Simple Summary
Ruminants are closely related to human life because they are able to convert plants into nutrients available to humans, including meat and milk. In modern agriculture, ruminant feeds include protein feeds, energy feeds, roughages, and additive feeds, where protein feeds can be categorised into plant proteins, animal proteins, microbial proteins, and non-protein nitrogen, according to the feed source. Only more plant protein feeds such as soybean meal can maintain a higher level of ruminant feeding to meet people’s demand for meat and milk; however, some anti-nutritional factors such as gossypol and tannins present in the plants themselves can affect the utilisation efficiency of ruminant feeds and even the health of ruminants, and it has become an imperative task to improve and solve this issue. Different plants contain different kinds of anti-nutritional factors, and different anti-nutritional factors have different physicochemical properties, so the corresponding treatment methods are also different. In order to minimise the impact of anti-nutritional factors in plants on ruminant production, we have to find out the distribution of anti-nutritional factors in different plants, the physicochemical properties of different anti-nutritional factors, and the most reasonable methods to eliminate them.
Abstract
In recent years, the rapid development of the ruminant feeding industry and the limited availability and rising prices of traditional protein feed ingredients have renewed the focus on protein feeds in ruminant diets. Plant protein feeds are a core component of protein feeds for ruminants; however, the utilisation of both conventional and non-conventional plant protein feeds is limited by the presence of anti-nutritional factors (ANFs). In order to maximise the use of plant protein feeds and to promote their application in ruminant production, it is important to have a comprehensive understanding of the types and nature of their ANFs, their anti-nutritional mechanisms, and current effective methods of eliminating ANFs. Therefore, the types, anti-nutritional mechanisms, and elimination methods of ANFs in major plant protein feeds for ruminants are initially summarised in this review, which provides a reference for anti-nutritional factor elimination and the production of full-price compound feeds for ruminants.
1. Introduction
In recent years, the price of feed raw materials has increased, the feed cost of farming has increased by 70~80%, and the increase in meat consumption requires more protein feed to maintain the level of animal production [1]. The overall shortage of feed raw materials and the expensive price of high-quality protein feed resources have become the main problems limiting the efficient farming of ruminants, and improving the digestive and absorptive efficiency of the feeds in ruminants is an effective way to alleviate the above problems. The feed formulation for ruminants is a complex and delicate process, aiming to fully satisfy their nutritional needs for growth, development, health maintenance, and performance enhancement. This integrated system consists of the following four main feed categories: protein feeds, energy feeds, roughages, and feed additives, each of which carries specific nutritional and physiological functions that together support a healthy feeding system for ruminants. Protein feeds are the core component of ruminant feeds. Protein feeds can be divided into plant protein, animal protein, microbial protein, and non-protein nitrogen according to the feed source. Plant protein feed sources mainly include all kinds of oilseed crops after the oil extraction of the remaining cake meal, including soybean meal (SBM), rapeseed meal (RSM), cottonseed meal (CSM), peanut meal, sunflower kernel meal, flax kernel meal, and so on. RSM, CSM, and other types of miscellaneous meal follows the ‘SBM reduction in feed’ trend. The application of the proportion of plant protein feed sources increased year by year, but it contains a variety of anti-nutritional components ANFs to limit the various types of miscellaneous meal as well as the application of SBM [2]. When livestock and poultry consume excessive amounts of ANFs, it not only affects the digestibility and absorption of nutrients, but it may even have adverse effects on growth and health [3,4]. For example, free gossypol, glucosinolates, trypsin inhibiting factor, antigenic proteins, and phytohemagglutinins present in cottonseed will inhibit the absorption of amino acids, and RSM contains a large amount of glucosinolates, tannins, phytic acid, erucic acid, and other ANFs [5,6]. At the same time, the nutrient composition of various types of miscellaneous meals is also defective, for instance, the lysine and threonine content of RSM is lower than that of SBM, and the digestibility in ruminants is significantly lower than that of SBM [7]. In order to improve the utilisation efficiency of plant protein feeds and their processing by-products, the inactivation of ANFs in them is a critical step. Currently, a number of different methods have been investigated to eliminate or inactivate these ANFs, mainly covering the following three techniques: physicochemical treatment, enzymatic treatment, and microbial decomposition [8]. This paper comprehensively reviews the classification, anti-nutritional mechanisms, and mitigation strategies of ANFs in plant-based protein feeds for ruminants. By synthesising current knowledge, this work aims to provide actionable insights for optimising feed formulations, enhancing nutrient utilisation, and advancing the development of balanced, high-performance compound feeds. Ultimately, this review serves as a foundational resource to guide future research and industry practices in sustainable ruminant nutrition.
