Simple Summary
Streptococcus equi subsp. zooepidemicus (SEZ) is a commensal bacterium of equids that can sometimes cause severe respiratory, reproductive, and even septicaemic disease; recently, it has been suggested that SEZ may even be responsible for human infections. The aim of this study was to describe the epidemiology and genomic diversity of SEZ strains by the isolating and sequencing of bacteria from nasal swabs of equids from central Italy (Abruzzo and Molise). Potential risk factors associated with the development of respiratory disease in equids were also considered.
Abstract
Streptococcus equi subsp. zooepidemicus (SEZ) is a major problem in equine veterinary medicine. Typically, a commensal in horses, SEZ can cause severe disease including respiratory infections, septicaemia and reproductive tract infections under certain conditions. Recent evidence suggests that humans can also develop severe disease infection through direct contact with infected animals or the consumption of contaminated unpasteurised milk and milk products. This study investigates SEZ strains isolated from nasal swabs of equidae in central Italy in 2023 to describe the epidemiology and genomic characteristics of circulating strains. A sampling plan was implemented to randomly collect nasal swabs from equid farms in the Abruzzo and Molise regions. In addition, a sampling form was designed to collect information on risk factors related to the presence of the bacterium and the potential development of respiratory disease. Relative risk was used to measure the association between the presence of SEZ in the samples and various variables. The swabs were analysed by real-time PCR and isolation. To confirm the identification and characterise the strains, the isolates were fully sequenced by next-generation sequencing (NGS) using the Illumina platform. A total of 478 animals from 99 farms were sampled and 30% of the animals tested positive for SEZ (79% horses, 20% donkeys, 1% mules). Forty-five percent of the farms tested positive for SEZ. Monitoring the clonal spread of SEZ is essential to understand the ecology of this emerging zoonotic pathogen, to assess the risk, and to implement effective control measures. In addition, genomic assessments are recommended to investigate the pathogenicity of circulating strains. This study provides a comprehensive understanding of the epidemiology and genomic diversity of SEZ strains isolated in central Italy.
1. Introduction
Streptococcus equi subsp. zooepidemicus (SEZ) is a commensal bacterium found on the skin and mucous membranes of horses. As an opportunistic pathogen, it can cause a variety of infections, including those of the respiratory tract and the reproductive and urinary tracts. These infections result in significant morbidity, economic loss, and complications in equine health management. Although SEZ can cause clinical respiratory disease in horses, not all exposed horses become ill. Horses carrying these bacteria can still infect other horses, even if they do not show any clinical signs of disease.
Recent evidence suggests that SEZ may also cause severe diseases in humans following contamination by infected animals or the consumption of unpasteurised milk and milk products [1,2,3]. Human infection with SEZ can result in symptoms ranging from mild skin infections to meningitis and sepsis, particularly in people with weakened immune systems [4,5,6]. Respiratory symptoms, including sore throat and pneumonia, can occur, especially if the bacteria are spread by direct contact with infected animals [7]. A large SEZ outbreak with 37 clinical cases was reported in Italy between November 2021 and May 2022 [8].
SEZ has also recently been identified as the causative agent of severe cases of pneumonia in donkeys in Italy, where a novel SEZ sequence type (ST525) was responsible for the death of four donkeys raised on a farm between March and April 2022 [9].
As a commensal bacterium, there is limited information available on its actual distribution. In a study by the United States Department of Agriculture, bacterial cultures from nasal swabs of 6000 healthy horses showed a SEZ prevalence of 9.2% [10].
In addition, little is known about the genetic factors that distinguish non-pathogenic strains from pathogenic strains, in both horses and humans.
The aim of this study was to assess the prevalence of SEZ at both individual and herd level in the Italian regions of Abruzzo and Molise using a cross-sectional study of nasal swab samples. The study also aimed to identify risk factors associated with the disease in horses and to characterise SEZ using whole-genome sequencing.
2. Materials and Methods
2.1. Study Area and Target Population
Regulation (EU) 2016/429 [11] requires the Member States of the European Union to establish a computerised database for terrestrial animals, including equidae. Following the entry into force of the above-mentioned law and related regulations, Italy implemented them with Legislative Decree No. 302 of 30 September 2021 and Legislative Decree No. 134 of 5 August 2022 [12,13], which establish the identification and registration system and define the management and operation of the national equine registry. The Italian Equine Register allows the search of individual equine information by unique identifiers such as the Single Lifetime Identification Document (SLID—also known as the passport), the Unique Equine Life Number (UELN) code, the transponder code, or the name of the animal. Through the specific interface with personal records, it is possible to visualise the above codes together with details of the species, breed, sex, date of birth, and whether or not the individual animal is intended for food production.
