included.