Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement, Experimental Birds, and Specimen Collection
2.2. Granulosa Cell Culture and Leptin Treatment
2.3. RNA Extraction and cDNA Synthesis
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Statistical Analyses
3. Results
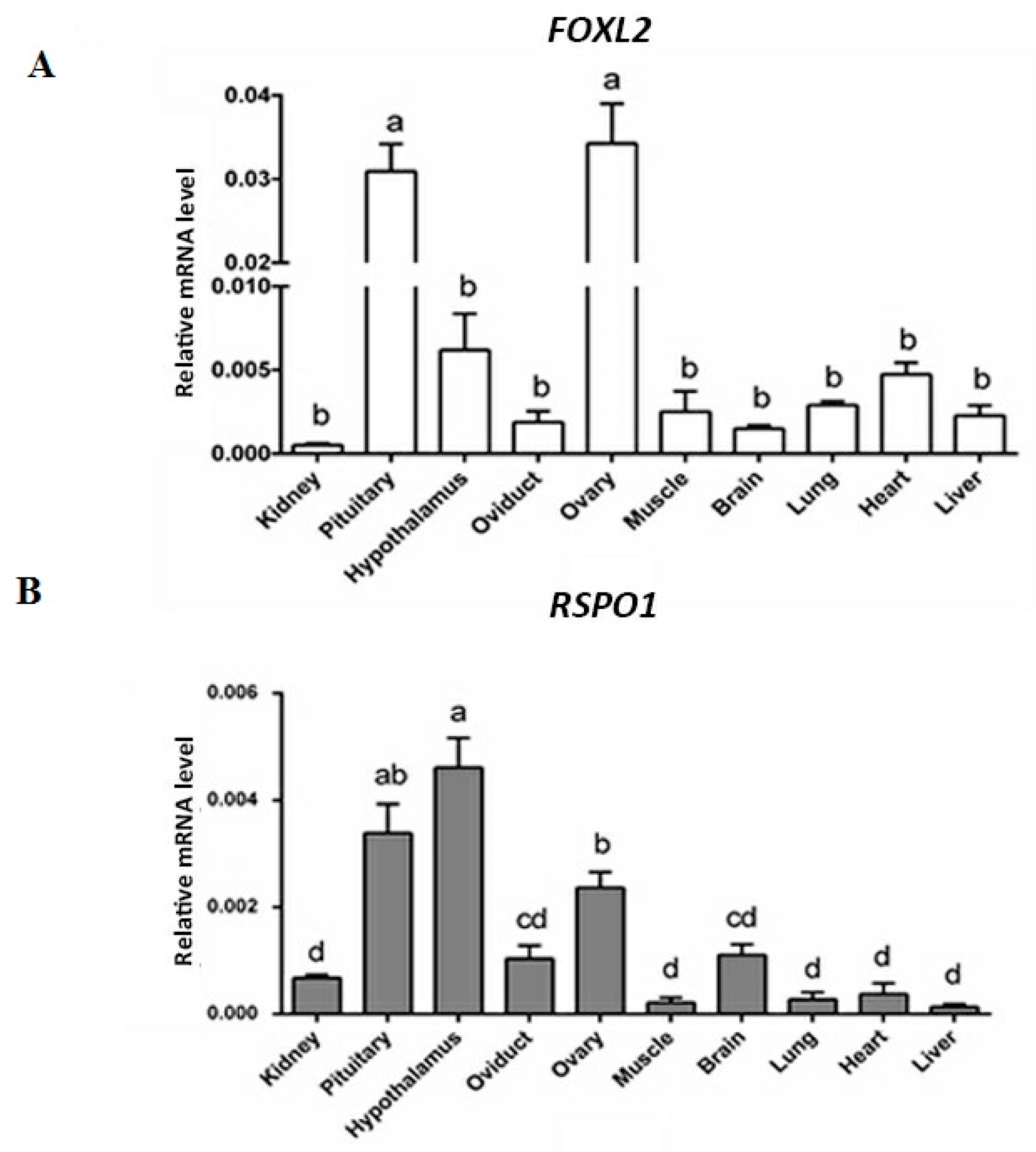
3.1. mRNA Expression of FOXL2 and RSPO1 in Different Central and Peripherial Tissues of Laying Hens
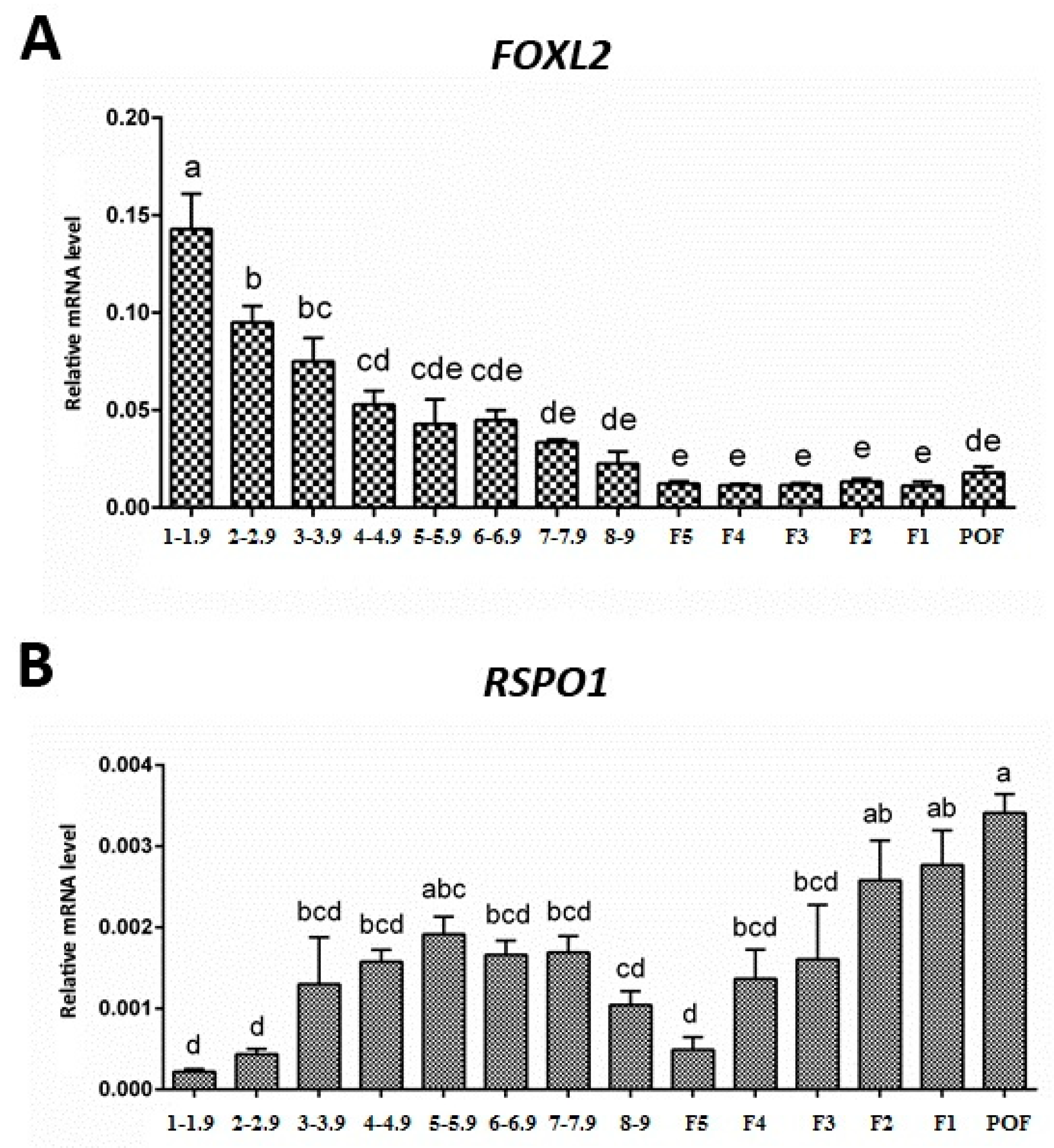
3.2. Expression of FOXL2 and RSPO1 in Ovarian Follicles at Different Stages of Development
3.3. mRNA Expression of FOXL2 and RSPO1 in Theca and Granulosa Cells of Ovarian Follicles
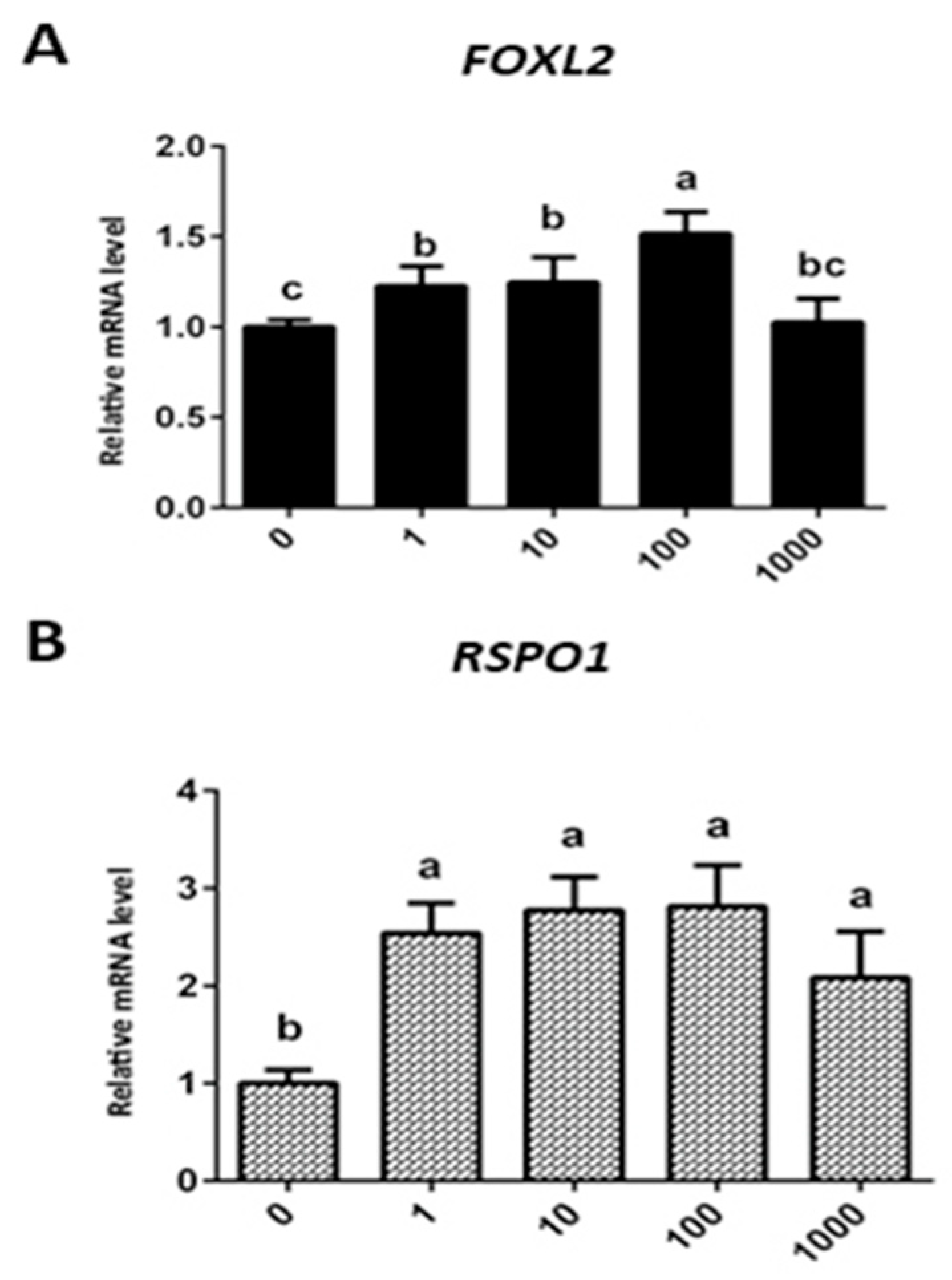
3.4. Effect of Exogenous Leptin Treatment on mRNA Expression of FOXL2 and RSPO1 in Granulosa Cells (In Vitro Cultured)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, A. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Woods, D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009, 163, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P. Follicle selection in the avian ovary. Reprod. Domest. Anim. 2012, 47, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gan, X.; Chen, D.; Huang, H.; Yuan, J.; Qiu, J.; Hu, S.; Hu, J.; Wang, J. Comparison of growth characteristics of in vitro cultured granulosa cells from geese follicles at different developmental stages. Biosci. Rep. 2018, 38, BSR20171361. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.O.; Bahr, J.M. Adenylyl cyclase system of the small preovulatory follicles of the domestic hen: Responsiveness to follicle-stimulating hormone and luteinizing hormone. Biol. Reprod. 1983, 29, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.C.; Johnson, A. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol. Reprod. 2005, 72, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. The avian ovary and follicle development: Some comparative and practical insights. Turk. J. Vet. Anim. Sci. 2014, 38, 660–669. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ritter, L.J.; Myllymaa, S.; Kaivo-Oja, N.; Dragovic, R.A.; Hickey, T.E.; Ritvos, O.; Mottershead, D.G. Molecular basis of oocyte-paracrine signalling that promotes granulosa cell proliferation. J. Cell Sci. 2006, 119, 3811–3821. [Google Scholar] [CrossRef]