According to official data from the Italian equine register, 474,759 equines were registered in the database on 31 December 2023, of which 18,933 (4%) were in Abruzzo and 4657 (1%) in Molise. The median number of animals per farm in the study population was 44. Of the farms in the areas included in the analysis, 43% had only 1 animal, 48% had up to 10 animals, 5% had between 11 and 20 animals, and 5% had more than 20 animals.
2.2. Sampling Design and Collection
A sampling plan was designed to collect nasal swabs from equidae randomly distributed in the regions of Abruzzo and Molise. To define the sample, the animals were considered as the sampling units, with an assumed a priori prevalence of 50%, a confidence level of 95%, and an absolute accuracy of 5%. Based on these parameters, the minimum required number of primary units required was 384 animals. Approximately 25% additional animals were sampled to provide a safety margin, bringing the total number of primary units to 478. Sampling parameters were defined based on the number of holdings with at least one animal (n = 5050) registered in the National Equine Register.
A model accompanying form was designed to collect anamnesis information on the sampled animals, such as the animal’s unique identifier, species (equine, asinine, or mule), age, sex, and breed, if intended for food production, and the presence of respiratory symptoms. In addition, information on the farm was collected such as the unique identification number, the species farmed, the type of activity carried out on the farm with animals of the same species or group of species, geographical location, and the name of the farm operator. Where information was missing from the sample accompanying form, it was obtained by consulting the National Equine Register.
Veterinarians collected samples from the nasal cavities of 478 adult horses using sterile swabs. The nostrils were cleaned with paper towels before the distal nasal cavity was swabbed, and then the swabs were placed in a transport medium for delivery to the laboratory. The. Istituto Zooprofilattico Sperimentale (IZS) in Teramo, Italy, performed the tests.
2.3. Data Management and Statistical Analysis
The unique identifier of the animal, the species sampled and the unique registration number of the facility reported on the form were also registered in the Laboratory Information System (LIMS) of the IZS Teramo. At the end of the registration process, a label with a sequential number was generated to uniquely identify the nasal swab collected from each animal. A database was created using data extracted from the LIMS, together with additional information collected from accompanying forms or obtained from the National Equine Register.
Each entry of animal, farm and laboratory into the database was double checked to ensure data quality. The spatial location of each sampled establishment, intended as the physical location where animals are kept, was plotted on a map using Geographic Information System (GIS) software, (QGIS 3.34.12-Prizren.QGIS.org, 2024. QGIS Geographic Information System. QGIS Association. http://www.qgis.org, accessed on 23 April 2025).
The prevalence of SEZ was calculated at both animal and farm level, with a 95% confidence interval determined using exact binomial confidence intervals.
Multivariate statistical analysis was performed using multivariate logistic regression, with the response variable being the presence or absence of SEZ on the collected swab, and the covariates including sex, species, breed, age category (less than or greater than 15 years, with 15 years serving as the threshold for classifying a horse as “old” [14]), symptoms, whether the animal was intended for food production, the simultaneous presence of multiple livestock species, and the presence of dairy species on the same farm. The potential relationship between the presence of SEZ and the covariates included in the model was also assessed using a chi-square test. A two-tailed Mann–Whitney test was used to assess age differences between negative and positive animals. All statistical analyses were performed using R v.4.3.2 (R Development Core Team, 2023).
2.4. Isolation, PCR, and Sequencing of SEZ
For isolation, nasal swabs were plated on 5% sheep blood agar plates (Microbiol srl, Cagliari, Italy) and incubated for 24–72 h at 37 ± 1 °C in a 5–10% CO2-enriched atmosphere. Suspect Streptococcus spp. colonies were subcultured and identified by MALDI-TOF (MALDI Biotyper®, Bruker Daltonics Gmbh & Co. KG, Bremen, Germany). DNA was then extracted from isolated colonies for sequencing using the Maxwell® RSC Genomic DNA Kit (Promega, Fitchburg, WI, USA) with minor protocol modifications. Nasal swabs were also subjected to DNA extraction using the Maxwell® RSC Genomic DNA Kit, followed by real-time PCR testing for SEZ using a commercial kit (Genesig® Primerdesign™ Ltd., Manchester, UK), targeting the SDR family oxidoreductase gene. The kit was validated prior to use and demonstrated 100% sensitivity and specificity (95% CI: 86.7–100%).
DNA extraction was performed with minor modifications for a total of 51 strains and NGS was performed using the Illumina platform (Illumina, San Diego, CA, USA). An in-house pipeline (https://github.com/genpat-it/ngsmanager/, accessed on 10 September 2024) was used for WGS data analysis and a quality check was performed. The KmerFinder tool [15] was used to confirm species identification.
Multilocus Sequence Typing (MLST) was performed according to the reference scheme (https://pubmlst.org/organisms/streptococcus-zooepidemicus, accessed on 15 September 2024). Virulence profiles were obtained using ABRicate (https://github.com/tseemann/abricate, accessed on 15 September 2024).