2. Overview of Plant Protein Feeds and ANFs
Plant protein feed sources mainly include the cake meal remaining after the oil extraction from various oilseed crops, including SBM, RSM, CSM, and so on. They have great potential as a protein source for ruminant protein feeds due to their richness in crude protein, amino acids, and good availability [9]. These plant protein feeds contain a variety of ANFs, such as protease inhibitors, antigenic proteins, and gossypol, which limit the efficiency of protein and carbohydrate utilisation in plant protein feeds and adversely affect the growth and reproduction of animals. The content and physicochemical properties of different ANFs in different feeds are varied, so the corresponding elimination methods are also different [10].
2.1. ANFs in SBM
SBM is the most commonly used plant-based protein feed in various animal feeds because of its high yield, high crude protein content, good quality, reasonable composition of essential amino acids, and high digestibility [3]. However, soybean also contains ANFs such as protease inhibitors, soybean antigens, soybean agglutinin, etc., which reduce its nutritional value.
2.1.1. Soybean Antigenic Protein
The main ANFs in soybeans are antigenic proteins, which are immunoreactive and thermostable, may cause allergic reactions in animals, and may affect their productive performance [11]. Soybean antigenic proteins can be classified into four categories according to the centrifugal sedimentation coefficient as follows: 2 S, 7 S, 11 S, and 15 S, with the highest proportion of soybean antigenic proteins being 11 S at approximately 40% [12]. The main components of soybeans that trigger the immune response of animals are β-accompanied by soybean globulin (7 S) and soybean globulin (11 S), with the 7 S protein having the greater immunoreactivity, and 11 S proteins consisting of six subunits [13]. Each of these is connected to a disulfide bond, subunits, connecting basic and acidic peptide chains via disulfide bonds [11].
2.1.2. Protease Inhibitors
Protease inhibitors are capable of hindering the function of a variety of proteases, such as trypsin, pepsin, and coagulation factors, etc. There are numerous types of protease inhibitors in nature, among which, the main ones contained in SBMs are trypsin inhibitors (TIs), classified into Bowman-Birk inhibitors (BBIs) and Kunitz inhibitors (KTIs) [14]. The KTI has a variable structure containing four cysteine residues, which form two disulfide bonds, a structure that makes the KTI easy to destroy when heated. The BBI contains a higher number of cysteines and seven disulfide bonds, and it has multiple reactive groups that can inhibit the activity of multiple proteases simultaneously. Although the content of TIs in soybean is only 2% of the total mass (about 0.6% for BBI and 1.4% for KTI), they account for 40% of the total anti-nutritional effect of all ANFs in soybean [4].
2.1.3. Soybean Agglutinin
Soybean agglutinin (SBA), as the name indicates, is a class of ANFs that promotes erythrocyte aggregation and is a major anti-nutrient in soybean, which is heat-insensitive, stable, and undamaged. In SBM, the concentration of SBA is typically 3% [15]. SBA impairs animal growth by interfering with the digestion and absorption of nutrients in the gastrointestinal tract. In addition, one of the most important characteristics of SBA considered to be ANFs is the remarkably high resistance and stability to protein hydrolysis over a large physiological pH range [16]. When SBA is ingested by livestock, SBA specifically binds to glycoproteins on the surface of small intestinal epithelial cells, thereby adversely affecting the animal’s intestinal epithelial cells and immune function. Some studies based on intestinal porcine epithelial cell lines (IPEC-J2) have shown that SBA exerts its toxic effects on the intestine through a variety of apoptosis-related pathways [17]. Other studies have shown that SBA have an effect on growth performance in young ruminants, but not in adult ruminants [18]. SBA may be degraded by rumen microorganisms.
2.1.4. Tannins
Tannins are water-soluble natural polyphenolic compounds, mainly found in the shell of soybeans, which can be divided into hydrolysed tannins (HTs) and condensed tannins (CTs). CTs cannot be hydrolysed and can react with amylase, trypsin, and their substrates, thus decreasing the utilisation rate of carbohydrates and proteins, and affecting the intake of the animal [19]. Tannins not only reduce the digestibility of feed proteins, but also affect the intestinal micro-ecosystem. When tannins are increased in feeds, they significantly reduce total gas production and the concentration of ammonia and volatile fatty acids in the intestinal tract, and they increase the number of viable bacteria in the enterococci and coliform flora [20].