- Ghanem, K.; Johnson, A.L. Response of hen pre-recruitment ovarian follicles to follicle stimulating hormone, in vivo. Gen. Comp. Endocrinol. 2019, 270, 41–47. [Google Scholar] [CrossRef]
- Kim, D.; Johnson, A.L. Differentiation of the granulosa layer from hen prehierarchal follicles associated with follicle-stimulating hormone receptor signaling. Mol. Reprod. Dev. 2018, 85, 729–737. [Google Scholar] [CrossRef]
- Johnson, A.; Lee, J. Granulosa cell responsiveness to follicle stimulating hormone during early growth of hen ovarian follicles. Poult. Sci. 2016, 95, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Liu, Z.; Guo, X.; Du, Y.; Yuan, Z.; Guo, M.; Kang, L.; Sun, Y.; Jiang, Y. Transcriptome Analysis on Single Small Yellow Follicles Reveals That Wnt4 Is Involved in Chicken Follicle Selection. Front. Endocrinol. 2017, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Bridgham, J.; Foster, D.; Johnson, A. Characterization of the chicken follicle-stimulating hormone receptor (cFSH-R) complementary deoxyribonucleic acid, and expression of cFSH-R messenger ribonucleic acid in the ovary. Biol. Reprod. 1996, 55, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.D.; Bae, J.; Klein, C.; Hsueh, A.J. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology 2004, 145, 3424–3433. [Google Scholar] [CrossRef]
- Pisarska, M.D.; Barlow, G.; Kuo, F.-T. Minireview: Roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology 2011, 152, 1199–1208. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Li, J.; Feng, Y.; Gong, Y. Transcriptome analysis of the potential roles of FOXL2 in chicken pre-hierarchical and pre-ovulatory granulosa cells. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 21, 56–66. [Google Scholar] [CrossRef]
