Physiological and Metabolic Responses of Mongolian Horses to a 20 km Endurance Exercise and Screening for New Oxidative-Imbalance Biomarkers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Experimental Animals
2.2. Blood Sample Collection Following High-Intensity Exercise
2.3. Heart Rate and Speed Monitoring
2.4. Hematological and Biochemical Analyses
2.5. Measurement of Blood Antioxidant Indices
2.6. Sample Preparation and LC-MS Analysis
2.7. Quality Controls and Validation of LC-MS Analysis Results
2.8. Statistical Analysis of Data
3. Results
3.1. Alterations in Heart Rate and Velocity of Mongolian Horses Across Varying Exercise Distances
3.2. Changes in Blood Routine Indexes of Mongolian Horses at Different Exercise Distances and Post-Exercise
3.3. Changes in Blood Biochemical Indexes of Mongolian Horses at Different Exercise Distances and Post-Exercise
3.4. Changes in Blood Antioxidant Indexes of Mongolian Horses at Different Exercise Distances and Post-Exercise
3.5. Mechanistic Changes in Blood Metabolomics of Mongolian Horses Pre- and Post-Exercise
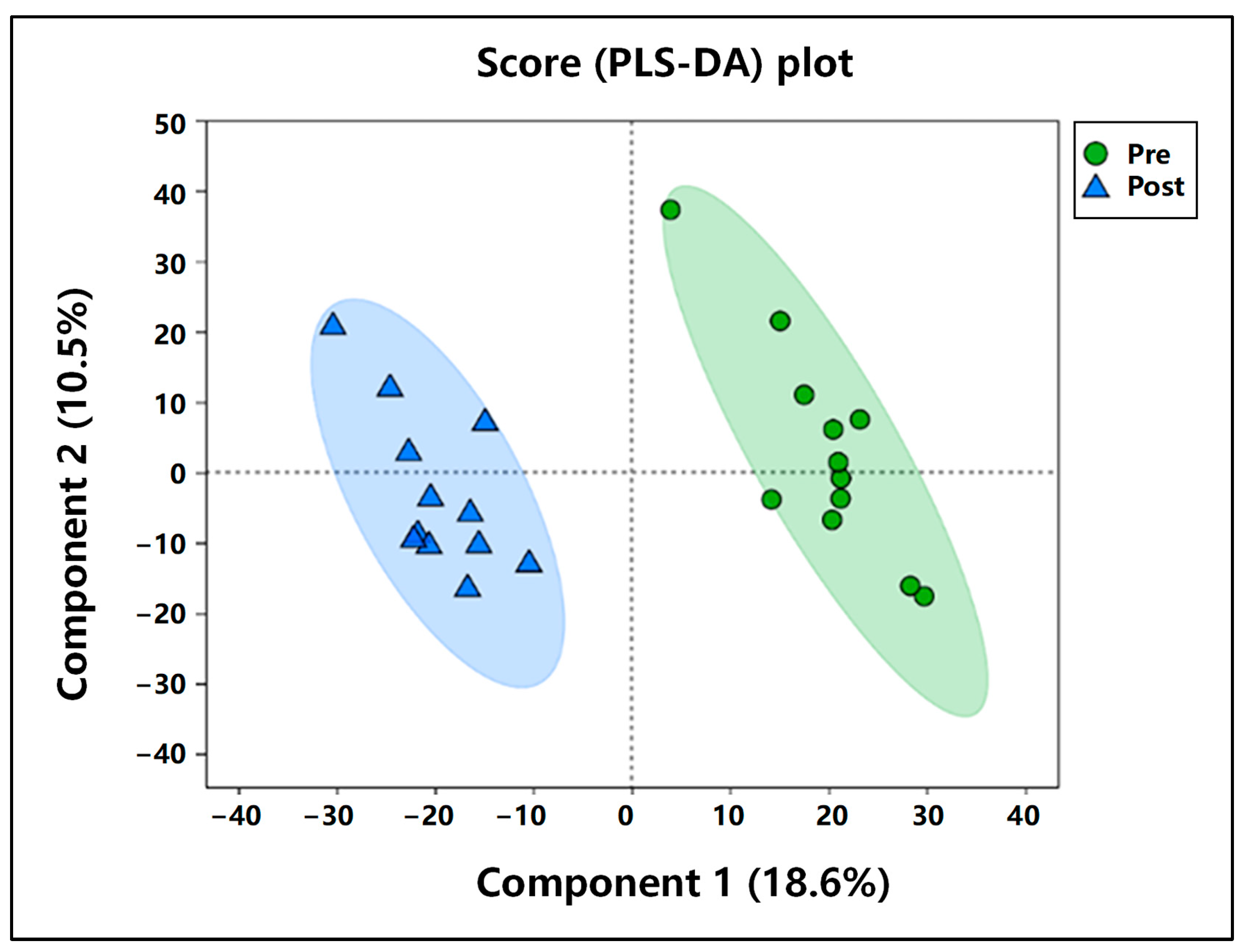
3.5.1. Comparative Analysis of PCA Pre- and Post-Exercise
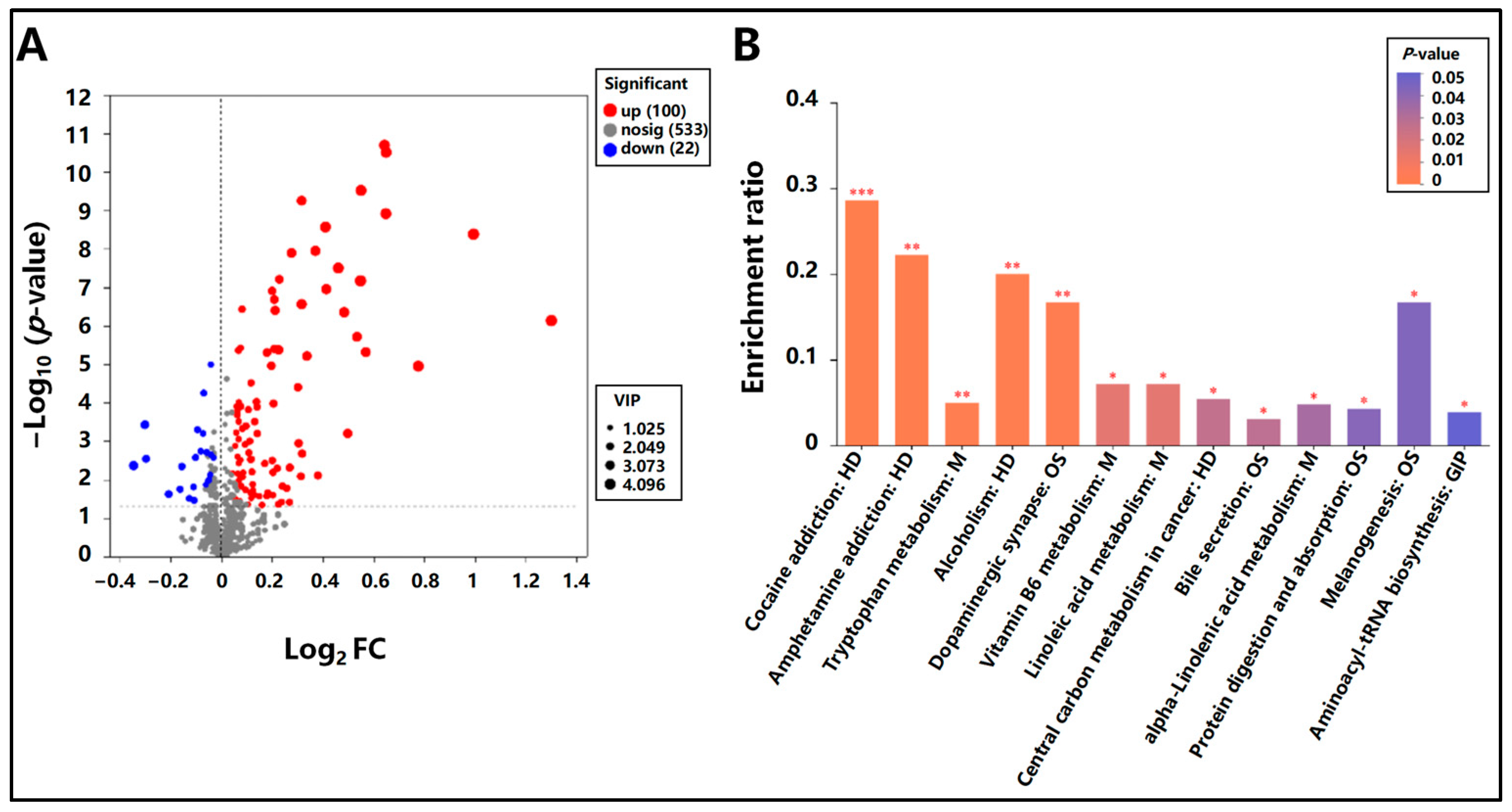
3.5.2. Differential Metabolite Identification and KEGG Pathway Enrichment Analysis in Mongolian Horses Pre- and Post-Exercise
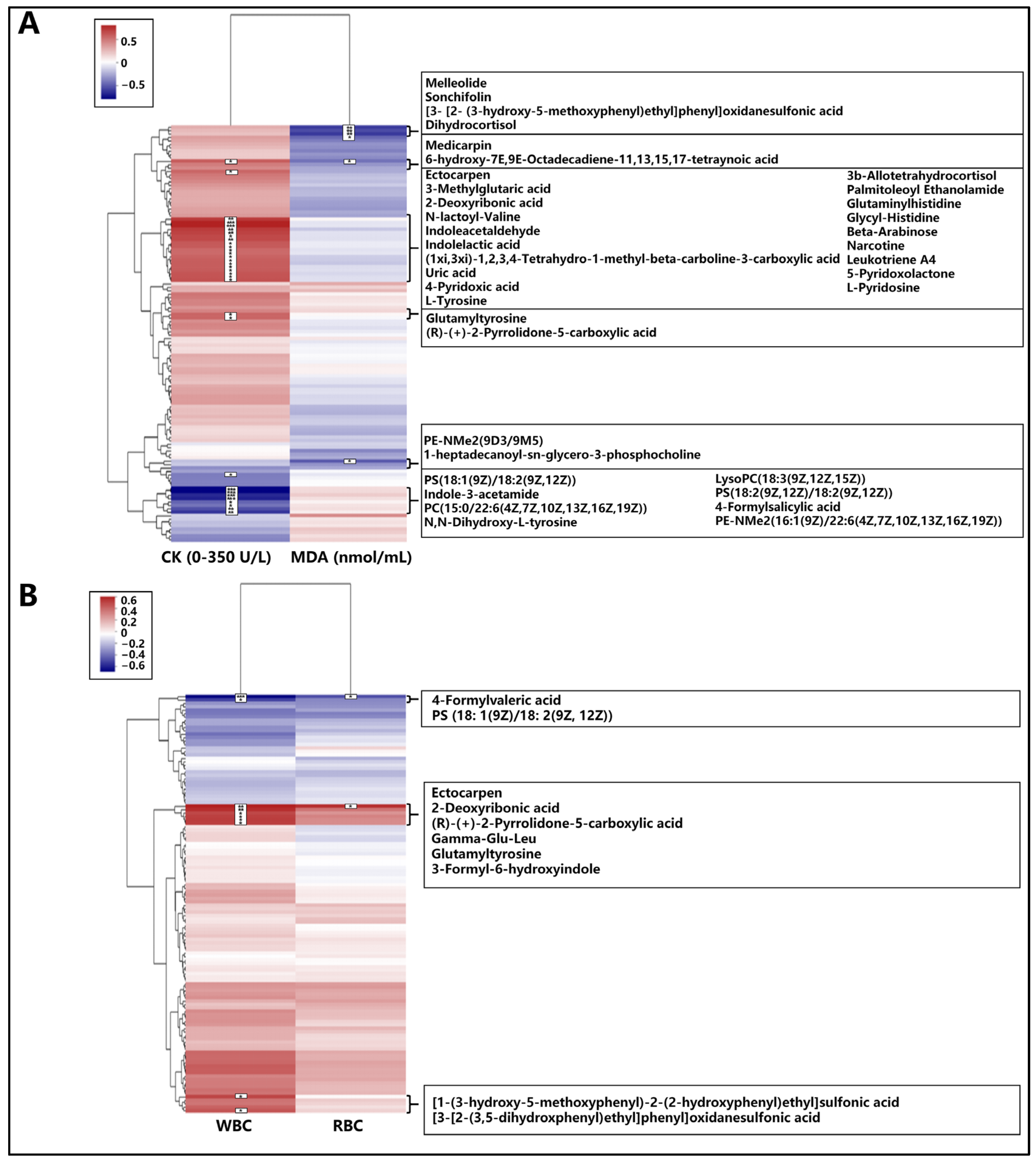
3.5.3. Correlation Analysis of Differential Metabolites with Blood Indicators
4. Discussion
4.1. Evaluation of Oxidative Imbalance
4.1.1. Effects of Mongolian Horses on Heart Rate and Speed at Different Exercise Distances and Post-Exercise
4.1.2. Effects of Mongolian Horses on Blood Antioxidant Index Levels at Different Exercise Distances and Post-Exercise
4.1.3. Effects of Blood Metabolomic Mechanisms in Mongolian Horses Pre- and Post-Exercise
4.2. Mechanisms of Endurance Adaptation Explained
4.2.1. Changes in Blood Routine Indexes of Mongolian Horses at Different Exercise Distances and Post-Exercise
4.2.2. Changes in Blood Biochemical Indexes of Mongolian Horses at Different Exercise Distances and Post-Exercise
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlando, L. The Evolutionary and Historical Foundation of the Modern Horse: Lessons from Ancient Genomics. Annu. Rev. Genet. 2020, 54, 563–581. [Google Scholar] [CrossRef]
