Effect of Morphine Administration on Social and Non-Social Play Behaviour in Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Design
2.2. Experimental Design
2.2.1. Behaviour
2.2.2. Cortisol Concentrations
2.3. Statistical Analysis
3. Results
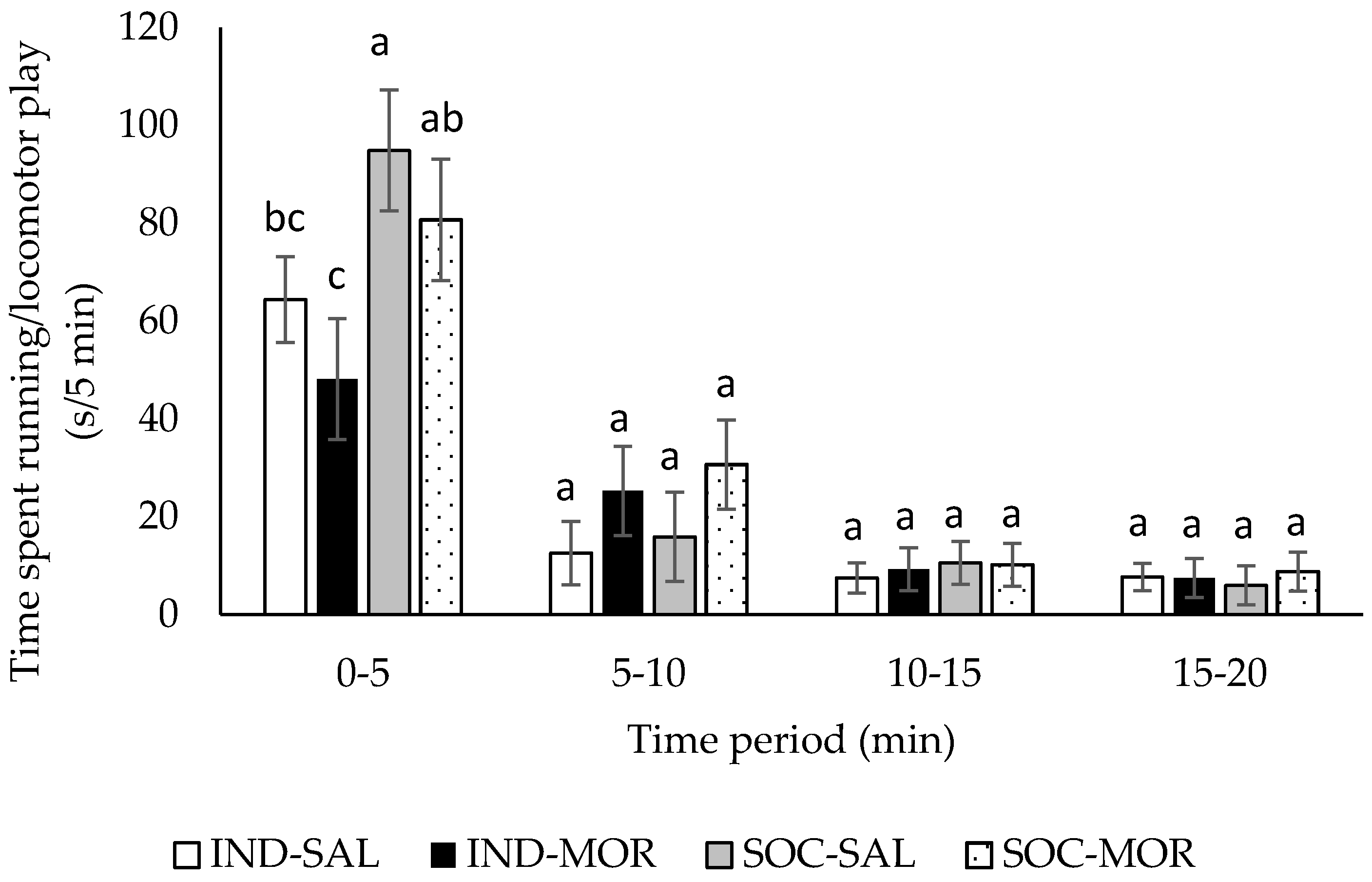
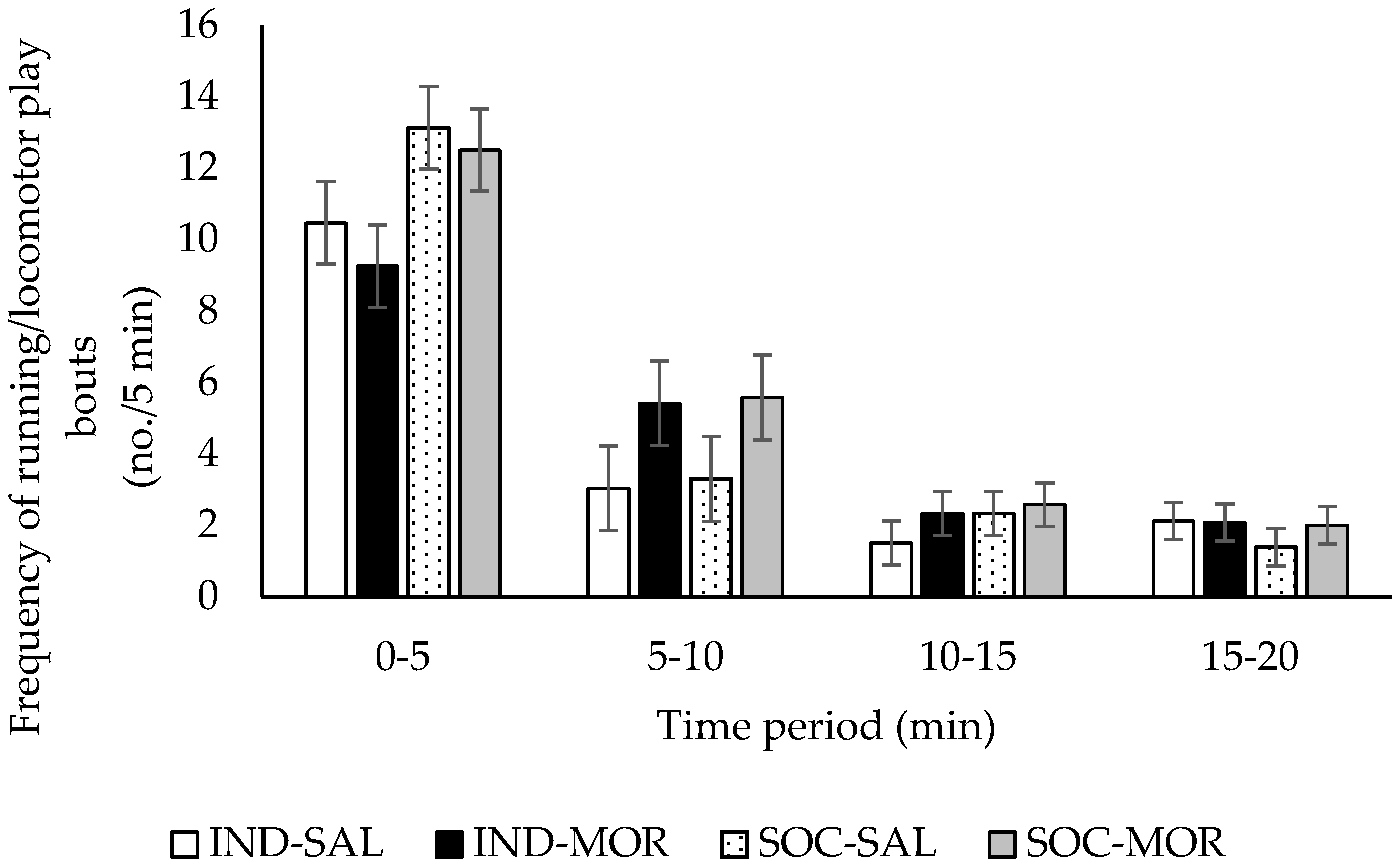
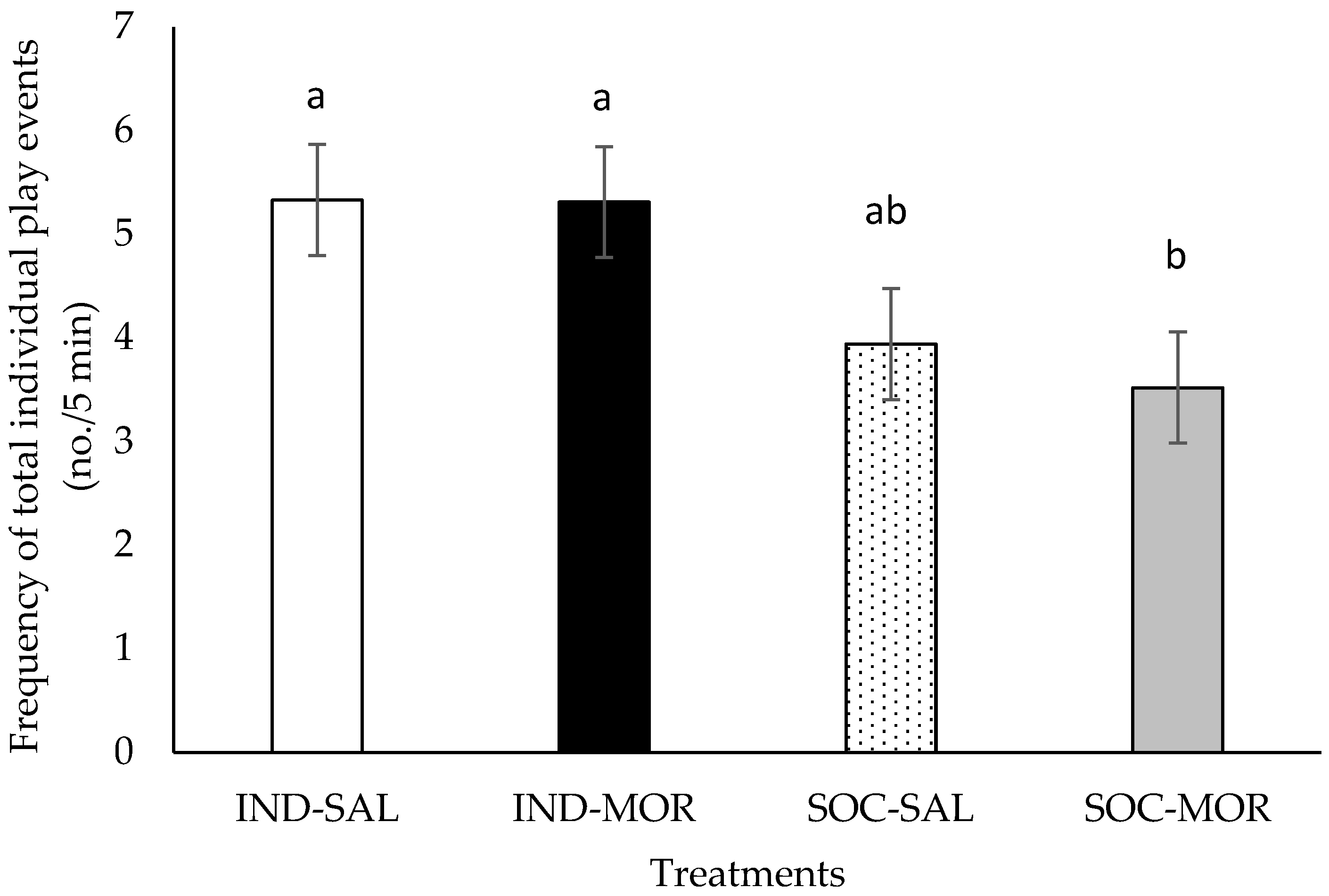
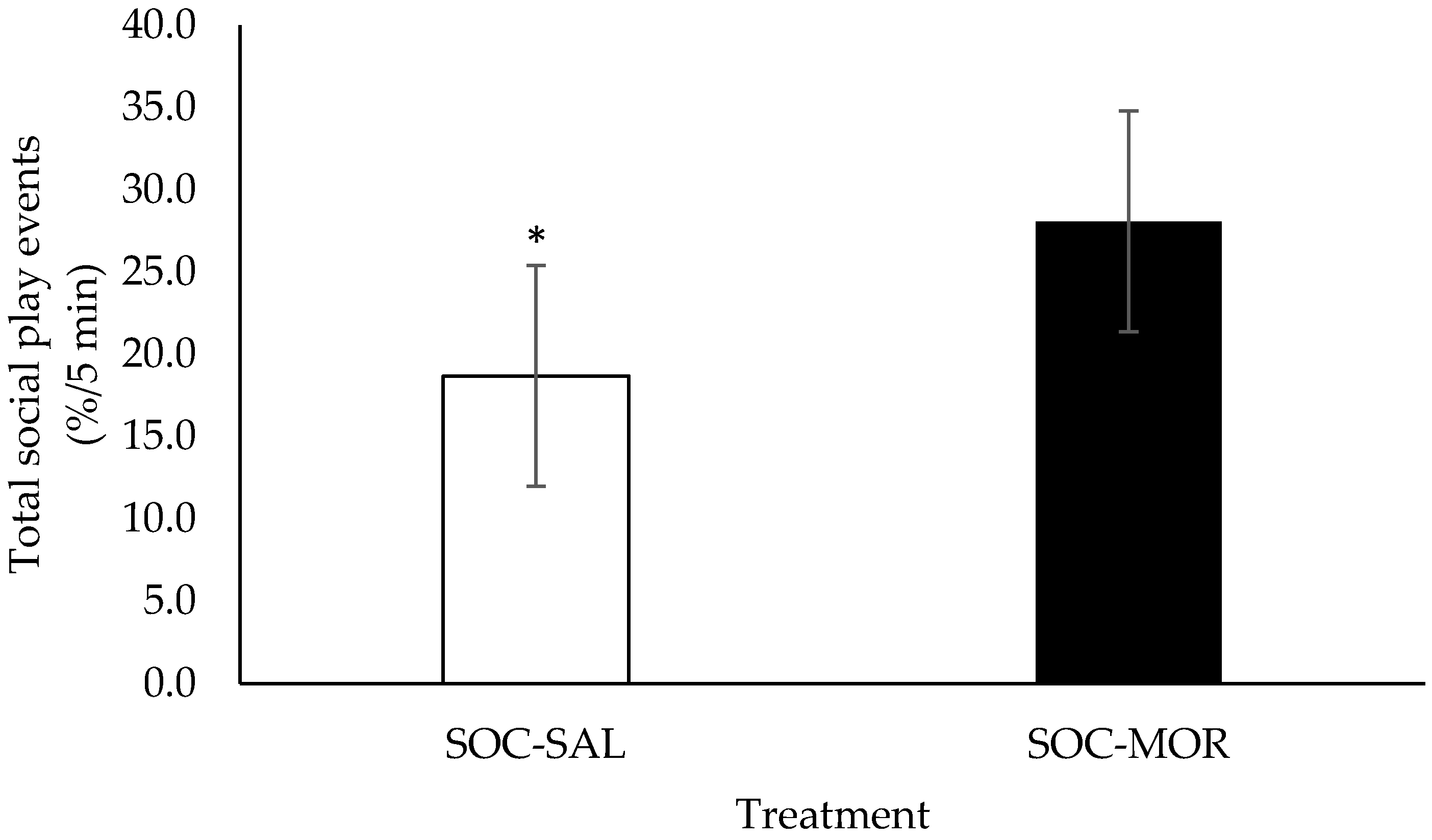
3.1. Behaviour
3.2. Cortisol Concentrations
4. Discussion
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Krachun, C.; Rushen, J.; de Passillé, A.M. Play behaviour in dairy calves is reduced by weaning and by a low energy intake. Appl. Anim. Behav. Sci. 2010, 122, 71–76. [Google Scholar] [CrossRef]
- Rushen, J.; de Passillé, A.M. Automated measurement of acceleration can detect effects of age, dehorning and weaning on locomotor play of calves. Appl. Anim. Behav. Sci. 2012, 139, 169–174. [Google Scholar] [CrossRef]
- Held, S.D.E.; Špinka, M. Animal play and animal welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Niesink, R.J.; Van Ree, J.M. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997, 21, 309–326. [Google Scholar] [CrossRef]
- Trezza, V.; Campolongo, P.; Vanderschuren, L.J.M.J. Evaluating the rewarding nature of social interactions in laboratory animals. Dev. Cogn. Neurosci. 2011, 1, 444–458. [Google Scholar] [CrossRef]