- Qin, N.; Fan, X.-C.; Xu, X.-X.; Tyasi, T.L.; Li, S.-J.; Zhang, Y.-Y.; Wei, M.-L.; Xu, R.-F. Cooperative effects of FOXL2 with the members of TGF-β superfamily on FSH receptor mRNA expression and granulosa cell proliferation from hen prehierarchical follicles. PLoS ONE 2015, 10, e0141062. [Google Scholar] [CrossRef]
- Qin, N.; Liu, Q.; Zhang, Y.; Fan, X.; Xu, X.; Lv, Z.; Wei, M.; Jing, Y.; Mu, F.; Xu, R. Association of novel polymorphisms of forkhead box L2 and growth differentiation factor-9 genes with egg production traits in local Chinese Dagu hens. Poult. Sci. 2015, 94, 88–95. [Google Scholar] [CrossRef]
- Hudson, Q.J.; Smith, C.A.; Sinclair, A.H. Aromatase inhibition reduces expression of FOXL2 in the embryonic chicken ovary. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 1052–1055. [Google Scholar]
- McDerment, N.A.; Wilson, P.W.; Waddington, D.; Dunn, I.C.; Hocking, P.M. Identification of novel candidate genes for follicle selection in the broiler breeder ovary. BMC Genom. 2012, 13, 494. [Google Scholar] [CrossRef]
- Govoroun, M.S.; Pannetier, M.; Pailhoux, E.; Cocquet, J.; Brillard, J.P.; Couty, I.; Batellier, F.; Cotinot, C. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 231, 859–870. [Google Scholar]
- Chassot, A.A.; Gillot, I.; Chaboissier, M.C. R-spondin1, WNT4, and the CTNNB1 signaling pathway: Strict control over ovarian differentiation. Reproduction 2014, 148, R97–R110. [Google Scholar] [CrossRef] [PubMed]
- Chassot, A.-A.; Ranc, F.; Gregoire, E.P.; Roepers-Gajadien, H.L.; Taketo, M.M.; Camerino, G.; De Rooij, D.G.; Schedl, A.; Chaboissier, M.-C. Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008, 17, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.; Harley, V.R. Wnt4 action in gonadal development and sex determination. Int. J. Biochem. Cell Biol. 2007, 39, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Capel, B. R-spondin1 tips the balance in sex determination. Nat. Genet. 