2.1.5. Phytic Acid
Phytic acid (PA), chemically known as inositol hexakisphosphate, exists mainly in the form of phosphorus storage from seeds of cereal and oilseed crops, and its molecular structure contains an inositol ring and six phosphoric acid groups. PA is present not only in legume feeds, but also in rapeseed and its cake meal [21]. The PA molecule consists of six phosphate groups, which can interact with metal ions and proteins to produce insoluble complexes, which are not easily digested and absorbed by animals, reducing the nutritional value of the feed and affecting its palatability, which has a negative impact on the healthy growth of animals [22].
2.1.6. Non-Starch Polysaccharides
SBM contains many non-starch polysaccharides (NSPs), of which β-glucan, arabinoxylan, and cellulose are the main components, usually accounting for less than 10% of its weight [23]. The high proportion of ether bonds in lignin makes it more difficult to degrade than cellulose and hemicellulose, and it requires 150–400 °C to break [24]. Degradation of NSPs in ruminants can occur in the rumen, small intestine, and large intestine, but the rumen is the most important degradation site for feed NSPs, most of which are rapidly degraded mainly by rumen microorganisms. It has been shown that the ingestion of NSPs can have a systemic viscosity effect, which can inhibit glucose absorption or form a physical barrier to inhibit starch digestion by amylase and reduce its digestion rate [25,26,27].
2.1.7. ANFs in Miscellaneous Meals
Miscellaneous meals contain high levels of protein and a variety of vitamins and minerals, but their amino acid ratios are unbalanced, and their use in ruminant feeds is limited by the presence of free lintol, thioglucosides, PA, and other ANFs in miscellaneous meals [24].
2.1.8. Gossypol
Gossypol is a phenolic substance that is particularly abundant in the seeds of the cotton plant, with the molecular formula C50H50O8. In CSM, gossypol exists in both free and bound forms, and the total amount of gossypol in both forms is referred to as total gossypol [28,29]. The World Health Organisation (WHO) in 2018 established a maximum threshold of 450 mg/kg for the content of free gossypol (FG) in CSM protein products [30]. Gossypol damages the gastrointestinal mucosa of animals and the reproductive capacity of males, and tolerance to gossypol varies in different animals, with pigs having a low tolerance and broilers and laying hens having a relatively high tolerance [29]. Ruminants have a better tolerance to gossypol due to their special digestive system, and it was found that CSM can replace SBM as the main protein source for beef cattle and goats, without affecting their growth and slaughter performance [31]. However, gossypol has toxic effects on the reproductive system of ruminants. Tiago et al. collected testicular samples from young male goats after feeding them with gossypol for 95 days, analysed them, and found that there was a significant decrease in the concentration of testosterone after feeding gossypol, as well as higher total sperm defects, poor sperm motility, and a high rate of lesions [32]. This indicates that the intake of gossypol in ruminants used for breeding is unsafe.
2.1.9. Glucosinolates
There are many glucosinolates in RSM that are decomposed by mustard enzymes to produce oxazolidinethione (OZT), isothiocyanate (ITC), and nitrile, with OZT being the main toxin in RSM, and it is more toxic to the thyroid gland. OZT is the main toxin in RSM and has a strong toxic effect on the thyroid gland [33]. ITC can damage the mucous membranes of the gastrointestinal tract of animals or cause substantial damage to the lungs and kidneys due to its own irritant properties [34]. Nitrile is the most toxic of the glucosinolates decomposition products, and prolonged or large intake can damage the liver and kidneys of animals [35]. Tolerance to glucosinolates varies among animals, being higher in ruminants and lower in pigs and poultry [36,37]. The treatment of CSM and RSM by physical, chemical, and biological methods can reduce the content of ANFs and improve the digestion and absorption of nutrients in animals [38]. The anti-nutritional mechanisms of ANFs in plant protein feeds are shown in Table 1.

Table 1.
Anti-nutritional mechanisms of ANFs in plant protein feeds.
3. Advances in ANF Elimination Methods
Currently, there are many techniques to eliminate ANFs in plants, such as high temperature and pressure, fermentation, etc., but they can be broadly categorised as physical, chemical, and biological methods [56]. Physical methods completely deactivate ANFs through high temperature, high pressure, radiation, etc. The chemical method involves adding chemical reagents (e.g., CuSO4 and H2O2, etc.) to feeds containing ANFs under appropriate conditions to inactivate ANFs through chemical reactions. Biological methods, on the other hand, include enzymatic degradation by enzymes and microbial fermentation to inactivate ANFs or degrade them into other small molecules.
