1. Introduction
Aquaculture is a crucial sector for global food security, supplying nearly 50% of the fish consumed worldwide, with an increasing trend due to the growing global population and declining wild fish stocks [
1]. Intensive aquaculture practices, while enhancing production efficiency, often lead to challenges such as high stocking densities, competition for resources, and poor water quality, which consequently result in physiological stress, oxidative damage, suppressed immunity, and heightened susceptibility to diseases in fish [
2,
3]. The overuse of antibiotics to manage bacterial infections has further exacerbated concerns regarding antimicrobial resistance, environmental contamination, and bioaccumulation in aquatic organisms, prompting regulatory restrictions on their application in aquaculture [
4,
5]. As a result, there has been a significant shift toward finding safer, eco-friendly alternatives, with probiotics emerging as a promising approach to enhancing fish health, growth, and disease resistance [
6,
7].
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts, primarily by modulating gut microbiota, improving nutrient assimilation, and strengthening immune responses [
8]. Among various probiotic candidates, Bacillus species have gained considerable attention due to their spore-forming ability, resilience to harsh environmental conditions, and diverse metabolic activities that contribute to improved fish health [
9]. The administration of Bacillus spp. in aquaculture has been shown to enhance intestinal microbial balance, increase digestive enzyme activity, promote growth performance, and strengthen the host immune defenses of Nile tilapia [
10,
11]. Furthermore, Bacillus-based probiotics have demonstrated the ability to inhibit pathogenic bacteria through competitive exclusion and the production of antimicrobial compounds such as bacteriocins and lipopeptides [
12,
13]. However, the efficacy of probiotics is strain-dependent, and inappropriate probiotic strains may lead to unintended consequences, such as immune overstimulation, horizontal gene transfer, and the potential for systemic infections [
14,
15]. Therefore, an ideal probiotic should be derived from the host’s microbiota, possess strong colonization abilities, promote beneficial bacteria, and suppress opportunistic pathogens without adverse effects [
16,
17].
Among Bacillus species, Bacillus subtilis has been widely studied for its multifaceted benefits in aquaculture. It has been reported to enhance antioxidant activity, improve digestive efficiency, and modulate immune responses in various fish species [
18,
19]. Specifically,
B. subtilis supplementation has been associated with increased feed conversion efficiency, higher growth rates, and improved survival in aquaculture species [
20]. Moreover, studies indicate that
B. subtilis enhances immune-related enzymatic activity, including lysozyme, peroxidase, superoxide dismutase (SOD), catalase (CAT), and immunoglobulin M (IgM), which are crucial for maintaining disease resistance in fish [
21]. Researchers documented that the dietary supplementation of
B. subtilis improved the growth performance, immune responses, and antioxidant status of Nile tilapia [
22,
23,
24]. For example, Won et al. [
22] reported the beneficial impact of combined
B. subtilis and L. lactis at 10
8 (CFU/g), while Hassaan et al. [
23] found improved performance at a dosage of 25% of the diet. Despite the well-documented benefits of
B. subtilis in aquaculture, there is still a dire need to explore the research on its application in Nile tilapia (
Oreochromis niloticus), particularly optimization of dietary levels during the fingerling stage, as there is variation regarding dosage recommendations. Moreover, the drastic climate change in Pakistan makes it most vulnerable to environmental stressors and disease outbreaks for aquaculture species, as Pakistan has been facing significantly increased temperatures, altered precipitation patterns, deforestation, and water scarcity. In this context, Nile tilapia (
Oreochromis niloticus) could be a promising species for aquaculture in Pakistan due to its adaptability to diverse environmental conditions, including varying water temperatures, low dissolved oxygen levels, and fluctuating water quality. Furthermore, the Nile tilapia’s relatively low environmental requirements and fast growth rate can contribute to food security and sustainable livelihoods and can enhance local economies while offering a viable solution to the increasing demand for fish protein in Pakistan. Keeping all this in view, the current study aimed to optimize the
B. subtilis dosage in the practical diet of Nile tilapia fingerlings. The objectives of the study were to evaluate the impact of dietary
B. subtilis supplementation at different inclusion levels on hematological and biochemical parameters, growth performance, antioxidant defense mechanisms, digestive enzyme activity, and the immune responses of Nile Tilapia.
