Geese Reared in Vineyard: Soil, Grass and Animals Interaction
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Geese Grazing
2.3. Soil Sampling
2.4. Chemical Soil Analysis
2.5. Soil Microbial Biomass C and Basal Respiration
2.6. Soil Microbial Community Structure
2.7. Cu Determination in Soil, Grass and Animal
2.8. Statistical Analysis
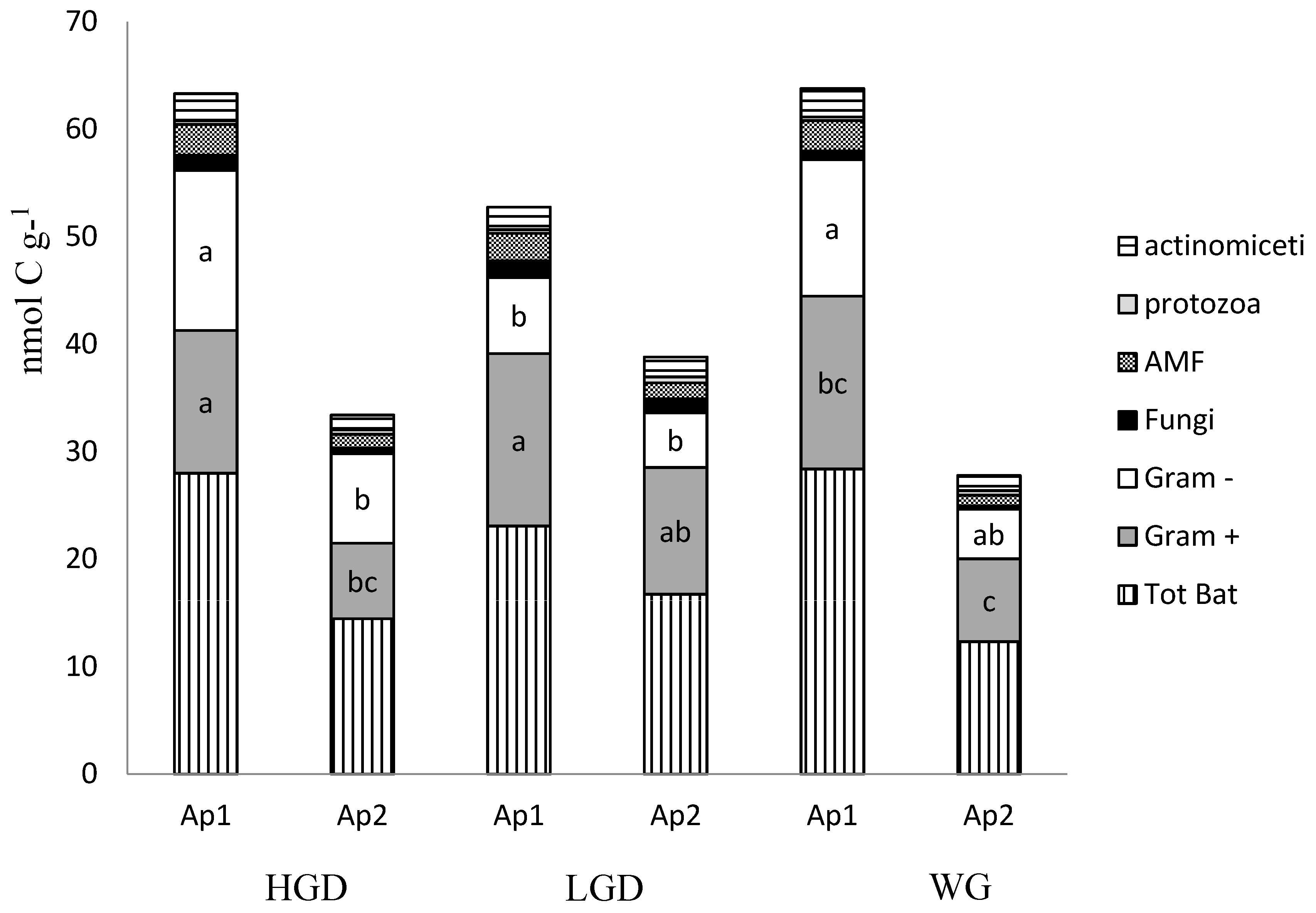
3. Results and Discussion
3.1. Soil Properties
- (i)
- the reduction of the input deriving from the grass cover due to the geese grazing (about 259 and 129 kg C/ha 100 d, respectively in HGD and LGD);
- (ii)
- a possible degradation of the geese droppings, which remains on the soil surface with a consequent loss of C in form of CO2 emission toward the atmosphere.