To verify the correlation between the 51 SEZ genomes, a single-nucleotide polymorphism (SNP) analysis was performed using the CFSAN pipeline [16] with CP001129 (Streptococcus equi subsp. zooepidemicus MGCS10565) as a reference.
3. Results
3.1. SEZ Prevalence
Of the samples collected from the 99 farms, 478 nasal swabs were analysed for the presence of SEZ. Of these farms, 56.6% had between 1 and 5 animals. Figure 1 shows the geographical distribution of the farms tested, highlighting those with positive results.
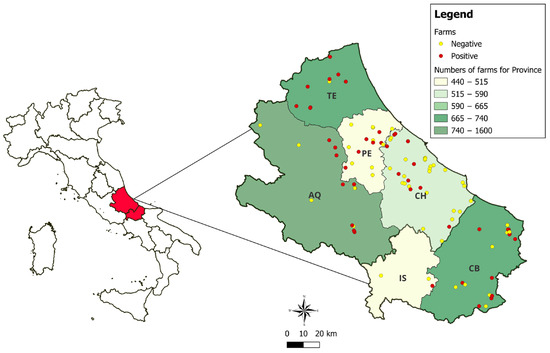
Figure 1.
Geographical distribution of the farms under investigation, respectively, for the provinces of L’Aquila (AQ), Chieti (CH), Pescara (PE), and Teramo (TE), and Molise, in the provinces of Campobasso (CB) and Isernia (IS).
A total of 144 horses tested positive for SEZ by PCR, resulting in an estimated prevalence of 30.1% (95% CI: 26.2–34.4%) at the animal level. Of the 478 nasal swabs tested by culture, 56 (11.7%) were positive. All of these were also positive by PCR. At the herd level, 45 farms were classified as positive because at least one animal on the farm tested positive by PCR (p = 45.5%; 95% CI: 36.0–55.3%). The distribution of farms and the number of tested and positive animals by province is shown in Table 1. For four animals, information on the province of the sampled farm could not be traced. As a result, the number of tested animals (n = 474) and the number of positive animals (n = 143) in Table 1 do not correspond to the total number of sampled animals (n = 478) and the total number of positive animals (n = 144).

Table 1.
Distribution of farms and animals sampled and number of testing positive by PCR for Abruzzo, in the provinces of L’Aquila (AQ), Chieti (CH), Pescara (PE), and Teramo (TE), and Molise, in the provinces of Campobasso (CB) and Isernia (IS).
The logistic model was not significant, and none of the covariates considered reached significance (p-value > 0.05), as shown in Table 2. The results of the chi-square test were not statistically significant (p-value < 0.05) for all variables examined except for the species. Statistical analysis revealed a significant association between species and the presence of SEZ, with asinine being significantly more positive than equine (chi-square = 4.70 p-value = 0.030). The median age of positive animals was 6.5 years, significantly lower (p-value = 0.000, two tailed Mann–Whitney Test) than that of negative animals (median age: 10 years).

Table 2.
Results of the covariate significance testing (null deviance: 499.11 on 402 degrees of freedom; residual deviance: 488.47 on 395 degrees of freedom; AIC: 504.47).
3.2. Genomic Characterisation and Cluster Analysis
A total of 56 strains were isolated from nasal swabs. Of these, 51 underwent whole-genome sequencing (WGS), while 5 could not be sequenced due to poor DNA extraction quality. All the 51 genomes were confirmed to be SEZ. MLST analysis showed that the SEZ genomes could be classified into 31 sequence types (STs) (Table 3). Among the calculated STs, 14 new STs were identified: ST536, ST538, ST539, ST540, ST541, ST542, ST543, ST544, ST546, ST547, ST549, ST550, ST551, and ST552. MLST profiles could not be calculated for two isolates (2024.TE.15857.1.2 and 2024.TE.15855.1.2) due to the genome fragmentation. The 51 sequenced genomes came from 23 different farms, including 7 from Molise and 16 from Abruzzo. Figure 2 shows the farms and the sequence types identified in each one. In almost half of the farms (n = 11), more than one sequence type was detected.

Table 3.
Metadata and genomic characteristics of 51 SEZ strains.
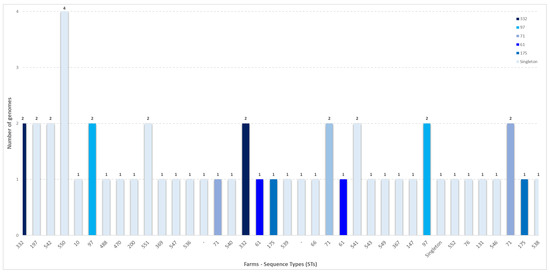
Figure 2.