2. Materials and Methods
2.1. Experimental Site, Approval, and Research Design
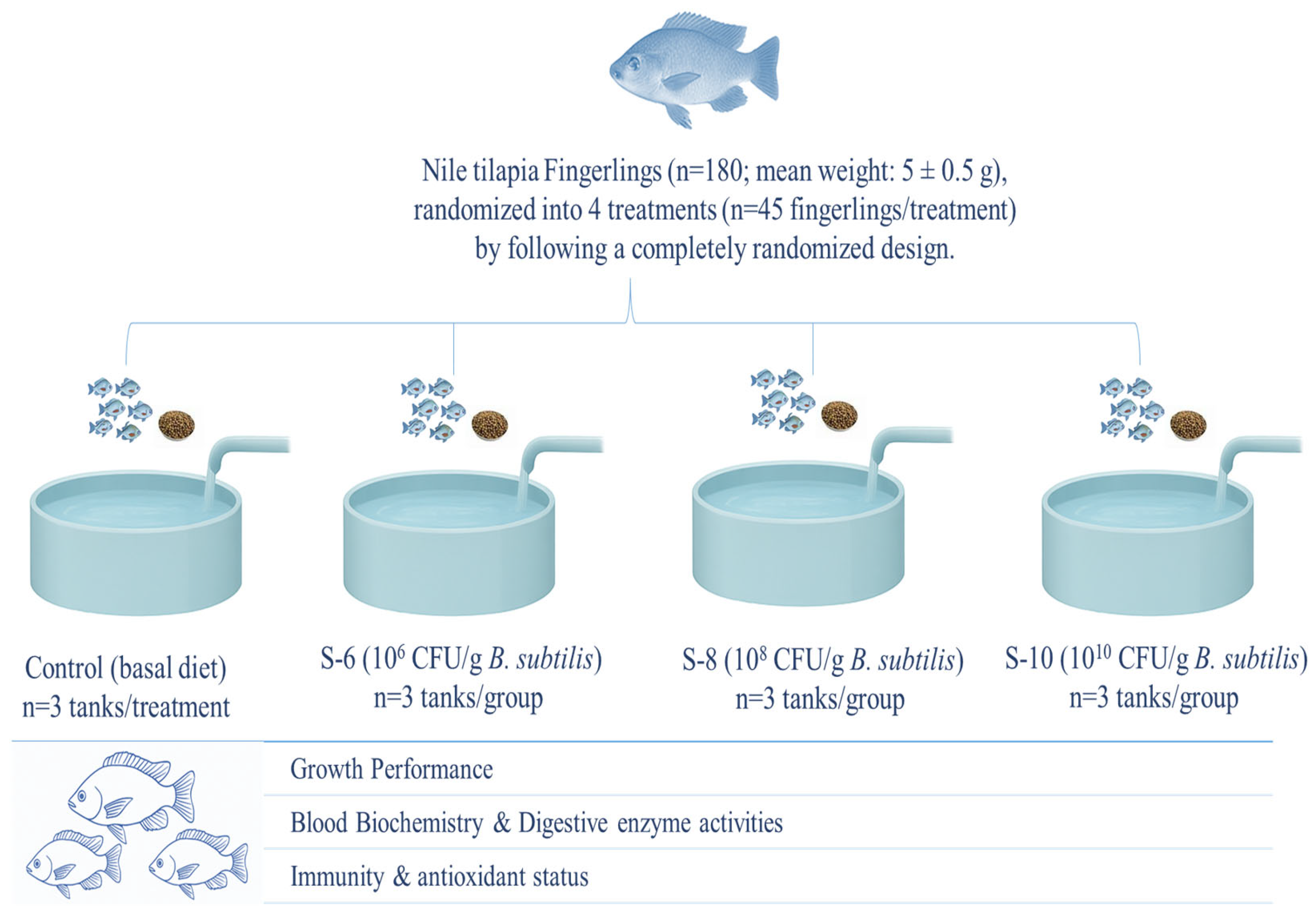
This experiment was carried out at the Fish Seed Rearing Unit, C block Ravi Campus Pattoki, and all experimental procedures were approved by the Animal Care and Use Committee of the University of Veterinary and Animal Sciences, Lahore, Pakistan (DR/163, 26-04-2021). In this experiment, 180 fingerlings (mean weight: 5 ± 0.5 g) were distributed across fifteen aquaria (n = 3 aquaria/treatment) and randomly assigned to four experimental treatments (n = 45 fingerlings/treatment) following a completely randomized design. The treatments consisted of a control group receiving a basal diet without supplementation and three groups fed probiotic-supplemented diets at concentrations of 106 (S-1), 108 (S-2), and 1010 (S-3) CFU g−1. These levels were selected based on the recommendation of the manufacturer. The probiotic, containing a single strain of Bacillus subtilis (BSN100) at a concentration of 1 × 109 CFU, was procured from ECOSH company (Estonia). To achieve final concentrations of 1 × 1010, 1 × 108, and 1 × 106 CFU of B. subtilis, one gram of the probiotic (1 × 1012 CFU) was appropriately diluted. The required volume of the probiotic was calculated using the following equation and subsequently mixed with sterile distilled water:
Probiotic Volume = [Target CFU/Initial CFU] × Initial volume
The calculated volumes were then diluted with sterile distilled water. To confirm the CFU concentration in each prepared solution, a plate count assay was performed using nutrient agar plates (HiMedia Ltd., Lahore, Pakistan). The plates were incubated at 37 °C for 24 h, and the resulting colonies were enumerated using a digital colony counter (Model: AVI-35).