3.2. Copper Cycle in the System Soil-Grass-Geese
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fraser, D. Animal welfare assurance programs in food production: A framework for assessing the options. Anim. Welf. 2006, 15, 93. [Google Scholar]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Cartoni Mancinelli, A.; Mattioli, S.; Dal Bosco, A.; Piottoli, L.; Ranucci, D.; Branciari, R.; Cotozzolo, E.; Castellini, C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim.Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Phelps, L.N.; Kaplan, J.O. Land use for animal production in global change studies: Defining and characterizing a framework. Glob. Chang. Biol. 2017, 23, 4457–4471. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, N.; Niccolucci, V.; Castellini, C.; Pulselli, F.M.; Bastianoni, S. Sustainability evaluation of agro-livestock integration: Implications and results of Emergy evaluation. Sci. Total Environ. 2018, 622–623, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.E. Benefits of re-integrating livestock and forages in crop production systems. J. Crop Improv. 2004, 12, 405–436. [Google Scholar] [CrossRef]
- Hanson, J.D.; Franzluebbers, A. Principles of integrated agricultural systems. Renew. Agric. Food Syst. 2008, 23, 263–264. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Hanson, J.D.; Tanaka, D.L.; Sassenrath, G. Principles of integrated agricultural systems: Introduction to processes and definition. Renewable Agric. Food Syst. 2008, 23, 265–271. [Google Scholar] [CrossRef]
- Hendrickson, J.R.; Liebig, M.A.; Sassenrath, G. Environment and integrated agricultural systems. Agric. Food Syst. 2008, 23, 304–313. [Google Scholar] [CrossRef]
- Russelle, M.P.; Entz, M.H.; Franzluebbers, A.J. Reconsidering integrated crop-livestock systems in North America. Agron. J. 2007, 99, 325–334. [Google Scholar] [CrossRef]
- Tanaka, D.L.; Anderson, R.L.; Rao, S.C. Crop sequencing to improve use of precipitation and synergize crop growth. Agron. J. 2005, 97, 385–390. [Google Scholar] [CrossRef]
- Watson, C.A.; Öborn, I.; Eriksen, J.; Edwards, A.C. Perspectives on nutrient management in mixed farming systems. Soil Use Manag. 2005, 21, 132–140. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Allen, V. Soil Microbial, Chemical and Physical Properties in Continuous Cotton and Integrated Crop–Livestock Systems. Soil Sci. Soc. Am. J. 2004, 68, 1875–1884. [Google Scholar] [CrossRef]
- Maughan, M.W.; Flores, J.P.C.; Anghinoni, I.; Bollero, G.; Fernandez, F.G.; Tracy, B.G. Soil quality and corn yield under crop-livestock integration in Illinois. Agron. J. 2009, 101, 1503–1510. [Google Scholar] [CrossRef]
- Lowy, P. Integrating poultry and sheep on vegetable cropping land for increased economic return and enhanced fertility. In Sustainable Agriculture Research and Education Project Database; Farmer/Rancher Project: Chesterfield, MO, USA, 2009. [Google Scholar]
- FAO. FAOSTAT. Commodities by Country. 2011. Available online: http://faostat/fao.org/ (accessed on 2 March 2019).
- OIV (International Organization of Vine and Wine). Statistical Report on World Viticulture 2012. Available online: http://www.oiv.int (accessed on 2 March 2019).