2006, 38, 1233–1234. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Wagle, M.; Tran, K.; Zhan, X.; Dixon, M.A.; Liu, S.; Gros, D.; Korver, W.; Yonkovich, S.; Tomasevic, N. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 2008, 19, 2588–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Charkraborty, T.; Zhou, Q.; Mohapatra, S.; Nagahama, Y.; Zhang, Y. Rspo1-activated signalling molecules are sufficient to induce ovarian differentiation in XY medaka (Oryzias latipes). Sci. Rep. 2016, 6, 19543. [Google Scholar] [CrossRef]
- Smith, C.A.; Shoemaker, C.M.; Roeszler, K.N.; Queen, J.; Crews, D.; Sinclair, A.H. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev. Biol. 2008, 8, 72. [Google Scholar] [CrossRef]
- Hausman, G.J.; Barb, C.R.; Lents, C.A. Leptin and reproductive function. Biochimie 2012, 94, 2075–2081. [Google Scholar] [CrossRef]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Beneš, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front. Endocrinol. 2018, 8, 367. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef] [PubMed]
- González, R.R.; Simón, C.; Caballero-Campo, P.; Norman, R.; Chardonnens, D.; Devoto, L.; Bischof, P. Leptin and reproduction. Hum. Reprod. Update 2000, 6, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Horev, G.; Einat, P.; Aharoni, T.; Eshdat, Y.; Friedman-Einat, M. Molecular cloning and properties of the chicken leptin-receptor (CLEPR) gene. Mol. Cell. Endocrinol. 2000, 162, 95–106. [Google Scholar] [CrossRef]
- Ohkubo, T.; Tanaka, M.; Nakashima, K. Structure and tissue distribution of chicken leptin receptor (cOb-R) mRNA. Biochim. Biophys. Acta Gene Struct. Expr. 2000, 1491, 303–308. [Google Scholar] [CrossRef]
- Paczoska-Eliasiewicz, H.; Gertler, A.; Proszkowiec, M.; Proudman, J.; Hrabia, A.; Sechman, A.; Mika, M.; Jacek, T.; Cassy, S.; Raver, N. Attenuation by leptin of the effects of fasting on ovarian function in hens (Gallus domesticus). Reproduction 2003, 126, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Paczoska-Eliasiewicz, H.E.; Proszkowiec-Weglarz, M.; Proudman, J.; Jacek, T.; Mika, M.; Sechman, A.; Rzasa, J.; Gertler, A. Exogenous leptin advances puberty in domestic hen. Domest. Anim. Endocrinol. 2006, 31, 211–226. [Google Scholar] [CrossRef]