2.2. Dietary Ingredient Selection, Recipe Formulation, and Feed Preparation
The dietary materials were purchased from the local market, and their composition and composite feed were assessed in accordance with Cunniff and Washington [
24] guidelines. The ingredients were passed through a 0.05 mm sieve (KENWOOD, AT284, Solihull, UK), batched according to the inclusion levels of the basal diet ingredients, and supplemented with the designated Bacillus subtilis (1 × 10
6, 1 × 10
8, and 1 × 10
10 CFU g
−1) levels. Each batch was then thoroughly mixed using a KENWOOD AT283 mixer (KENWOOD, Solihull, UK), moistened with water to form a dough, and pelleted using a meat mincer (ANEX, AG 3060, Karachi, Pakistan). Bacillus subtilis was applied post-pelleting using an oil coating, ensuring minimal heat damage. The viability loss was less than 10%, as confirmed by CFU counts. The pelleted feeds were air-dried at room temperature and stored at 4 °C. To maintain probiotic viability, diets were freshly prepared weekly, and bacterial counts in the feed were assessed every three days using the plate count assay. The basal diet was formulated based on the nutrient requirements of Nile tilapia as per the Council [
25]. If the concentration dropped below the desired level, freshly prepared probiotic feed was reapplied. Details of the dietary ingredient composition and chemical analysis are presented in
Table 1. Moreover, the experimental design illustration is given in
Figure 1.
2.3. Fish Husbandry Practices
At the start of the experiment, the fish were treated with a potassium permanganate solution (5 g/L) for 1–2 min to ensure biosecurity. The fish were divided into four groups and acclimatized in 120 L concrete tanks with a continuous water flow system. Tank water conditions were maintained at a pH of 7.2, a temperature of 27 °C, and a flow rate of 500 L/h. During the two-week acclimatization period, the fish were fed a basal diet (control) containing 36% crude protein at a feeding rate of 2% of their body weight per day. Health monitoring during acclimatization included visual assessment of skin and gill coloration, detection of external wounds, and observation of signs of illness. Only fish exhibiting normal skin and gill coloration, the absence of visible injuries or infections, and normal swimming behavior in flowing water were selected for the experimental trial. The fish selection criteria for uniformity in weight and health status were adapted and slightly modified from the method described by [
26].
After acclimatization, the fingerlings were stocked in 15 aquaria (n = 15 fingerlings/100 L aquaria). Healthy fish were shifted into aquaria that were triple-stocked for each diet following the acclimatization period. During the experimental period, each aquarium’s dissolved oxygen level, temperature, and pH were maintained @ 5.8–7.3 mg/L, 24.9–28.7 °C, and 7.4–8.6, respectively. Fish were maintained under a 12 h light/12 h dark photoperiod throughout the experimental period. Moreover, each aquarium was subjected to a water change once a day. For 8 weeks, the experimental fish were fed twice a day at a feeding rate of 2% of their body weight.
2.4. Growth Performance Measurements
The mean weight of fish in all aquaria was recorded before the initiation of the feeding trial. Subsequently, the total weight of an equal number of fish from each aquarium (n = 15 fish) was measured weekly, and their averages were used to calculate the following growth performance. In addition, at the end of the trial, five fish from each aquarium (15 per dietary group) were randomly selected, and their individual body weights were recorded before blood collection to calculate the covariance. Growth performance parameters, including average weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), and survival rate (SR), were then calculated using the formulas previously described by Mushtaq et al. [
27].