- Trezza, V.; Baarendse, P.J.J.; Vanderschuren, L.J.M.J. The pleasures of play: Pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010, 31, 463–469. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [PubMed]
- Berridge, K.C. Pleasures of the brain. Brain Cogn. 2003, 52, 106–128. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Tuominen, L. Opioid system and human emotions. Brit. J. Pharmacol. 2018, 175, 2737–2749. [Google Scholar]
- Ragen, B.J.; Maninger, N.; Mendoza, S.P.; Bales, K.L. The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus). Neuroscience 2015, 287, 32–42. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Niesink, R.J.M.; Spruijt, B.M.; Van Ree, J.M. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology 1995, 117, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Normansell, L.; Panksepp, J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev. Psychobiol. 1990, 23, 75–83. [Google Scholar] [CrossRef]
- Guard, H.J.; Newman, J.D.; Roberts, R.L. Morphine administration selectively facilitates social play in common marmosets. Dev. Psychobiol. 2002, 41, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Tančin, V.; Schams, D.; Kraetzl, W.-D. Cortisol and ACTH release in dairy cows in response to machine milking after pretreatment with morphine and naloxone. J. Dairy. Res. 2000, 67, 467–474. [Google Scholar]
- Parrott, R.F.; Goode, J.A. Effects of Intracerebroventricular Corticotropin-Releasing Hormone and Intravenous Morphine on Cortisol, Prolactin and Growth-Hormone Secretion in Sheep. Dom. Anim. Endocrinol. 1992, 9, 141–149. [Google Scholar] [CrossRef]
- Parrott, R.F.; Thornton, S.N. Opioid influences on pituitary function in sheep under basal conditions and during psychological stress. Psychoneuroendocrinology 1989, 14, 451–459. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.A.; Schütz, K.E.; Stewart, M. Rearing substrate and space allowance influences locomotor play behaviour of dairy calves in an arena test. Appl. Anim. Behav. Sci. 2014, 154, 8–14. [Google Scholar] [CrossRef]
- Verbeek, E.; Ferguson, D.; de Monjour, P.Q.; Lee, C. Opioid control of behaviour in sheep: Effects of morphine and naloxone on food intake, activity and the affective state. Appl. Anim. Behav. Sci. 2012, 142, 18–29. [Google Scholar] [CrossRef]
- Worth, G.A.; Stewart, M.; Cave, V.; Schütz, K.E.; Foster, M.; Sutherland, M.A. Dairy calves’ preference for rearing substrate. Appl. Anim. Behav. Sci. 2015, 168, 1–9. [Google Scholar] [CrossRef]