- Hu, S.; Gan, C.; Wen, R.; Xiao, Q.; Gou, H.; Liu, H.; Zhang, Y.; Li, L.; Wang, J. Role of leptin in the regulation of sterol/steroid biosynthesis in goose granulosa cells. Theriogenology 2014, 82, 677–685. [Google Scholar] [CrossRef]
- Gilbert, A.B.; Evans, A.J.; Perry, M.M.; Davidson, M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 1977, 50, 179–181. [Google Scholar] [CrossRef]
- Sirotkin, A.; Grossmann, R. Leptin directly controls proliferation, apoptosis and secretory activity of cultured chicken ovarian cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 422–429. [Google Scholar] [CrossRef]
- Seroussi, E.; Cinnamon, Y.; Yosefi, S.; Genin, O.; Smith, J.G.; Rafati, N.; Bornelov, S.; Andersson, L.; Friedman-Einat, M. Identification of the Long-Sought Leptin in Chicken and Duck: Expression Pattern of the Highly GC-Rich Avian leptin Fits an Autocrine/Paracrine Rather Than Endocrine Function. Endocrinology 2016, 157, 737–751. [Google Scholar] [CrossRef]
- Seroussi, E.; Pitel, F.; Leroux, S.; Morisson, M.; Bornelov, S.; Miyara, S.; Yosefi, S.; Cogburn, L.A.; Burt, D.W.; Anderson, L.; et al. Mapping of leptin and its syntenic genes to chicken chromosome 1p. BMC Genet. 2017, 18, 77. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Duan, J.; Wu, H.; Li, J.; Jiang, Y.; Tang, H.; Li, X.; Kang, L. Expression dynamics of gonadotropin-releasing hormone-I and its mutual regulation with luteinizing hormone in chicken ovary and follicles. Gen. Comp. Endocrinol. 