Weight gain (g) = final body weight (g) − initial body weight (g)
SGR (%) = [(Final weight (g) − Initial weight (g)/days of growth trial] × 100
Feed conversion ratio = Feed intake (g)/weight gain (g)
Survival rate (%) = [Number of surviving fish/Initial number of fish] × 100
2.5. Sample Collection
At the end of the experiment, five randomly selected fish per aquarium were subjected to blood sampling via the caudal veins using 3 mL syringes and stored in EDTA vacutainers for hematological analysis. Additional blood samples were collected in Eppendorf tubes without anticoagulant and centrifuged at 3000 rpm for 15 min to collect serum, which was stored at −80 °C for biochemical analyses. Furthermore, three fish per aquarium (nine fish/group) were anesthetized with MS-22 following Mushtaq et al. [
27], aseptically dissected, and their liver and gastrointestinal tracts (stomach, pyloric caeca, intestine) were collected for antioxidant and enzymatic analyses.
2.6. Hematological and Biochemical Analysis
Hematological parameters, including red blood cell (RBC) count, white blood cell (WBC) count, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), hemoglobin (HGB), and platelet (PLT) count, were analyzed using an autohematological analyzer (Celltac α, MEK-6550, Nihon Kohden Ltd., Shinjuku, Japan) at the General Laboratory, Department of Fisheries and Aquaculture, UVAS Ravi Campus, Pattoki. The serum was analyzed for AST (BioAST631), ALT (BioALT94), and ALP (BioALP2) using commercial kits (BioScien kits, Karachi, Pakistan) by following the protocols of Hossain et al. [
28]. Additionally, serum samples were analyzed using an automatic biochemical analyzer (Hitachi 7600-110, Hitachi High-Technologies Corporation., Tokyo, Japan) to assess stress biomarkers, including cortisol and glucose levels. Immunological parameters, such as immunoglobulin concentration, total serum protein, lysozyme activity, and aminotransferase activity, were measured following the methodologies described by Ullah et al. [
29]. Furthermore, fresh heparinized blood samples were analyzed for respiratory burst activity and phagocytic activity according to the protocols established by Solem et al. [
30]. Briefly, isolated macrophages were incubated with NBT solution (0.1%) for 30 min at 25 °C. The reaction was stopped with methanol, and the cells were washed and air-dried. The reduced NBT (formazan) was then solubilized with 2 M KOH and DMSO, and absorbance was read at 620 nm using a microplate reader (Thermo Fisher, Waltham, MA, USA). For phagocytic analysis, isolated macrophages were incubated with heat-killed
Aeromonas hydrophila stained with trypan blue at a ratio of 10:1 (bacteria/macrophage) for 1 h at 25 °C. The cells were then washed, fixed with methanol, and stained with Giemsa. At least 200 macrophages were observed under a microscope for the percentage of phagocytic activity.
2.7. Estimation of Digestive Enzyme Activities
The collected samples of gastrointestinal tissues were homogenized (1:10
w/
v) in ice-cold phosphate buffer (0.1 M, pH 7.4), centrifuged (12,000×
g, 20 min, 4 °C), and supernatants were used for enzyme assays. Protease activity was measured using casein as a substrate [
31], with absorbance recorded at 280 nm, and one unit defined as the enzyme releasing 1 μmol of tyrosine/min. Lipase activity was determined using p-nitrophenyl palmitate [
32], with absorbance recorded at 410 nm, and one unit defined as the enzyme releasing 1 μmol of p-nitrophenol/min. Amylase activity was assessed via the 3,5-dinitrosalicylic acid (DNS) method [
33], with absorbance recorded at 540 nm, and one unit defined as the enzyme releasing 1 μmol of maltose/min. Total protein concentration was determined using the Bradford method for normalization.