- Bonk, S.; Burfeind, O.; Suthar, V.S.; Heuwieser, W. Technical note: Evaluation of data loggers for measuring lying behavior in dairy calves. J. Dairy. Sci. 2013, 96, 3265–3271. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Larive, J.; Cave, V.; Zobel, G. Behavioural and physiological responses to clove oil injected under the horn bud of calves. Appl. Anim. Behav. Sci. 2018, 204, 29–36. [Google Scholar] [CrossRef]
- AWP UBC. UBC Animal Welfare Program: SOP—HOBO Data Loggers, SAS CODE Version 1; UBC: Vancouver, BC, Canada, 2016. [Google Scholar]
- Vanderschuren, L.J.M.J.; Achterberg, E.J.M.; Trezza, V. The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehavioral. Rev. 2016, 70, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Ahloy-Dallaire, J.; Espinosa, J.; Mason, G. Play and optimal welfare: Does play indicate the presence of positive affective states? Behav. Process. 2018, 156, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzo, J.W.; Sutton-smith, B.; Atkins, M.; Meyers, R.; Stevenson, H.; Coolahan, K.; Weiss, A.; Manz, P. Community-based resilient peer treatment of withdrawn maltreated preschool children. J. Consult. Clin. Psychol. 1996, 64, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Gariépy, N.; Howe, H. The therapeutic power of play: Examining the play of young children with leukaemia. Child Care Heal. Dev. 2003, 29, 523–537. [Google Scholar] [CrossRef]