- Puchalska, M.; Witkowska-Piłaszewicz, O. Gene doping in horse racing and equine sports: Current landscape and future perspectives. Equine Vet. J. 2025, 57, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.C.; Hayek, A.R.; Jones, B.; Evans, D.L.; McGreevy, P.D. Number, causes and destinations of horses leaving the Australian Thoroughbred and Standardbred racing industries. Aust. Vet. J. 2014, 92, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, X.; Zhao, Y.; Bai, D.; Du, M.; Song, L.; Liu, Z.; Yin, Z.; Manglai, D. Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse. BMC Genet. 2020, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Mongke, T.; Budsuren, U.; Tiemuqier, A.; Bozlak, E.; Wallner, B.; Dulamsuren, S.; Daidiikhuu, D.; Amgalan, S.; An, T.; Mongkejargal, B.; et al. Genomic conservation of Mongolian horses promoted by preservation of the intangible cultural heritage of Naadam in Mongolia. Conserv. Lett. 2024, 17, e13019. [Google Scholar] [CrossRef]
- Bou, T.; Han, H.; Mongke, T.; Zhao, R.; La, X.; Ding, W.; Jia, Z.; Liu, H.; Tiemuqier, A.; An, T.; et al. Fast and slow myofiber-specific expression profiles are affected by noncoding RNAs in Mongolian horses. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100942. [Google Scholar] [CrossRef]
- White, A.; Estrada, M.; Walker, K.; Wisnia, P.; Filgueira, G.; Valdés, F.; Araneda, O.; Behn, C.; Martıínez, R. Role of exercise and ascorbate on plasma antioxidant capacity in thoroughbred race horses. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 99–104. [Google Scholar] [CrossRef]
- Marlin, D.J.; Fenn, K.; Smith, N.; Deaton, C.D.; Roberts, C.A.; Harris, P.A.; Dunster, C.; Kelly, F.J. Changes in circulatory antioxidant status in horses during prolonged exercise. J. Nutr. 2002, 132, 1622S–1627S. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 489354. [Google Scholar] [CrossRef]
- Johansson, L.; Ringmark, S.; Bergquist, J.; Skiöldebrand, E.; Jansson, A. A metabolomics perspective on 2 years of high-intensity training in horses. Sci. Rep. 2024, 14, 2139. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Hou, C.; Xing, Y.; Cao, J.; Wu, K.; Liu, C.; Zhang, D.; Zhang, L.; Zhang, Y.; et al. Genome-wide detection of copy number variations among diverse horse breeds by array CGH. PLoS ONE 2014, 9, e86860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ortega, E.; Wang, C.J.K. Pre-performance Physiological State: Heart Rate Variability as a Predictor of Shooting Performance. Appl. Psychophysiol. Biofeedback 2018, 43, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A. The effect of oxidative stress during exercise in the horse. J. Anim. Sci. 2016, 94, 4067. [Google Scholar] [CrossRef] [PubMed]