3.1. Physical Methods
For heat-stable ANFs, such as PA and tannins, physical methods such as peeling and crushing can be used to reduce their content. For example, soybean can remove most of the tannins and reduce PA content by removing the outer hulls [58]. For thermally unstable ANFs, such as agglutinins and protease inhibitors, a common method is heating [59].
Heating methods include dry heat treatments (e.g., baking, microwave heating, and infrared irradiation) and moist heat treatments (e.g., boiling, autoclaving, and extrusion heating), which are only suitable for thermally unstable ANFs. They are not effective for thermally stable ANFs, such as PA, cyanogenic compounds, and oligosaccharides. RSM heated at 100 °C for 5 min or 2 h increased the removal of glucosinolates from 24% to 95% but protein digestibility decreased from 79% to 71% [60]. Duodu et al. used soaking and autoclaving techniques, leading to a reduction in FG levels by 34.48% and 27.59%, respectively, as well as a significant reduction in PA [61]. Water immersion also removed soluble NSPs [62]. In addition to this, studies have also reported the elimination of NSPs using electron beam (EB) irradiation methods [63]. The method of electron radiation also effectively eliminates FG and total gossypol in CSM. Bahraini et al. compared the effects of EB and gamma ray (GR) irradiation treatments at doses of 10, 20, and 30 kGy on the chemical composition, protein quality, and protein digestibility of CSM, significantly reducing FG and total gossypol content in CSM in a dose-dependent manner compared with unirradiated CSM. In addition, ER irradiation induced a greater decrease in FG and total gossypol content than GR irradiation [64].
The processing of feeds also affects the degradation or deposition of ANFs over time. The first step in feed production is comminution, which also destroys the fibrous structure of the raw material and increases the solubility of NSPs, making them available to digestive enzymes. Extrusion is a high-temperature, short-duration process that combines several processes, including heat and mass transfer, mixing, shearing, particle size reduction, melting, texturizing, caramelising, and moulding. Extrusion is also a very effective method to inactivate amylase inhibitors, trypsin, pancreatic rennet, and SBA activity without altering the protein levels in the food [65]. In general, the moisture content and composition of raw materials, barrel temperature, and extruder feed rate are the most important factors affecting the extrusion process in terms of reducing the amount of ANFs [66]. In another study, the effectiveness of extrusion processing was compared with conventional non-thermal processing methods such as dehulling, soaking (in double deionised water at 30 °C for 12 h), and germination (at 25 °C for 72 h) in an attempt to reduce ANFs in broad beans and kidney beans. The results showed that germination for 72 h resulted in a significant reduction in phytates in broad beans (60.8%) and kidney beans (30.2%), whereas extrusion alone resulted in a reduction in phytates by about 26.7% and 21.4% in broad beans and kidney beans, respectively [67].
3.2. Chemical Methods
Chemical methods refer to the addition of acids, bases, alcohols, ammonia, and special substances to react with ANFs or purify ANFs by extraction. Chemical methods can deactivate ANFs by decomposing them, neutralising them, dissolving them, binding them, destroying the structure of the ANFs, or breaking the bonds that maintain the structure. For example, the treatment of SBM with ascorbic acid, CuSO4, FeSO4, and urea can inactivate TIs, and the addition of FeSO4 and CuSO4 to RSM results in a glucosinolates detoxification rate of up to 85.2%, which is less costly, but also suffers from poor palatability, severe nutrient loss, and some contamination [68].
Barraza et al. found that the addition of 0.5%, 1%, and 2% calcium hydroxide to CSM led to a reduction in FG by 21.25%, 28.15%, and 40.52%, respectively, but the use of calcium hydroxide often reduces the bioactivity and detoxification efficiency of the vitamins in the feed [69]. In addition, supercritical CO2 extraction and solvent extraction were more effective in removing FG. Bhattacharjee et al. used supercritical fluid extraction with CO2 to extract oil from CSM and found that when the extraction parameters were set at a pressure higher than 550 bar, a temperature range of 70–80 °C, and an extraction time of 2–3 h, it was possible to maximise the oil yield while minimising FG extraction [70]. Pelitire et al. used acetone and ethanol as solvents to remove FG from CSM and found that both solvents were effective in reducing the total FG level in CSM to between 5% and 10% of its initial value, with the rate of FG removal being much faster via ethanol extraction than via acetone extraction [71]. It is suggested that the process of supercritical CO2 extraction and solvent extraction can be used to produce CSM with low FG.