- Altmann, E.O.; Gotlib, I.H. The social behavior of depressed children: An observational study. J. Abnorm. Child Psychol. 1988, 16, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Trombin, T.F.; Procópio-Souza, R.; Kameda, S.R.; Zanlorenci, L.H.F.; Fukushiro, D.F.; Calzavara, M.B.; Wuo-Silva, R.; Mári-Kawamoto, E.; Costa, J.M.; Zanier-Gomes, P.H.; et al. Environmental novelty modulates the induction and expression of single injection-induced behavioral sensitization to morphine. Pharmacol. Biochem. Behav. 2018, 173, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Vestergaard, K.S.; Krohn, C.C. Play behaviour in dairy cattle kept in pens: The effect of social contact and space allowance. Appl. Anim. Behav. Sci. 1998, 56, 97–108. [Google Scholar] [CrossRef]
- Jensen, M.B. Short communication: A note on the effect of isolation during testing and length of previous confinement on locomotor behaviour during open-field test in dairy calves. Appl. Anim. Behav. Sci. 2001, 70, 309–315. [Google Scholar] [CrossRef]
- Nanda, A.S.; Dobson, H.; Ward, W.R. Opioid modulation of the hypothalamo-pituitary-adrenal axis in dairy cows. Dom. Anim. Endocrinol. 1992, 9, 181–186. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.-C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Vanderschuren, L.J.M.J. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur. Neuropsychopharmacol. 