Sequence types identified across sampled farms, with each farm represented by a unique letter. Isolates identified as singletons are highlighted in light blue.
The results of the virulence gene analyses are shown in Table 3. The fbp54 gene, which codes for fibronectin-binding proteins, was present in 41 isolates. Meanwhile, mf2 and mf3 were identified in 19 and 29 strains, respectively. Interestingly, one isolate (2024.TE.6603.1.55) carried the spel gene, which encodes the precursor of streptococcal exotoxin L precursor, and the spek gene, which is involved in the production of streptococcal toxin associated with phages. The hasC gene, whose product is a cell wall surface anchor family protein, was found in 16 isolates.
Clustering analysis highlighted the presence of different clusters according to MLST analysis (Figure 3). Specifically, a strain isolated from a donkey (2024.TE.6815.2.5) and belonging to ST197 clustered with another strain isolated from another donkey from the same farm. Two further clusters were identified on the same farm. Specifically, four strains isolated from donkeys belonged to cluster ST550 and two strains belonging to novel ST542 were isolated from two horses.
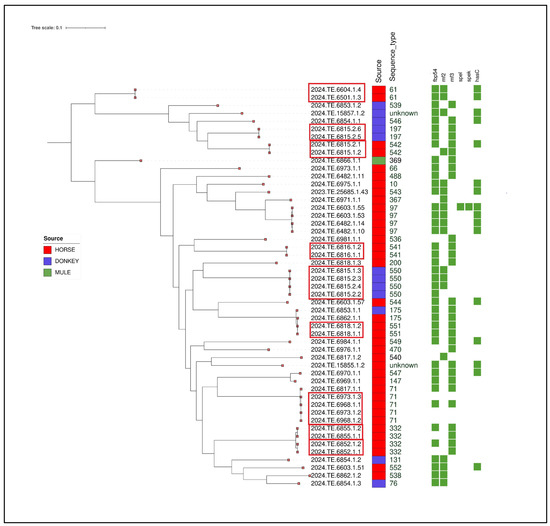
Figure 3.
Maximum Likelihood (ML) midpoint-rooted tree obtained from CFSAN pipeline of the 51 SEZ isolates. The first layer represents the source of isolation as shown in the legend. The second layer represents MLST analysis results. Virulence genes are shown in the heatmap with a green colour. The clusters identified with SNP analysis were highlighted with a red box. The visualisation of genes profiles and genes presence/absence was visualised using the Interactive Tree of Life (iTOL) online tool (https://itol.embl.de/, accessed on 15 September 2024).
Another cluster was detected between two strains (ST541) isolated from two different horses belonging to the same breeder.
Furthermore, an SNP analysis revealed another cluster involving four SEZ strains (ST71) isolated from four horses sampled from two different farms in the provinces of Chieti and L’Aquila; meanwhile, another strain (2024.TE.6817.1.1) belonging to ST71, isolated from another farm in the province of L’Aquila, was distant from the rest of the cluster. No epidemiological link was found between the three farms.
A similar situation was observed for the ST332 strains. Specifically, one cluster was identified for two strains isolated from two horses belonging to the same farm in the province of Isernia, while the isolated strain (2024.TE.6852.1.1) was correlated with another strain (2024.TE.6852.1.2), both originating from two horses belonging to the same farm in the province of Campobasso. Furthermore, a cluster (ST541) was identified between two horses sampled from the same farm in the province of Teramo.
Another cluster was identified between two strains belonging to ST551 and isolated from two different horses.
Finally, two strains (ST61) isolated from two different horses on the same farm in the province of Campobasso were correlated. Given the relevance of this clone following the outbreak in the “Vestina” area in the period November 2021–May 2022 [8], a comparative genomics analysis was performed on these two strains with those belonging to the aforementioned outbreak. The results showed that the strains isolated in this study are not correlated. Animal movement records also that there were no connections between the farm in Campobasso and the farm where the outbreak occurred.
The remaining isolates were singletons.
4. Discussion
Streptococcus equi subsp. zooepidemicus (SEZ) is a commensal bacterium found in the pharyngeal mucosa of various animal species. Studies of SEZ in equids are mostly limited to post-symptomatic observations or seroprevalence data, where the detection of antibodies indicates prior exposure but does not confirm carrier status. However, the literature does not allow for population-wide inferences, as it often relies on opportunistic sampling. The only probabilistic study, conducted by Libardoni et al. in Brazil [17] and focusing on estimating the prevalence of Streptococcus equi subsp. equi, reported a prevalence of 2.37% at the animal level and 5.86% at the herd level.