- Dojana, N. Electrocardiographic Parameters of the Sport Horse. Available online: https://www.researchgate.net/publication/307884277_Electrocardiographic_parameters_of_the_sport_horse (accessed on 20 April 2025).
- Cho, J.M.; Manandhar, S.; Lee, H.R.; Park, H.M.; Kwak, M.K. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: Implication to cancer cell resistance. Cancer Lett. 2008, 260, 96–108. [Google Scholar] [CrossRef]
- Baker, A.; Lin, C.C.; Lett, C.; Karpinska, B.; Wright, M.H.; Foyer, C.H. Catalase: A critical node in the regulation of cell fate. Free Radic. Biol. Med. 2023, 199, 56–66. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.Z.; Wang, Y.; Tian, Y.; Zheng, H.P.; Zhou, Z.K.; Zhou, Y.B.; Tang, X.D.; Zhao, X.H.; Wu, T.; et al. The protein phosphatase PC1 dephosphorylates and deactivates CatC to negatively regulate H2O2 homeostasis and salt tolerance in rice. Plant. Cell. 2023, 35, 3604–3625. [Google Scholar] [CrossRef]
- Kosmalski, M.; Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. Non-Alcoholic Fatty Liver Disease Is Associated with a Decreased Catalase (CAT) Level, CT Genotypes and the T Allele of the -262 C/T CAT Polymorphism. Cells 2023, 12, 2228. [Google Scholar] [CrossRef]
- Del Angel-Meza, A.R.; Dávalos-Marín, A.J.; Ontiveros-Martinez, L.L.; Ortiz, G.G.; Beas-Zarate, C.; Chaparro-Huerta, V.; Torres-Mendoza, B.M.; Bitzer-Quintero, O.K. Protective effects of tryptophan on neuro-inflammation in rats after administering lipopolysaccharide. Biomed. Pharmacother. 2011, 65, 215–219. [Google Scholar] [CrossRef]
- Arber, S.; Costa, R.M. Networking brainstem and basal ganglia circuits for movement. Nat. Rev. Neurosci. 2022, 23, 342–360. [Google Scholar] [CrossRef]
- Zhou, L.; Mozaffaritabar, S.; Kolonics, A.; Kawamura, T.; Koike, A.; Kéringer, J.; Gu, Y.; Karabanov, R.; Radák, Z. Long-term iron supplementation combined with vitamin B6 enhances maximal oxygen uptake and promotes skeletal muscle-specific mitochondrial biogenesis in rats. Front. Nutr. 2024, 10, 1335187. [Google Scholar] [CrossRef]
- Kalicki, B.; Lewicka, A.; Jęderka, K.; Leśniak, M.; Marszałkowska-Jakubik, J.; Lewicki, S. Vitamin B6 improves blood parameters in rats fed a protein-deficient diet and subjected to moderate, long-term exercise. Cent. Eur. J. Immunol. 2019, 44, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Virk, R.S.; Dunton, N.J.; Young, J.C.; Leklem, J.E. Effect of vitamin B-6 supplementation on fuels, catecholamines, and amino acids during exercise in men. Med. Sci. Sports Exerc. 