2008, 18, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Otovic, P.; Hutchinson, E. Limits to using HPA axis activity as an indication of animal welfare. ALTEX 2015, 32, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, A.C.; Taylor, J.H.; Birnie, A.K.; Huffman, M.C.; French, J.A. Gestational cortisol and social play shape development of marmosets’ HPA functioning and behavioral responses to stressors. Dev. Psychobiol. 2014, 56, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Norscia, I.; Palagi, E. When play is a family business: Adult play, hierarchy, and possible stress reduction in common marmosets. Primates 2011, 52, 101–104. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, C.L.; Hol, T.; Van Ree, J.M.; Spruijt, B.M.; Everts, H.; Koolhaas, J.M. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999, 34, 129–138. [Google Scholar] [CrossRef]
| Behaviour | Description |
|---|---|
| Running/locomotor play 1 | Rapid forward or sideways movement, including trotting (two-beat gait), cantering (three-beat gait) and galloping (fast four-beat gait). Lasting longer than 1 s in real time. |
| Individual Play Events | |
| Kicking 2 | One or both hind legs lifted off the ground in one rapid movement, legs extended outwards from the body. The calf can be stationary or moving. |
| Bucking 2 | One or both hind legs lifted off the ground in one rapid movement and extended outwards from the body. Hooves are raised as high as or higher than the front knees of the forelegs. |
| Jumping 1 | Both forelegs lifted from the ground and stretched forward. Movement upwards but not forwards. The hind legs may be elevated. |
| Social Play Events | |
| Frontal pushing 1 | Two calves are standing front to front, butting head against head/neck. |
| Mounting 1 | A calf mounts another calf’s head or body from front, side or back. |
| Variable | Treatment | SAL vs. MOR | IND vs. SOC | Time | Treatment × Time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F(3,29)-Value | p-Value | F(1,29)-Value | p-Value | F(1,29)-Value | p-Value | F(3,120)-Value | p-Value | F(9,120)-Value | p-Value | |
| Duration of running/locomotor play | 4.266 | 0.013 | 0.003 | 0.956 | 13.391 | 0.001 | 5.781 | 0.001 | 2.492 | 0.012 |
| Frequency of running/locomotor play | 2.730 | 0.062 | 2.625 | 0.116 | 5.506 | 0.026 | 5.781 | 0.001 | 1.265 | 0.263 |
| Frequency of total individual play events | 3.101 | 0.042 | 0.168 | 0.685 | 9.230 | 0.005 | 5.781 | 0.001 | 1.563 | 0.134 |
| Percentage of total social play events | 2.854 | 0.091 | - | - | - | - | 1.602 | 0.195 | 0.830 | 0.590 |
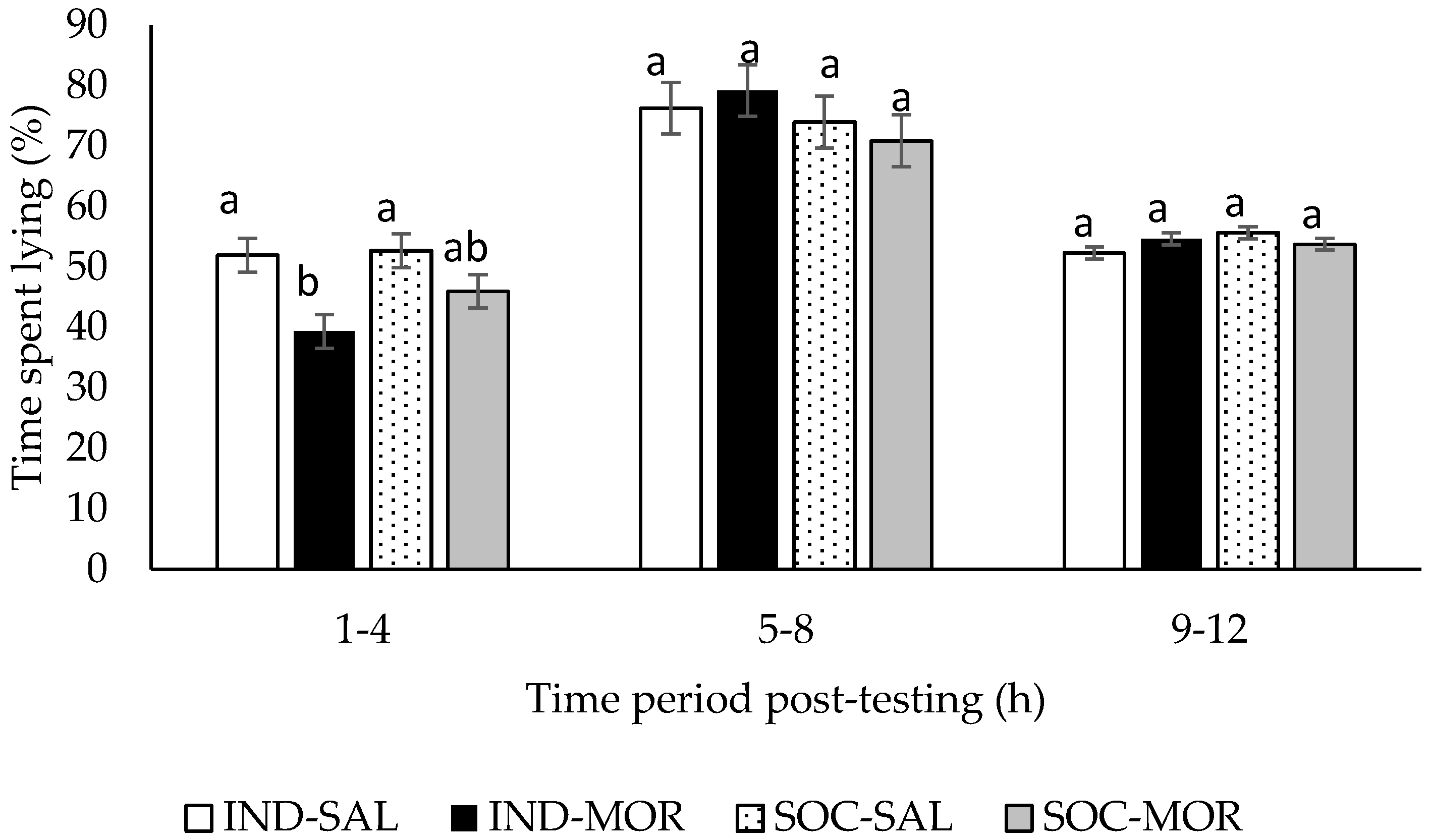
| Time spent lying (1–4 h post-treatment) | 4.914 | 0.007 | 11.534 | 0.002 | 1.704 | 0.202 | - | - | - | - |