2019, 270, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Hu, S.; Xiao, Q.; Han, C.; Gan, C.; Gou, H.; Liu, H.; Li, L.; Xu, H.; He, H. Leptin exerts proliferative and anti-apoptotic effects on goose granulosa cells through the PI3K/Akt/mTOR signaling pathway. J. Steroid Biochem. Mol. Biol. 2015, 149, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Justice, N.J.; Blount, A.L.; Pelosi, E.; Schlessinger, D.; Vale, W.; Bilezikjian, L.M. Impaired FSHβ expression in the pituitaries of Foxl2 mutant animals. Mol. Endocrinol. 2011, 25, 1404–1415. [Google Scholar] [CrossRef]
- Wang, D.-S.; Kobayashi, T.; Zhou, L.-Y.; Paul-Prasanth, B.; Ijiri, S.; Sakai, F.; Okubo, K.; Morohashi, K.-I.; Nagahama, Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol. Endocrinol. 2007, 21, 712–725. [Google Scholar] [CrossRef]
- Wang, J.; Gong, Y. Transcription of CYP19A1 is directly regulated by SF-1 in the theca cells of ovary follicles in chicken. Gen. Comp. Endocrinol. 2017, 247, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, X.; Wang, L.; Li, R.; Yang, M.; Wang, S. Associations between Forkhead Box L2 Expression and Ovary Development in Laying Hens. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 2019, 25, 305–309. [Google Scholar] [CrossRef]
- Pannetier, M.; Chassot, A.A.; Chaboissier, M.C.; Pailhoux, E. Involvement of FOXL2 and RSPO1 in Ovarian Determination, Development, and Maintenance in Mammals. Sex. Dev. Genet. Mol. Biol. Evol. Endocrinol. Embryol. Pathol. Sex Determ. Differ. 2016, 10, 167–184. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Wang, C.; Lv, L.; Chen, Y.; Zuo, Z. Exogenous leptin promotes the recovery of regressed ovary in fasted ducks. Anim. Reprod. Sci. 2009, 110, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, C.; Wang, T.; Li, H.; Li, Y.; Ren, J.; Tian, Y.; Li, Z.; Jiao, Y.; Kang, X. Discovery and functional characterization of leptin and its receptors in Japanese quail (Coturnix japonica). Gen. Comp. Endocrinol. 2016, 225, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Friedman-Einat, M.; Seroussi, E. Avian Leptin: Bird’s-Eye View of the Evolution of Vertebrate Energy-Balance Control. Trends Endocrinol. Metab. 2019, 30, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Hen, G.; Yosefi, S.; Ronin, A.; Einat, P.; Rosenblum, C.I.; Denver, R.J.; Friedman-Einat, M. Monitoring leptin activity using the chicken leptin receptor. J. Endocrinol. 2008, 197, 325–333. [Google Scholar] [CrossRef]