This study not only assesses the prevalence of SEZ in equine populations in the regions of Abruzzo and Molise (Italy), but also investigates the risk factors associated with the presence of SEZ and the genetic characteristics of the circulating SEZ strains. Traditional strain typing methods do not capture the genomic diversity of SEZ and only Streptococcus equi subsp. zooepidemicus from other streptococci. Following a local human outbreak associated with SEZ from raw milk cheeses [8] and the emergence of a highly virulent strain that caused significant mortality in a donkey herd [9], it became essential to assess whether these strains differed genetically from typical circulating strains.
Our results show a prevalence of SEZ of 30.1% at the animal level and 45.5% at the herd level, which is significantly higher than the prevalence of Streptococcus equi subsp. equi reported by Libardoni et al. [17], despite our smaller sample size. The higher percentage of animals sampled in this study (456/23,588 versus 1010/522,578 in the Brazilian study) may account for this difference, together with the fact that SEZ is considered a commensal bacterium, unlike Streptococcus equi subsp. equi. Notably, our multivariable logistic regression analysis showed no significant associations between the risk factors studied and the presence of SEZ. Variables such as respiratory symptoms or the presence of dairy species on the farm did not correlate with SEZ prevalence, suggesting that pharyngeal SEZ colonisation does not necessarily lead to respiratory symptoms. While some variables, such as age and species, showed significant associations in univariable analyses (e.g., chi-square and Mann–Whitney tests), these associations did not persist in the multivariable model. There may be several reasons for this discrepancy. First, the inclusion of multiple predictors, some of which may be correlated or weakly associated with the outcome, may have introduced multicollinearity and diluted the apparent effects of individual variables. Second, the true effect sizes of certain variables may be modest and require a larger sample size to be detected with adequate statistical power in a multivariable context. Third, unmeasured confounders or effect modifiers—such as variations in animal health status, management practices, or environmental exposures—may have masked potential associations. In addition, intermittent bacterial shedding and inconsistencies in sampling techniques between independent professionals may have affected detection rates.
Interestingly, donkeys had a significantly higher prevalence of SEZ than horses. This may reflect the role of donkeys as asymptomatic carriers, as they are generally susceptible to respiratory pathogens, although they often present with milder symptoms than horses. This species-specific susceptibility highlights a potential area for further research, particularly to identify mechanisms that could inform more tailored management practices for donkeys to limit SEZ transmission.
Another important observation is the significantly younger age of SEZ-positive animals compared to negative animals, suggesting age-related susceptibility factors. This association invites further investigation into the genetic, environmental, and nutritional influences on younger animals, which may help to improve management strategies across different age groups.
A genomic analysis revealed a high degree of variability among circulating strains. Fourteen new STs were identified, and these results highlight the importance of using whole-genome sequencing (WGS) to characterise SEZ strains, contributing to the expanding knowledge of this still poorly understood pathogen, as shown by Nocera et al., 2023 [18]. The remaining seventeen had been previously reported. In particular, ST200 and ST10 had been detected in cervico-uterine swabs from mares with endometritis [18], as well as in both healthy dogs and those with respiratory symptoms [19,20]. In contrast, ST147 was previously isolated from a horse breeding facility in China and from a mare in Argentina in 2017 [21,22]. ST71 has been isolated from the respiratory tract of horses in the UK and USA over an extended period [23]. ST369 was detected in cervico-uterine swabs from mares with endometritis in Italy [18].
Finally, ST61 was isolated from the clitoris of a mare in 2013 [24]. Although this clone has been identified as the source of local human outbreaks [8], the strains isolated in this study and belonging to the same sequence type did not show any correlation with those previously mentioned.
The fact that the identified genotypes have also been reported in different countries and host species suggests that there is no specific adaptation to a particular host.
Emerging clones such as ST194 and ST132 were not isolated in this study. These two clones have been associated with several epidemic outbreaks in different regions of the world. ST194 was first documented in a swine mortality outbreak in China in the 1970s [25], where the ATCC 35246 strain was responsible for a large-scale infection that resulted in the death of over 300,000 pigs. Subsequently, this ST has been implicated in significant outbreaks in North America, notably in Ohio and Tennessee in 2019 [26], where high mortality rates were observed in pig populations. Although ST132 has been less extensively studied, it has also been identified in outbreaks predominantly affecting livestock, with notable public health and economic impacts.
Genomic characterisation revealed a significant similarity to group A streptococci (GAS), particularly those associated with GAS infections. Most SEZ strains carried the fpb54 gene, which encodes a streptococcal fibronectin-binding protein—a critical factor in GAS infection, facilitating attachment to human buccal epithelial cells [27,28]. In addition, 19 of the 51 SEZ strains carried the mf2 gene, a virulence factor associated with Streptococcus pyogenes prophages that is rarely detected in SEZ [9]. Another GAS-associated virulence factor, mf3, encoding mitogen factor 3 (MF3) with DNase activity, has also been identified [29].