| Time spent lying (5–8 h post-treatment) | 0.646 | 0.592 | 0.000 | 0.985 | 1.464 | 0.236 | - | - | - | - |
| Time spent lying (9–12 post-treatment) | 2.041 | 0.130 | 0.050 | 0.825 | 1.558 | 0.222 | - | - | - | - |
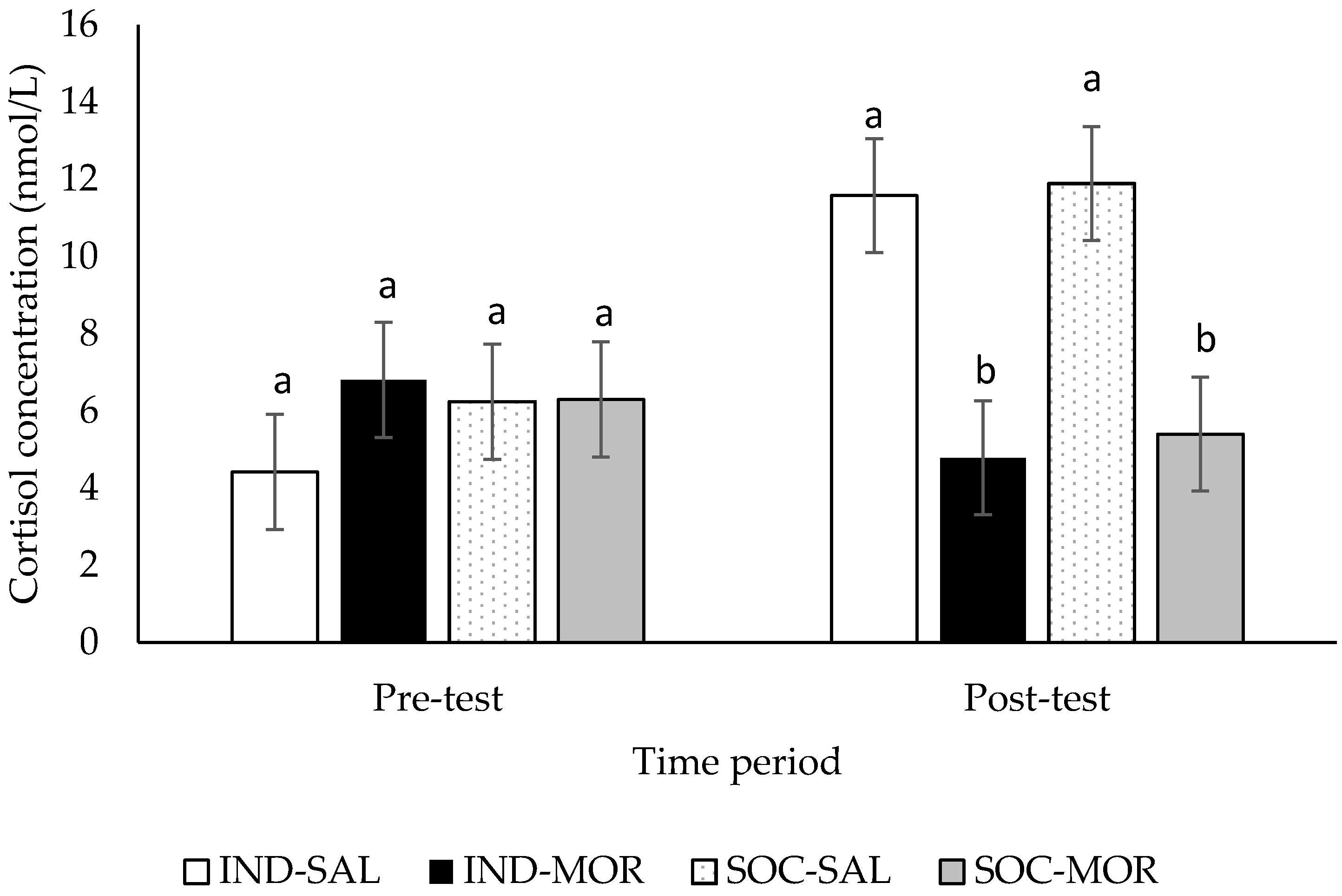
| Cortisol concentrations (pre-test) | 0.500 | 0.685 | 0.678 | 0.417 | 0.200 | 0.658 | - | - | - | - |
| Cortisol concentrations (post-test) | 7.121 | 0.001 | 13.391 | 0.001 | 0.104 | 0.749 | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutherland, M.; Worth, G.; Cameron, C.; Verbeek, E. Effect of Morphine Administration on Social and Non-Social Play Behaviour in Calves. Animals 2019, 9, 56. https://doi.org/10.3390/ani9020056
Sutherland M, Worth G, Cameron C, Verbeek E. Effect of Morphine Administration on Social and Non-Social Play Behaviour in Calves. Animals. 2019; 9(2):56. https://doi.org/10.3390/ani9020056
Chicago/Turabian StyleSutherland, Mhairi, Gemma Worth, Catherine Cameron, and Else Verbeek. 2019. "Effect of Morphine Administration on Social and Non-Social Play Behaviour in Calves" Animals 9, no. 2: 56. https://doi.org/10.3390/ani9020056
APA StyleSutherland, M., Worth, G., Cameron, C., & Verbeek, E. (2019). Effect of Morphine Administration on Social and Non-Social Play Behaviour in Calves. Animals, 9(2), 56. https://doi.org/10.3390/ani9020056