1999, 31, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Schmidt, C.; Seibel, R.; Wehsling, M.; Le Mignon, M.; Wille, G.; Fischer, M.; Zimmer, A. Keto leucine and keto isoleucine are bioavailable precursors of their respective amino acids in cell culture media. J. Biotechnol. 2020, 321, 1–12. [Google Scholar] [CrossRef]
- Torres, N.; Tobón-Cornejo, S.; Velazquez-Villegas, L.A.; Noriega, L.G.; Alemán-Escondrillas, G.; Tovar, A.R. Amino acid catabolism: An overlooked area of metabolism. Nutrients 2023, 15, 3378. [Google Scholar] [CrossRef]
- Gao, X.; Chang, S.; Liu, S.; Peng, L.; Xie, J.; Dong, W.; Tian, Y.; Sheng, J. Correlations between α-Linolenic Acid-Improved Multitissue Homeostasis and Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00391-20. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, X.; Li, L.; Tang, Y.; Zheng, M.; Zeng, W.; Lei, X. Improving obesity and lipid metabolism using conjugated linoleic acid. Vet. Med. Sci. 2022, 8, 2538–2544. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Starner, R.J.; McClelland, L.; Abdel-Malek, Z.; Fricke, A.; Scott, G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp. Dermatol. 2010, 19, 682–684. [Google Scholar] [CrossRef]
- Tennakoon, R.; Cui, H. Aminoacyl-tRNA synthetases. Curr. Biol. 2024, 34, R884–R888. [Google Scholar] [CrossRef]
- Qin, X.; Hao, Z.; Tian, Q.; Zhang, Z.; Zhou, C.; Xie, W. Cocrystal structures of glycyl-tRNA synthetase in complex with tRNA suggest multiple conformational states in glycylation. J. Biol. Chem. 2014, 289, 20359–20369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wang, H.; Mao, X.; Luo, L. Noncanonical function of threonyl-tRNA synthetase regulates vascular development in zebrafish. Biochem. Biophys. Res. Commun. 2016, 473, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Lakunina, V.; Tarassov, I.; Krasheninnikov, I.A.; Kamenski, P.A. Noncanonical functions of aminoacyl-tRNA synthetases. Biochemistry 2012, 77, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Green, H.J.; Fraser, I.G. Differential effects of exercise intensity on serum uric acid concentration. Med. Sci. Sports Exerc. 1988, 20, 55–59. [Google Scholar] [CrossRef]
- Janssen, G.M.; Degenaar, C.P.; Menheere, P.P.; Habets, H.M.; Geurten, P. Plasma urea, creatinine, uric acid, albumin, and total protein concentrations before and after 15-, 25-, and 42-km contests. Int. J. Sports Med. 1989, 10, S132–S138. [Google Scholar] [CrossRef]