- Hilimire, K. Integrated Crop/Livestock Agriculture in the United States: A Review. J. Sustain. Agric. 2011, 35, 376–393. [Google Scholar] [CrossRef]
- Paolotti, L.; Boggia, A.; Castellini, C.; Rocchi, L.; Rosati, A. Combining livestock and tree crops to improve sustainability in agriculture: A case study using the Life Cycle Assessment (LCA) approach. J. Clean. Prod. 2016, 131, 351–363. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastingsa, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA–Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Lantinga, E.A.; Neuteboom, J.H.; Meijs, J.A.C. Sward methods. In Herbage Intake Hand Book, 2nd ed.; Penning, P.D., Ed.; The British Grassland Society: Reading, PA, USA, 2004; pp. 23–52. [Google Scholar]
- Dal Bosco, A.; Mugnai, C.; Rosati, A.; Paoletti, A.; Caporali, S.; Castellini, C. Effect of range enrichment on performance, behavior, and forage intake of free-range chickens. J. Appl. Poult. Res. 2014, 23, 137–145. [Google Scholar] [CrossRef]
- Kear, J. The agricultural importance of wild goose droppings. Wildfowl 1963, 14, 72–77. [Google Scholar]
- AOAC. Official Methods of Analysis of the AOAC International, 16th ed.; Method 970.12; Association of Official Analytical Chemists International: Washington, DC, USA, 1995. [Google Scholar]
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C. Soil Survey Staff. In Field Book for Describing and Sampling Soils; Version 3.0.; Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2012. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3. Chemical Methods; Sparks, D.L., Ed.; SSSA and ASA: Madison WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Agnelli, A.; Bol, R.; Trumbore, S.E.; Dixon, L.; Cocco, S.; Corti, G. Carbon and nitrogen in soil and vine roots in harrowed and grass-covered vineyards. Agric. Ecosyst. Environ. 2014, 193, 70–82. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegård, Å. Changes in soil fungal:bacterial biomass following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlid, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Fritze, H.; Pietikainen, J.; Pennanen, T. Distribution of microbial biomass and phospholipid fatty acids in Podzol profiles under coniferous forest. Eur. J. Soil Sci. 2000, 51, 565–573. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variation in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Federle, T.W. Microbial distribution in soil new techniques. In Perspectives in Microbial Ecology; Megusar, F., Gantar, M., Eds.; Slovene Society for Microbiology: Ljubljana, Slovenia, 1986; pp. 493–498. [Google Scholar]
- Massaccesi, L.; Benucci, G.M.N.; Gigliotti, G.; Cocco, S.; Corti, G.; Agnelli, A. Rhizosphere effect of three plant species of environment under periglacial conditions (Majella Massif, central Italy). Soil Biol. Biochem. 2015, 89, 184–195. [Google Scholar] [CrossRef]
- De Deyn, G.; Quirk, H.; Bardgett, R. Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility. Biol. Lett. 2011, 7, 75–78. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Chung, H.; Zak, D.R.; Reich, P.B.; Ellsworth, D.S. Plant species richness, elevated CO2, and atmospheric nitrogen deposition alter soil microbial community composition and function. Glob. Chang. Biol. 2007, 13, 980–989. [Google Scholar] [CrossRef]
- Kroppenstedt, R.M. Fatty acid and menaquinone analysis of actinomycetes and related organisms. In Chemical Methods in Bacterial Systematics, Society for Applied Bacteriology (Technical Series No. 20); Goodfellow, M., Minnikin, D.E., Eds.; Academic Press: London, UK, 1985; pp. 173–199. [Google Scholar]
- Box, G.E.P.; Cox, D.R. Analysis of transformations. J. R. Stat. Soc. Ser. B Stat. Methodol. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Agnelli, A.; Massaccesi, L.; De Feudis, M.; Cocco, S.; Courchesne, F.; Corti, G. Holm oak (Quercus ilex L.) rhizosphere affects limestone-derived soil under a multi-centennial forest. Plant Soil 2016, 400, 297–314. [Google Scholar] [CrossRef]