2.8. Antioxidant Analysis
For antioxidant status, a 2 g liver sample was mixed with 6 mL of phosphate buffer (pH 7.4), filtered through Whatman filter paper no. 1, and centrifuged at 10,000×
g for 15 min. The supernatant was separated, and all enzyme isolation procedures were performed at 4 °C. Catalase (CAT) activity was measured using the Ac [
34] method by monitoring the decrease in H
2O
2 concentration at 240 nm with an Analytik Jena Specord 200 Plus UV/VIS spectrophotometer. Superoxide dismutase (SOD) activity was determined following Giannopolitis and Ries [
35] by assessing its ability to prevent the photoreduction of nitroblue tetrazole (NBT). Glutathione peroxidase (GPx) activity was measured using the method of Civello et al. [
36] by evaluating its ability to reduce H
2O
2 at 470 nm. Lipid peroxidation in muscles and liver was determined according to Mushtaq et al. [
27], where samples were mixed with a KCl and Tris-maleate solution, followed by ascorbic acid, and heated with trichloroacetic acid. After cooling and centrifugation, thiobarbituric acid (TBA) and HCl were added, and TBA values were measured at 530 nm, expressed as µg malondialdehyde equivalents/mg tissue.
2.9. Statistical Analysis
The raw data were normalized using QQ plots (SPSS, Chicago, 18.0 version). The data were analyzed by one-way analysis of variance in SPSS software. The variation in the final body weight was used as a covariate for blood and other parameters. The means were compared using the Tukey test after adjusting the significance level set at p < 0.05.
4. Discussion
Most researchers have been actively investigating alternative, safer solutions to antibiotics for use in aquaculture, aiming to enhance fish production while minimizing negative impacts on the environment and human health. Among these alternatives, probiotics have emerged as natural bio-enhancers with strong potential to replace antibiotics due to their significant effects on fish health, growth, and disease resistance [
37]. Probiotics benefit fish primarily by modulating gut microbiota, improving digestive efficiency, stimulating immune responses, and offering protection against pathogenic microorganisms. Among various probiotic candidates, Bacillus species are particularly noteworthy due to their growth-promoting, antimicrobial, and immunostimulatory properties. These bacteria are non-pathogenic and spore-forming, allowing them to survive harsh environmental conditions, which makes them ideal for inclusion in animal diets [
38]. Their safety has been well documented, with no known pathogenic effects on host organisms. Several Bacillus species, including
B. subtilis,
B. amyloliquefaciens,
B. cereus,
B. thuringiensis,
B. pumilus,
B. clausii,
B. mojavensis,
B. circulans, and
B. licheniformis, have demonstrated probiotic potential across various animal species, including fish [
39,
40,
41]. These strains exert diverse beneficial effects, particularly on growth performance, immune function, and gut health. Despite its widespread use, limited research has focused specifically on
B. subtilis as a probiotic for
Oreochromis niloticus (Nile tilapia), especially during the vulnerable fingerling stage. Since fingerlings represent a critical growth phase, supplementation during this period may significantly influence early development, immune function, and disease resistance. Moreover, Nile tilapia possess a moderately developed stomach and relatively long intestine, consistent with their omnivorous feeding habits [
10]. Therefore, this study aimed to evaluate the effects
of B. subtilis supplementation at three different concentrations (10
6, 10
8, and 10
10 CFU/g) on growth performance and health indicators in
O. niloticus fingerlings over two months. The results demonstrated that
B. subtilis supplementation significantly enhanced key growth performance parameters, including final body weight, average weight gain, specific growth rate, and feed conversion ratio, with the most pronounced effects observed at the highest concentration of 10
10 CFU/g. These findings are consistent with previous studies reporting similar effects of
B. amyloliquefaciens in
O. niloticus [
41],
B. licheniformis in
O. mossambicus [
42], and
B. subtilis in
Pangasius hypophthalmus [
43]. This supports the broader role of Bacillus species in enhancing fish growth and productivity. The improvement in growth performance can be attributed to the diverse bioactive mechanisms of
B. subtilis, including the secretion of extracellular enzymes such as proteases, amylases, and lipases, which facilitate the breakdown of macronutrients and improve nutrient digestibility [
11]. Additionally, the production of short-chain fatty acids, including acetate, propionate, and butyrate, contributes to gut health by supporting epithelial cell proliferation and serving as additional energy sources [
2]. However, in some parameters, no statistically significant differences were observed between the control and treatment groups. This might be due to inter-individual variability, possible imbalances in gut microbiota, or enzyme activity, all of which could impact nutrient absorption and growth performance [
44]. Moreover, the relatively short six-week duration may not have been sufficient to capture the long-term benefits of probiotic supplementation, indicating that extended trials are warranted.