- Wołyniec, W.; Ratkowski, W.; Kasprowicz, K.; Malgorzewicz, S.; Aleksandrowicz, E.; Witek, K.; Grzywacz, T.; Zmijewski, P.; Renke, M. Changes in electrolytes and uric acid excretion during and after a 100 km run. J. Biol. Regul. Homeost. Agents 2018, 32, 1205–1210. [Google Scholar]
- Kayacan, Y.; Yazar, H.; Kisa, E.C.; Ghojebeigloo, B.E. A novel biomarker explaining the role of oxidative stress in exercise and l-tyrosine supplementation: Thiol/disulphide homeostasis. Arch. Physiol. Biochem. 2018, 124, 232–236. [Google Scholar] [CrossRef]
- Zaragoza, J.; Tinsley, G.; Urbina, S.; Villa, K.; Santos, E.; Juaneza, A.; Tinnin, M.; Davidson, C.; Mitmesser, S.; Zhang, Z.; et al. Effects of acute caffeine, theanine and tyrosine supplementation on mental and physical performance in athletes. J. Int. Soc. Sports Nutr. 2019, 16, 56. [Google Scholar] [CrossRef]
- D’Ascola, A.; Bruschetta, G.; Zanghi, G.; Campo, S.; Medica, P.; Campana, S.; Ferlazzo, G.; Gibbs, B.F.; Ferlazzo, A.M. Changes in plasma 5-HT levels and equine leukocyte SERT expression in response to treadmill exercise. Res. Vet. Sci. 2018, 118, 184–190. [Google Scholar] [CrossRef]
- Lowry, O.H.; Bessey, O.A. The interrelationship of dietary, serum, white blood cell, and total body ascorbic acid. J. Biol. Chem. 1946, 166, 111–119. [Google Scholar] [CrossRef]
- Xu, H.C.; Huang, J.; Pandyra, A.A.; Lang, E.; Zhuang, Y.; Thöns, C.; Timm, J.; Häussinger, D.; Colonna, M.; Cantor, H.; et al. Lymphocytes Negatively Regulate NK Cell Activity via Qa-1b following Viral Infection. Cell Rep. 2017, 21, 2528–2540. [Google Scholar] [CrossRef] [PubMed]
- Qasim, N.; Mahmood, R. Diminution of Oxidative Damage to Human Erythrocytes and Lymphocytes by Creatine: Possible Role of Creatine in Blood. PLoS ONE 2015, 10, e0141975. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, J.; Mangalmurti, N.S. Immunomodulatory roles of red blood cells. Curr. Opin. Hematol. 2022, 29, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Lei, X.; He, D.; Zhang, H.; Yang, Z.; Mu, W.; Fang, K.; Xue, D.; Li, H.; Li, X.; et al. Ferrous hemoglobin and hemoglobin-based oxygen carriers acting as a peroxidase can inhibit oxidative damage to endothelial cells caused by hydrogen peroxide. Artif. Organs 2021, 45, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Gurecká, R.; Podracká, Ľ. Association of Leukocyte, Erythrocyte, and Platelet Counts with Metabolic Syndrome and Its Components in Young Individuals without Overt Signs of Inflammation: A Cross-Sectional Study. Children 2024, 11, 66. [Google Scholar] [CrossRef]