- Khaleel, R.; Reddy, K.R.; Overcash, M.R. Transport of potential pollutants in runoff water from land areas receiving animal wastes: A review. Water Res. 1980, 14, 421–436. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Guenet, B.; Neill, C.; Bardoux, G.; Abbadie, L. Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl. Soil Ecol. 2010, 46, 436–442. [Google Scholar] [CrossRef]
- De Nobili, M.; Contin, M.; Mondini, C.; Brookes, P.C. Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol. Biochem. 2001, 33, 1163–1170. [Google Scholar] [CrossRef]
- Drake, J.E.; Darby, B.A.; Giasson, M.A.; Kramer, M.A.; Phillips, R.P.; Finzi, A.C. Stoichiometry constrains microbial response to root exudation—Insights from model and a field experiment in a temperate forest. Biogeochem. Discuss. 2012, 9, 6899–6945. [Google Scholar] [CrossRef]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Banyikwa, F.F.; McNaughton, M.M. Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 1997, 278, 1798–1800. [Google Scholar] [CrossRef]
- Frank, D.A.; Groffman, P.M. Ungulate vs. Landscape control of soil c and n processes in grasslands of Yellowstone national park. Ecology 1998, 79, 2229–2241. [Google Scholar] [CrossRef]
- Frank, D.A.; Evans, R.D. Effects of native grazers on grassland N cycling in Yellowstone National Park. Ecology 1997, 78, 2238–2248. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A.; Yeates, G.W. Linking above-ground and below-ground interactions: How plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 1998, 30, 1867–1878. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Herbivore-mediated linkages between aboveground and belowground communities. Ecology 2003, 84, 2258–2268. [Google Scholar] [CrossRef]
- de Faccio Carvalho, P.C.; Anghinoni, I.; de Moraes, A.; de Souza, E.D.; Sulc, R.M.; Lang, C.R.; Flores, J.P.; Lopes, M.L.; da Silva, J.L.; Conte, O.; et al. Managing grazing animals to achieve nutrient cycling and soil improvement in no-till integrated systems. Nutr. Cycl. Agroecosyst. 2010, 88, 259–273. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Lu, J.; SantoDomingo, J.W.; Hill, S.; Edge, T.A. Microbial Diversity and Host-Specific Sequences of Canada Goose Feces. Appl. Environ. Microbiol. 2009, 75, 5919–5926. [Google Scholar] [CrossRef]
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996; ISBN 0-9631172-1-1. [Google Scholar]
- Mor, G.; Kwon, J.-Y. Trophoblast-microbiome interaction: A new paradigm on immune regulation. Am. J. Obstet. Gynecol. 2015, 213, S131–S137. [Google Scholar] [CrossRef]
- Parat, C.; Chaussod, R.; Lévéque, J.; Dousset, S.; Andreux, F. The relationship between cupper accumulated in vineyard calcareous soil and soil organic matter and iron. Eu. J. Soil Sci. 2002, 53, 663–669. [Google Scholar] [CrossRef]
- Viti, C.; Quaranta, D.; De Philippis, R.; Corti, G.; Agnelli, A.; Cuniglio, R.; Giovannetti, L. Characterizing cultivable soil microbial communities from copper fungicide-amended olive orchard and vineyard soils. World J. Microbiol. Biotechnol. 2008, 24, 309–318. [Google Scholar] [CrossRef]
- Duplay, J.; Semhi, K.; Errais, E.; Imfeld, G.; Babcsanyi, I.; Perrone, T. Copper, zinc, lead and cadmium bioavailability and retention in vineyard soils (Rouffach, France): The impact of cultural practices. Geoderma 2014, 230–231, 318–328. [Google Scholar] [CrossRef]
- Besnard, E.; Chenu, C.; Robert, M. Influence of organic amendments on copper distribution among particle-size and density fractions in Champagne vineyard soils. Environ. Pollut. 2000, 112, 329–337. [Google Scholar] [CrossRef]
- Lucia, M.; André, J.M.; Bernadet, M.D.; Gontier, K.; Gérard, G.; Davail, S. Concentrations of metals (zinc, copper, cadmium, and mercury) in three domestic ducks in France: Pekin, muscovy, and mule ducks. J. Agric. Food Chem. 2007, 56, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chiou, P.W.S.; Chen, K.L.; Yu, B. Toxicity, tissue accumulation and residue in egg and excreta of copper in laying hens. Anim. Feed Sci. Technol. 1997, 67, 49–60. [Google Scholar] [CrossRef]