In one isolate, both the spel and spek genes associated with streptococcal exotoxin production were detected. The spel gene, first detected in SEZ in 2005, shares over 98% identity with the spel gene of S. pyogenes, suggesting possible horizontal gene transfer. The superantigenic toxin encoded by the spel gene is a common virulence factor in S. equi subsp. equi, but is less common in SEZ [30]. The spek gene, which is highly similar to the seeL gene, plays a role in immune evasion by suppressing phagocyte recruitment [31]. Consequently, isolate 2024.TE.6603.1.55 has considerable virulence potential.
Finally, this study confirmed the presence of the hasC gene from the has operon, encoding UDP-glucose dehydrogenase, which is required for the synthesis of the SEZ hyaluronic acid capsule [32]. This capsule, which mimics human connective tissue, reduces the host immune response and represents another virulence mechanism [33].
5. Conclusions
Overall, this study provides valuable insights into distribution of SEZ in the target regions, showing considerable strain variability and a higher prevalence in donkeys. The lack of association with cohabitation with dairy species contradicts previous assumptions about risk factors. These findings highlight the need for further research to determine species-specific susceptibility mechanisms and to identify factors that activate SEZ virulence, which could have implications for both animal and public health.
Author Contributions
Conceptualization, F.C., A.P. and C.E.D.F.; methodology, F.C., A.P. and M.C.; software, F.C., M.C. and A.C.; validation, D.A., A.A., M.R., M.C.C., M.D.D. and A.P.; formal analysis, F.C. and M.C.; investigation, M.D.D.B., A.G. and G.V.; resources, A.P.; data curation, F.C., M.C. and A.C.; writing—original draft preparation, F.C., M.C. and A.C.; writing—review and editing, A.P., A.A. and D.A.; visualisation, A.C., M.C. and F.C.; supervision, A.P. and C.E.D.F.; project administration, A.P.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Welfare Body of the Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise "G. Caporale" (protocol n° 10/2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequences are publicly available in the National Center for Biotechnology Information (NCBI) database. The accession numbers are detailed in Table 3.
Acknowledgments
We would like to express our sincere gratitude to our independent professional colleagues operating in the field for their invaluable collaboration and expertise throughout this study. Their contributions have been essential to the successful completion of this research. We also wish to thank the staff at the peripheral diagnostic units of the IZSAM for their continuous support and dedication. Their hard work and commitment were fundamental to the successful execution of the study, and we greatly appreciate their involvement.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SEZ | Streptococcus equi subsp. zooepidemicus |
| IZSAM | Istituto Zooprofilattico Sperimentale dell’Abruzzo e Molise |
| WGS | Whole Genome Sequencing |
References
- Kuusi, M.; Lahti, E.; Virolainen, A.; Hatakka, M.; Vuento, R.; Rantala, L.; Vuopio-Varkila, J.; Seuna, E.; Karppelin, M.; Hakkinen, M.; et al. An outbreak of Streptococcus equi subspecies zooepidemicus associated with consumption of fresh goat cheese. BMC Infect. Dis. 2006, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Balter, S.; Benin, A.; Pinto, S.W.; Teixeira, L.M.; Alvim, G.G.; Luna, E.; Jackson, D.; LaClaire, L.; Elliott, J.; Facklam, R.; et al. Epidemic nephritis in Nova Serrana, Brazil. Lancet 2000, 355, 1776–1780. [Google Scholar] [CrossRef]
- Torres Rosângela, S.L.A.; Santos Talita, Z.; Bernardes Andre, F.L.; Soares Patricia, A.; Soares Ana, C.C.; Dias Ricardo, S. Outbreak of Glomerulonephritis Caused by Streptococcus zooepidemicus SzPHV5 Type in Monte Santo de Minas, Minas Gerais, Brazil. J. Clin. Microbiol. 2018, 56, 10-1128. [Google Scholar] [CrossRef]