- Kirschvink, N.; Moffarts, B.D.; Lekeux, P. The oxidant/antioxidant equilibrium in horses. Vet. J. 2008, 177, 178–191. [Google Scholar] [CrossRef]
- Teng, F.; Zhu, R.; Zou, C.; Xue, Y.; Yang, M.; Song, H.; Liang, J. Interaction between serum uric acid and triglycerides in relation to blood pressure. J. Hum. Hypertens. 2011, 25, 686–691. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Fazio, F.; Casella, S.; Caola, G. A comparison of daily rhythm of creatinine and creatine kinase in the sedentary and athlete horse. J. Equine Vet. Sci. 2009, 29, 575–580. [Google Scholar] [CrossRef]
- Suzuki, T.; Shimizu, M.; Yamauchi, Y.; Sato, R. Polymethoxyflavones in orange peel extract prevent skeletal muscle damage induced by eccentric exercise in rats. Biosci. Biotechnol. Biochem. 2021, 85, 440–446. [Google Scholar] [CrossRef]
- Heinzelmann, S.; Bauer, G. Multiple protective functions of catalase against intercellular apoptosis-inducing ROS signaling of human tumor cells. Biol. Chem. 2010, 391, 675–693. [Google Scholar] [CrossRef]
- Tu, H.; Li, Y.L. Inflammation balance in skeletal muscle damage and repair. Front. Immunol. 2023, 14, 1133355. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
| Items | Travel Distance | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 km | 5 km | 10 km | 15 km | 20 km | |||
| Average heart rate (bpm) | 40.00 | 127.00 | 125.25 | 126.42 | 122.75 | 7.54 | 0.979 |
| Maximum heart rate (bpm) | - | 187.08 | 168.08 | 163.58 | 162.83 | 10.58 | 0.338 |
| Average speed (km/h) | - | 17.85 | 19.15 | 19.47 | 18.41 | 1.18 | 0.764 |
| Maximum speed (km/h) | - | 37.68 | 34.82 | 38.61 | 38.68 | 2.76 | 0.733 |
| Items | Pre-Exercise | During Exercise | Post-Exercise | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 km | 10 km | 15 km | 20 km | 1 h | 2 h | 4 h | 6 h | ||||
| WBC (109/L) | 10.13 B | 11.63 AB | 12.48 AB | 12.82 A | 13.01 A | 14.30 A | 14.33 A | 14.03 A | 12.70 A | 0.29 | 0.010 |
| Lymph (109/L) | 4.59 BC | 5.81 A | 6.22 A | 5.55 AB | 5.40 AB | 4.12 CD | 3.33 D | 3.68 CD | 3.90 CD | 0.15 | <0.001 |
| Mon (109/L) | 0.54 CD | 0.54 CD | 0.53 D | 0.61 BCD | 0.65 ABCD | 0.81 A | 0.80 A | 0.73 AB | 0.71 ABC | 0.02 | <0.001 |
| Gran (109/L) | 5.00 D | 5.28 D | 5.23 D | 5.99 D | 6.96 CD | 9.38 AB | 10.21 A | 9.62 AB | 8.09 BC | 0.28 | <0.001 |
| Lymph (%) | 46.18 AB | 50.20 A | 52.22 A | 46.93 AB | 42.47 B | 28.76 C | 23.73 C | 26.49 C | 30.84 C | 1.29 | <0.001 |