- Skřivan, M.; Skřivanová, V.; Marounek, M. Effect of various copper supplements to feed of laying hens on Cu content in eggs, liver, excreta, soil, and herbage. Arch. Environ. Contam. Toxicol. 2006, 50, 280–283. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Yohannes, Y.B.; Mizukawa, H.; Ishizuka, M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J. Manganese, copper, zinc, iron, cadmium, mercury and lead in muscle meat, liver and kidneys of poultry, rabbit and sheep slaughtered in the northern part of Poland, 1987. Food Addit. Contam. 1991, 8, 71–83. [Google Scholar] [CrossRef]
| SITE 1 | pH | TOC 2 | WEOC 3 |
|---|---|---|---|
| Horizons | g kg−1 | ||
| HGD | |||
| Ap1 | 7.85 (0.00) c | 14 (2) a | 0.4 (0.1) a |
| Ap2 | 7.97 (0.01) a | 9.9 (0.4) ab | 0.53 (0.02) a |
| LGD | |||
| Ap1 | 7.88 (0.00) bc | 9.9 (0.3) ab | 0.45 (0.03) a |
| Ap2 | 7.92 (0.02) ab | 6.6 (0.7) b | 0.36 (0.01) b |
| WG | |||
| Ap1 | 7.64 (0.01) d | 12.9 (0.1) a | 0.38 (0.08) a |
| Ap2 | 7.83 (0.01) c | 9.2 (0.9) ab | 0.37 (0.08) a |
| Compounds 1 | K | N | P | C | |
|---|---|---|---|---|---|
| SITE 2 | kg d.m./ha | ||||
| Input | HGD | 18.7 | 43.05 | 18.72 | 1310 |
| LGD | 9.3 | 21.52 | 9.36 | 655 | |
| Intake | HGD | - | 45.28 | 9.81 | 259 |
| LGD | - | 22.64 | 4.90 | 129 | |
| SITE 1 | Compounds 2 | |||
|---|---|---|---|---|
| Total N | NH4+-N | NO3−-N | Organic N | |
| Horizons | g kg−1 | mg kg−1 | mg kg−1 | g kg−1 |
| HGD | ||||
| Ap1 | 0.9 (0.1) a | 27 (1) b | 6.5 (0.1) a | 0.9 (0.1) a |
| Ap2 | 0.8 (0.1) a | 23.8 (0.5) b | 0.74 (0.06) c | 0.8 (0.1) a |
| LGD | ||||
| Ap1 | 1.08 (0.03) a | 23.6 (0.7) b | 0.81 (0.02) c | 1.06 (0.03) a |
| Ap2 | 0.98 (0.01) a | 23 (2) b | 1.75 (0.09) b | 0.96 (0.02) a |
| WG | ||||
| Ap1 | 1.15 (0.06) a | 27 (1) b | 2.6 (0.3) b | 1.12 (0.06) a |
| Ap2 | 1.16 (0.05) a | 41 (2) a | 7 (2) a | 1.11 (0.05) a |
| SITE 2 | Cmic | ΣCO2-C | Cmic/TOC Ratio |
|---|---|---|---|
| Horizons | mg kg−1 | mg kg−1 | |
| HGD | |||
| Ap1 | 1705 (213) ab | 420 (11) c | 0.12 (0.00) ab |
| Ap2 | 1255 (58) ab | 584 (15) b | 0.13 (0.00) ab |
| LGD | |||
| Ap1 | 2229 (453) a | 644 (16) b | 0.22 (0.05) a |
| Ap2 | 1827 (170) a | 315 (2) d | 0.27 (0.06) a |
| WG | |||
| Ap1 | 1041 (113) bc | 875 (32) a | 0.08 (0.01) b |
| Ap2 | 796 (19) c | 326 (2) d | 0.09 (0.01) b |
| Site 1 | LGD | HWG | WG |
|---|---|---|---|
| Feed | 8.0 (1) | 8.0 (1) | 8.0 (1) |
| Soil Ap1 | 45 (2) | 39 (3) | 58 (3) |
| Soil Ap2 | 40 (3) | 36 (3) | 52 (5) |
| Roots | 25 (1) | 27 (1) | 22 (2) |
| Grass | 11 (0.9) | 11 (0.8) | 11 (0.5) |
| Traits | Unit of Measure | LGD | HGD | Control |
|---|---|---|---|---|
| Estimated Cu intake | mg/d | 4 (1) | 3 (1) | 1 (0.9) |
| Liver | mg kg−1 | 152 (5) b | 144 (5) b | 95 (3) a |
| Breast meat | mg kg−1 | 3 (1) | 3 (1) | 3 (1) |
| Drumstick meat | mg kg−1 | 1 (1) | 1 (0.8) | 0.9 (0.5) |
| Feces | mg kg−1 | 59 (4) b | 55 (3) b | 23 (3) a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaccesi, L.; Cartoni Mancinelli, A.; Mattioli, S.; De Feudis, M.; Castellini, C.; Dal Bosco, A.; Marongiu, M.L.; Agnelli, A. Geese Reared in Vineyard: Soil, Grass and Animals Interaction. Animals 2019, 9, 179. https://doi.org/10.3390/ani9040179
Massaccesi L, Cartoni Mancinelli A, Mattioli S, De Feudis M, Castellini C, Dal Bosco A, Marongiu ML, Agnelli A. Geese Reared in Vineyard: Soil, Grass and Animals Interaction. Animals. 2019; 9(4):179. https://doi.org/10.3390/ani9040179
Chicago/Turabian StyleMassaccesi, Luisa, Alice Cartoni Mancinelli, Simona Mattioli, Mauro De Feudis, Cesare Castellini, Alessandro Dal Bosco, Maria Laura Marongiu, and Alberto Agnelli. 2019. "Geese Reared in Vineyard: Soil, Grass and Animals Interaction" Animals 9, no. 4: 179. https://doi.org/10.3390/ani9040179
APA StyleMassaccesi, L., Cartoni Mancinelli, A., Mattioli, S., De Feudis, M., Castellini, C., Dal Bosco, A., Marongiu, M. L., & Agnelli, A. (2019). Geese Reared in Vineyard: Soil, Grass and Animals Interaction. Animals, 9(4), 179. https://doi.org/10.3390/ani9040179