- Madzar, D.; Hagge, M.; Moller, S.; Regensburger, M.; Lee, D.; Schwab, S.; Jantsch, J. Endogenous endophthalmitis complicating Streptococcus equi subspecies zooepidemicus meningitis: A case report. BMC Res. Notes 2015, 8, 184. [Google Scholar] [CrossRef]
- Franceschi, G.; Soffritti, A.; Mantovani, M.; Digaetano, M.; Prandini, F.; Sarti, M.; Bedini, A.; Meschiari, M.; Mussini, C. Streptococcus equi Subspecies zooepidemicus Endocarditis and Meningitis in a 62-Year-Old Horse Rider Patient: A Case Report and Literature Review. Microorganisms 2024, 12, 2201. [Google Scholar] [CrossRef]
- Minces, L.R.; Brown, P.J.; Veldkamp, P.J. Human meningitis from Streptococcus equi subsp. zooepidemicus acquired as zoonoses. Epidemiol. Infect. 2011, 139, 406–410. [Google Scholar] [CrossRef]
- Pelkonen, S.; Lindahl, S.B.; Suomala, P.; Karhukorpi, J.; Vuorinen, S.; Koivula, I.; Vaisanen, T.; Pentikainen, J.; Autio, T.; Tuuminen, T. Transmission of Streptococcus equi subspecies zooepidemicus infection from horses to humans. Emerg. Infect. Dis. 2013, 19, 1041–1048. [Google Scholar] [CrossRef]
- Bosica, S.; Chiaverini, A.; De Angelis, M.E.; Petrini, A.; Averaimo, D.; Martino, M.; Rulli, M.; Saletti, M.A.; Cantelmi, M.C.; Ruggeri, F.; et al. Severe Streptococcus equi Subspecies zooepidemicus Outbreak from Unpasteurized Dairy Product Consumption, Italy. Emerg. Infect. Dis. 2023, 29, 1020–1024. [Google Scholar] [CrossRef]
- Cantelmi, M.C.; Merola, C.; Averaimo, D.; Chiaverini, A.; Cito, F.; Cocco, A.; Di Teodoro, G.; De Angelis, M.E.; Di Bernardo, D.; Auzino, D.; et al. Identification of the Novel Streptococcus equi subsp. zooepidemicus Sequence Type 525 in Donkeys of Abruzzo Region, Italy. Pathogens 2023, 12, 750. [Google Scholar] [CrossRef]
- Centers for Epidemiology and Animal Health; USDA; Animal and Plant Health Inspective Service (APHIS). Infectious Upper Respiratory Disease in U.S. Horses: Laboratory Results for Influenza Serology and Nasal Swab Culture for Streptococcus Isolation. 2001. Available online: https://www.aphis.usda.gov/sites/default/files/equine98_is_iurd.pdf (accessed on 6 January 2024).
- European Parliament and of the Council. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’). 2016. Available online: http://data.europa.eu/eli/reg/2016/429/oj (accessed on 10 January 2024).
- Ministero della Salute di concerto con il Ministero delle Politiche Agricole, Alimentari e Forestali. DECRETO 30 Settembre 2021 Gestione e Funzionamento Dell’anagrafe Degli Equini. (21A07453) (GU Serie Generale n.302 del 21-12-2021). 2021. Available online: https://www.gazzettaufficiale.it/eli/id/2021/12/21/21A07453/sg (accessed on 15 January 2024).
- Il Presidente della Repubblica. Decreto Legislativo 5 Agosto 2022, n.134, Disposizioni in Materia di Sistema di Identificazione e Registrazione Degli Operatori, Degli Stabilimenti e Degli Animali per L’adeguamento della Normativa Nazionale alle Disposizioni del Regolamento (UE) 2016/429, ai Sensi Dell’articolo 14, Comma 2, Lettere a), b), g), h), i) e p), della Legge 22 Aprile 2021, n. 53. 2022. Available online: https://www.gazzettaufficiale.it/eli/id/2022/09/12/22G00142/sg (accessed on 18 January 2024).
- Mcgowan, C. Welfare of Aged Horses. Animals 2011, 1, 366–376. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Lukjancenko, O.; Saputra, D.; Rasmussen, S.; Hasman, H.; Sicheritz-Pontén, T.; Aarestrup, F.M.; Ussery, D.W.; Lund, O. Benchmarking of Methods for Genomic Taxonomy. J. Clin. Microbiol. 2014, 52, 1529–1539. [Google Scholar] [CrossRef]
- Davis, S.; Pettengill, J.B.; Luo, Y.; Payne, J.; Shpuntoff, A.; Rand, H.; Strain, E. CFSAN SNP Pipeline: An Automated Method for Constructing SNP Matrices from Next-Generation Sequence Data. PeerJ Comput. Sci. 2015, 1, e20. [Google Scholar] [CrossRef]
- Libardoni, F.; Machado, G.; Gressler, L.T.; Kowalski, A.P.; Diehl, G.N.; dos Santos, L.C.; Corbellini, L.G.; de Vargas, A.C. Prevalence of Streptococcus equi subsp. equi in horses and associated risk factors in the State of Rio Grande do Sul, Brazil. Res. Vet. Sci. 2016, 104, 53–57. [Google Scholar] [CrossRef]