In addition to growth, hemato-immunological indicators provided important insights into fish health. Hematological parameters such as HCT, RBC count, and WBC count are reliable indicators of the physiological and immune status of fish [
45,
46]. In the current study, fish supplemented with
B. subtilis, especially at 10
10 CFU/g, showed significantly elevated HCT, RBC, and WBC levels compared to controls. These findings align with prior research in
Cirrhinus mrigala [
47],
Oplegnathus fasciatus [
46], and
O. niloticus [
48,
49], where probiotic supplementation enhanced hematological parameters. The increase in WBC count reflects immunostimulation and enhanced disease resistance [
50], as probiotics such as Bacillus spp. are known to stimulate immune cell activity and modulate innate immune responses [
50,
51]. Liver and kidney biomarkers, including ALT, AST, and ALP, serve as key indicators of organ function and physiological well-being in fish [
27]. In this study,
B. subtilis-fed fish exhibited significantly lower ALT and AST levels, indicating reduced hepatic stress and improved liver function. This is consistent with earlier studies showing that probiotic supplementation, including
B. subtilis, B. licheniformis, and
Enterococcus faecalis, can decrease serum liver enzyme levels in fish [
52,
53]. Interestingly, ALP activity was higher in the probiotic groups, which may reflect increased macrophage enzyme activity and heightened immune readiness [
54,
55,
56].
Key immune molecules, such as immunoglobulin M (IgM) and lysozymes, were also positively influenced by
B. subtilis supplementation. IgM plays a pivotal role as the primary immunoglobulin in fish, while lysozyme contributes to bacterial cell wall degradation. In this study, fish receiving higher doses of
B. subtilis displayed significantly increased serum IgM and lysozyme activity, particularly in the 10
10 CFU/g group. This enhancement in immune function corroborates findings from
O. mossambicus supplemented with
B. subtilis [
42] and reinforces the immunostimulatory capacity of the Bacillus species. Moreover, respiratory burst and phagocytic activities—key aspects of innate immunity—were significantly elevated in the probiotic-fed groups, echoing previous findings that suggest improved bactericidal capacity and immune defense following Bacillus supplementation [
57,
58,
59,
60,
61]. Digestive capacity, another critical health indicator, was markedly improved through probiotic intervention in this study. Fish fed
B. subtilis displayed significantly higher digestive enzyme activities, including protease, lipase, and amylase, enhancing nutrient breakdown and assimilation. These findings are in agreement with studies in
Litopenaeus vannamei [
62],
P. hypophthalmus [
63],
O. niloticus [
52], and
O. mossambicus [
42], highlighting the ability of Bacillus spp. to enhance digestive processes, thereby contributing to better feed utilization and overall performance. Probiotic supplementation also modulated antioxidant responses in this experiment. Fish fed
B. subtilis showed higher activities of antioxidant enzymes such as SOD, GPx, and catalase, along with lower levels, indicating reduced oxidative stress and improved antioxidant defense. These results are consistent with earlier studies reporting similar protective effects of probiotics in various fish species [
64,
65,
66]. The ability of probiotics to produce antioxidant metabolites, including glutathione and exopolysaccharides, further supports their role in mitigating oxidative damage [
67]. Interestingly, no significant changes were observed in serum cortisol or glucose levels across the groups, suggesting that
B. subtilis did not induce stress during the trial. These findings align with previous research showing no alterations in stress biomarkers in healthy, unstressed fish following probiotic supplementation [
68]. These health-associated effects of
B. subtilis can be attributed to its ability to secrete antimicrobial peptides such as subtilin, bacillomycin, surfactin, fengycin, and iturin, which inhibit pathogenic bacteria and help maintain gut microbial balance [
7]. Additionally, the synthesis of bacteriocins enhances the competitive exclusion of harmful microbes [
14].
B. subtilis also produces B-complex vitamins (e.g., B12, riboflavin, biotin), essential cofactors, and immunostimulatory metabolites that support metabolic and immune functions. Furthermore, quorum-sensing inhibitors produced by
B. subtilis may reduce pathogen virulence, contributing to better intestinal health and immunity [
16]. Collectively, these mechanisms underpin the observed improvements in nutrient utilization, immune defense, oxidative balance, and gut integrity, ultimately enhancing the growth performance and overall health status of
O. niloticus fingerlings.