- Denver, R.J.; Bonett, R.M.; Boorse, G.C. Evolution of leptin structure and function. Neuroendocrinology 2011, 94, 21–38. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′→3′) | Annealing Temperature (°C) | Accession Number | Product Length (bp) |
|---|---|---|---|---|
| FOXL2-F | CCTCAACGAGTGCTTCATCA | 60 | NM_001012612 | 299 |
| FOXL2-R | ACATCTGGCAAGAGGCGTAG | |||
| RSPO1-F | AAGGCTACTCTGCTGCCAAC | 60 | NM_001318444 | 295 |
| RSPO1-R | CGATTTCTGTTCCCGTTTGT | |||
| GAPDH-F | CTTTCCGTGTGCCAACCC | 61 | NM_204305.1 | 136 |
| GAPDH-R | CATCAGCAGCAGCCTTCACTAC | |||
| ACTB-F | TGGGTATGGAGTCCTGTGGT | 60 | L08165.1 | 160 |
| ACTB-R | AGGGCTGTGATCTCCTTCTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, W.; Qazi, I.H.; Li, S.; Zhao, X.; Yin, H.; Wang, Y.; Zhu, Q.; Han, H.; Zhou, G.; Du, X. Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells. Animals 2019, 9, 1083. https://doi.org/10.3390/ani9121083
Niu W, Qazi IH, Li S, Zhao X, Yin H, Wang Y, Zhu Q, Han H, Zhou G, Du X. Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells. Animals. 2019; 9(12):1083. https://doi.org/10.3390/ani9121083
Chicago/Turabian StyleNiu, Weihe, Izhar Hyder Qazi, Sichen Li, Xiaoling Zhao, Huadong Yin, Yan Wang, Qing Zhu, Hongbing Han, Guangbin Zhou, and Xiaohui Du. 2019. "Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells" Animals 9, no. 12: 1083. https://doi.org/10.3390/ani9121083
APA StyleNiu, W., Qazi, I. H., Li, S., Zhao, X., Yin, H., Wang, Y., Zhu, Q., Han, H., Zhou, G., & Du, X. (2019). Expression of FOXL2 and RSPO1 in Hen Ovarian Follicles and Implication of Exogenous Leptin in Modulating Their mRNA Expression in In Vitro Cultured Granulosa Cells. Animals, 9(12), 1083. https://doi.org/10.3390/ani9121083