| Mon (%) | 5.29 AB | 4.74 BC | 4.44 C | 4.94 ABC | 5.24 AB | 5.67 A | 5.44 AB | 5.37 AB | 5.66 A | 0.08 | 0.002 |
| Gran (%) | 48.53 BC | 45.07 BC | 47.58 C | 51.73 BC | 52.29 B | 65.58 A | 70.83 A | 68.14 A | 63.50 A | 1.26 | <0.001 |
| RBC (1012/L) | 6.88 B | 7.95 A | 7.83 A | 8.10 A | 7.80 A | 6.69 B | 6.88 B | 6.93 B | 6.92 B | 0.08 | <0.001 |
| HGB (g/L) | 96.92 B | 112.25 A | 112.00 A | 114.42 A | 110.25 A | 95.00 B | 95.33 B | 97.00 B | 97.55 B | 1.18 | <0.001 |
| HCT (%) | 30.83 B | 35.99 A | 35.65 A | 36.93 A | 35.37 A | 29.98 B | 30.52 B | 30.65 B | 30.55 B | 0.44 | <0.001 |
| PLT (109/L) | 236.75 | 248.00 | 239.75 | 229.00 | 243.08 | 219.83 | 225.08 | 226.36 | 229.73 | 0.27 | 0.961 |
| MCV (fL) | 44.93 | 45.35 | 45.70 | 45.67 | 45.53 | 44.95 | 44.48 | 44.35 | 44.28 | 0.12 | 0.884 |
| MCH (pg) | 14.18 | 14.09 | 14.38 | 14.07 | 14.16 | 14.29 | 13.85 | 14.00 | 14.20 | 2.10 | 0.992 |
| MCHC (g/L) | 316.50 | 312.50 | 315.50 | 309.42 | 312.17 | 317.92 | 312.67 | 316.55 | 322.00 | 0.08 | 0.952 |
| RDW (%) | 17.75 | 17.98 | 17.83 | 17.85 | 17.96 | 17.71 | 17.88 | 17.79 | 17.69 | 5.47 | 0.994 |
| MPV (fL) | 6.65 | 6.73 | 6.67 | 6.92 | 6.98 | 6.86 | 6.77 | 6.77 | 6.65 | 0.05 | 0.798 |
| PDW | 16.05 | 16.05 | 16.40 | 16.28 | 16.08 | 16.18 | 16.20 | 16.05 | 16.20 | 0.05 | 0.816 |
| PCT (%) | 0.16 | 0.17 | 0.16 | 0.16 | 0.17 | 0.15 | 0.15 | 0.15 | 0.15 | 0.01 | 0.952 |
| Items | Pre-Exercise | During Exercise | Post-Exercise | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 km | 10 km | 15 km | 20 km | 1 h | 2 h | 4 h | 6 h | ||||
| TG (mmol/L) | 0.22 C | 0.32 B | 0.38 AB | 0.44 A | 0.40 AB | 0.20 C | 0.16 C | 0.16 C | 0.14 C | 0.01 | <0.001 |
| UREA (mmol/L) | 5.64 | 5.59 | 5.69 | 6.23 | 6.28 | 5.96 | 5.87 | 5.83 | 6.01 | 0.07 | 0.218 |
| Ca (mmol/L) | 2.72 | 2.65 | 2.67 | 2.58 | 2.53 | 2.61 | 2.66 | 2.63 | 2.59 | 0.02 | 0.405 |
| LDH (U/L) | 254.57 | 261.10 | 274.62 | 282.25 | 293.71 | 303.14 | 305.38 | 313.64 | 320.20 | 8.73 | 0.635 |
| AST (U/L) | 314.36 | 319.19 | 324.96 | 323.18 | 319.43 | 330.91 | 336.25 | 343.02 | 344.15 | 7.86 | 0.991 |
| ALP (U/L) | 193.98 | 206.88 | 200.90 | 205.23 | 206.91 | 209.18 | 216.68 | 216.51 | 221.35 | 4.24 | 0.897 |
| TP (g/L) | 73.80 | 73.72 | 73.35 | 73.42 | 70.86 | 73.60 | 73.97 | 75.93 | 75.55 | 0.49 | 0.469 |
| ALB (g/L) | 29.93 | 30.40 | 30.79 | 30.54 | 30.18 | 31.25 | 31.80 | 30.64 | 31.43 | 0.20 | 0.361 |
| CREA (μmoI/L) | 57.29 C | 63.73 BC | 69.26 AB | 75.82 A | 75.94 A | 68.33 AB | 64.56 BC | 62.74 BC | 59.27 C | 1.04 | <0.001 |