- Nocera, F.P.; Capozzi, L.; Simone, D.; Pizzano, F.; Iovane, V.; Bianco, A.; Parisi, A.; De Martino, L. Multi-locus sequence typing and in vitro antimicrobial resistance of equine Streptococcus equi subspecies zooepidemicus strains. Vet. Res. Commun. 2024, 48, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Chalker, V.J.; Waller, A.; Webb, K.; Spearing, E.; Crosse, P.; Brownlie, J.; Erles, K. Genetic diversity of Streptococcus equi subsp. zooepidemicus and doxycycline resistance in kennelled dogs. J. Clin. Microbiol. 2012, 50, 2134–2136. [Google Scholar] [CrossRef]
- Mangano, E.R.; Jones, G.M.C.; Suarez-Bonnet, A.; Waller, A.S.; Priestnall, S.L. Streptococcus zooepidemicus in dogs: Exploring a canine pathogen through multilocus sequence typing. Vet. Microbiol. 2024, 292, 110059. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Wang, L.; Zhang, B.; Su, L. Whole-Genome Sequencing and Phenotypic Analysis of Streptococcus equi subsp. zooepidemicus Sequence Type 147 Isolated from China. Microorganisms 2024, 12, 110059. [Google Scholar] [CrossRef]
- Retamar, G.C.; Bustos, C.P.; Guillemi, E.C.; Becu, T.; Ivanissevich, A.; Mesplet, M.; Munoz, A.J. Streptococcus equi subsp. zooepidemicus: High molecular diversity of Argentinian strains isolated from mares with endometritis. Res. Vet. Sci. 2024, 173, 105242. [Google Scholar] [CrossRef]
- Webb, K.; Jolley, K.A.; Mitchell, Z.; Robinson, C.; Newton, J.R.; Maiden, M.C.J.; Waller, A. Development of an unambiguous and discriminatory multilocus sequence typing scheme for the Streptococcus zooepidemicus group. Microbiology 2008, 154, 3016–3024. [Google Scholar] [CrossRef]
- Rasmussen, C.D.; Haugaard, M.M.; Petersen, M.R.; Nielsen, J.M.; Pedersen, H.G.; Bojesen, A.M. Streptococcus equi subsp. zooepidemicus isolates from equine infectious endometritis belong to a distinct genetic group. Vet. Res. 2013, 44, 26. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Zhang, X.; Tian, X.; Liang, Y.; Pan, F.; Song, H.; Xia, L.; Wu, Z.; Wang, W.; et al. The clinical characteristics of Streptococcus equi ssp. zooepidemicus causing acute death in pigs and its prevention with chimeric monoclonal antibodies. Vet. Microbiol. 2025, 302, 110420. [Google Scholar] [CrossRef]
- Chen, X.; Mou, K.; Lu, W.; Schumacher, L.; Resende-De-Macedo, N.; Sitthicharoenchai, P.; Derscheid, R.; Burrough, E.; Li, G. Genomic characterization of Streptococcus equi subspecies zooepidemicus from a 2021 outbreak in Indiana with increased sow mortality. mSphere 2023, 8, e00404-23. [Google Scholar] [CrossRef] [PubMed]
- Courtney, H.S.; Dale, J.B.; Hasty, D.L. Host cell specific adhesins of group A streptococci. Adv. Exp. Med. Biol. 1997, 418, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Courtney, H.S.; Dale, J.B.; Hasty, D.I. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect. Immun. 1996, 64, 2415–2419. [Google Scholar] [CrossRef]
- Wen, Y.; Tsou, C.; Kuo, H.; Wang, J.; Wu, J.; Liao, P. Differential secretomics of Streptococcus pyogenes reveals a novel peroxide regulator (PerR)-regulated extracellular virulence factor mitogen factor 3 (MF3). Mol. Cell. Proteom. 2011, 10, M110.007013. [Google Scholar] [CrossRef]
- Alber, J.; El-Sayed, A.; Estoepangestie, S.; Lammler, C.; Zschock, M. Dissemination of the superantigen encoding genes seeL, seeM, szeL and szeM in Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus. Vet. Microbiol. 2005, 109, 135–141. [Google Scholar] [CrossRef]
- Morris, E.R.A.; Wu, J.; Bordin, A.I.; Lawhon, S.D.; Cohen, N.D. Differences in the Accessory Genomes and Methylomes of Strains of Streptococcus equi subsp. equi and of Streptococcus equi subsp. zooepidemicus Obtained from the Respiratory Tract of Horses from Texas. Microbiol. Spectr. 2022, 10, e00764-21. [Google Scholar] [CrossRef]
- Blank, L.M.; Hugenholtz, P.; Nielsen, L.K. Evolution of the hyaluronic acid synthesis (has) operon in Streptococcus zooepidemicus and other pathogenic streptococci. J. Mol. Evol. 2008, 67, 13–22. [Google Scholar] [CrossRef]
- Crater, D.L.; van de Rijn, I. Hyaluronic Acid Synthesis Operon (has) Expression in Group A Streptococci. J. Biol. Chem. 1995, 270, 18452–18458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).