| GLU (mmol/L) | 4.22 B | 5.49 A | 5.51 A | 5.38 A | 5.23 A | 4.18 B | 4.17 B | 3.9 B | 3.99 B | 0.11 | <0.001 |
| TC (mmol/L) | 1.58 | 1.54 | 1.56 | 1.60 | 1.53 | 1.57 | 1.62 | 1.62 | 1.63 | 0.02 | 0.970 |
| Mg (mmol/L) | 0.74 | 0.82 | 0.84 | 0.79 | 0.80 | 0.76 | 0.85 | 0.76 | 0.79 | 0.02 | 0.726 |
| P (mmol/L) | 1.50 | 1.66 | 1.70 | 1.75 | 1.76 | 1.51 | 1.60 | 1.79 | 1.75 | 0.03 | 0.352 |
| GGT (U/L) | 13.59 | 11.98 | 13.85 | 13.93 | 11.84 | 14.31 | 14.66 | 14.95 | 14.77 | 0.61 | 0.931 |
| CK (U/L) | 164.51 CD | 143.06 D | 166.17 CD | 199.32 BCD | 271.55 ABC | 321.43 A | 341.62 A | 297.95 AB | 264.41 ABC | 13.49 | <0.001 |
| Items | Pre-Exercise | 5 km | 10 km | 15 km | 20 km | Post-Exercise 1 h | 2 h | 4 h | 6 h | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-AOC (mM) | 0.52 | 0.50 | 0.48 | 0.46 | 0.44 | 0.45 | 0.48 | 0.51 | 0.52 | 0.08 | 0.975 |
| SOD (U/mL) | 18.11 | 18.06 | 17.97 | 17.60 | 17.12 | 17.58 | 17.83 | 18.10 | 18.11 | 0.99 | 0.827 |
| CAT (U/mL) | 8.84 A | 8.31 AB | 7.85 AB | 7.31 AB | 6.77 B | 7.24 AB | 7.71 AB | 8.48 AB | 8.83 A | 2.07 | 0.046 |
| GSH-Px (U) | 318.06 | 316.36 | 314.95 | 312.98 | 310.86 | 313.92 | 315.26 | 317.96 | 318.06 | 7.20 | 0.265 |
| MDA (nmol/mL) | 3.06 | 3.28 | 3.42 | 3.52 | 3.60 | 3.59 | 3.58 | 3.40 | 3.18 | 0.26 | 0.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Li, L.; Ma, W.; Bai, D.; Dugarjaviin, M. Physiological and Metabolic Responses of Mongolian Horses to a 20 km Endurance Exercise and Screening for New Oxidative-Imbalance Biomarkers. Animals 2025, 15, 1350. https://doi.org/10.3390/ani15091350
Zhang X, Liu Y, Li L, Ma W, Bai D, Dugarjaviin M. Physiological and Metabolic Responses of Mongolian Horses to a 20 km Endurance Exercise and Screening for New Oxidative-Imbalance Biomarkers. Animals. 2025; 15(9):1350. https://doi.org/10.3390/ani15091350
Chicago/Turabian StyleZhang, Xinzhuang, Yuanyi Liu, Lianhao Li, Wei Ma, Dongyi Bai, and Manglai Dugarjaviin. 2025. "Physiological and Metabolic Responses of Mongolian Horses to a 20 km Endurance Exercise and Screening for New Oxidative-Imbalance Biomarkers" Animals 15, no. 9: 1350. https://doi.org/10.3390/ani15091350
APA StyleZhang, X., Liu, Y., Li, L., Ma, W., Bai, D., & Dugarjaviin, M. (2025). Physiological and Metabolic Responses of Mongolian Horses to a 20 km Endurance Exercise and Screening for New Oxidative-Imbalance Biomarkers. Animals, 15(9), 1350. https://doi.org/10.3390/ani15091350