The chemical method is convenient in operation, has a good inactivation effect, and saves equipment and energy, but the chemical reagents may remain in the raw materials and need to be cleaned and dried after treatment, which not only increases the production cost, but it may also cause pollution to the environment. This limits the use of chemical methods in feed processes.
3.3. Biological Methods
3.3.1. Enzymatic Method
The enzymatic method utilises enzyme preparations such as protease, NSPs enzyme, phytase, and tannase to degrade ANFs and thus reduce their content. Since the implementation of China’s policy of banning antibiotics in feed in 2020, enzyme preparation has been more and more widely used in the feed industry, which not only serves to eliminate ANFs in feed ingredients but also solves the problem of insufficient endogenous enzymes in young animals, promotes the secretion of endogenous enzymes, and improves the utilisation rate of proteins. Phytase is a critical feed additive for monogastric animals, as their gastrointestinal tracts naturally lack this enzyme. To enhance phytate hydrolysis in diets, exogenous phytase must be supplemented—primarily derived from fungal and bacterial strains [72]. Up to dozens more enzymes are known to catalyse PA cleavage, including phytase secreted by grains, intestinal mucosa, and gastrointestinal microbial communities and a large number of commercial preparations of phytase with modified properties, with the role of each phytase type and its maximum activity depending on the pH of the enzymatic environment [73]. On this basis, phytase is classified as either acidic or alkaline. Acidic phytase shows broad substrate specificity for metal-free phytates, while alkaline phytase is specific for metal-bound PA. Phytase promotes balanced animal mineral nutrition and significantly reduces phosphorus excretion from animal faeces with the addition of appropriate amounts of phytase to feeds, thus mitigating the ecological problem of phosphorus pollution [74].
3.3.2. Microbial Degradation
Microbial fermented feed is a type of feed produced by the metabolism of microorganisms, which has the characteristics and effects of reducing the content of ANFs in raw materials, producing beneficial metabolites, and improving the digestion and absorption of feed nutrients by animals [75,76]. Fermentation is a dynamic process that converts complex substrates into simple compounds, and its products are mainly influenced by the microbial species, fermentation substrate characteristics, and fermentation parameters [77]. Probiotic species commonly used in fermented feeds include yeasts, bacilli, lactobacilli, and moulds [78]. Zheng et al. fermented SBM using Bacillus sphaericus and found that Bacillus sphaericus was able to degrade ANFs as well as change the microstructure of fermented SBM proteins to improve the nutritional quality of fermented SBM [79]. The fermentation of whole feeds is a more convenient way compared to the fermentation of single protein feed ingredients, but this type of fermentation results in a higher loss of amino acids such as lysine [80]. The fermentation process is catalysed by enzymes, and temperature is an important factor to ensure enzyme activity. Appropriate fermentation temperatures can help shorten the reaction stabilisation time, and Dujardin et al. showed that increasing the temperature of liquid fermented feeds from 15 °C to 30 °C reduced the time taken for the pH of the feeds to drop to 4.0 by six-fold [81]. As understood from the perspective of enzyme kinetics, increasing temperature accelerates microbial growth and metabolism [82]. In conclusion, microbial fermentation is a complex and dynamic fermentation process, and the fermentation end products are regulated by the interactions of its influencing factors. In order to obtain the desired fermentation products, the precise optimisation of fermentation conditions is imperative. The process of bacterial growth can decompose some of the ANFs, which significantly improves the efficiency of feed utilisation [83]. Protozoa and bacteria in the rumen of ruminants will compete in the decomposition of starch granules, slowing down the process of rumen fermentation and thus reducing problems such as acidosis [84].
The degradation of ANFs by microbial fermentation seems to be a promising and environmentally friendly method compared to physicochemical methods. The key to the degradation of ANFs by microbial degradation is the selection of bacterial strains. Many strains of bacteria (including Aspergillus, yeasts, and Bacillus) have been used in the microbial fermentation of plant protein feeds, since the fermentation process can secrete a variety of enzymes, including proteases, cellulases, and growth factors [85]. Improvement varies between strains, with some strains only improving roughage nutrient content and positively affecting the reduction in ANFs. Some strains affect only positively the improvement of enzyme activity in the gut, while other microorganisms can improve both [86]. For example, Bacillus sp. is able to degrade ANFs [79]. The effect of mixed-strain fermentation is generally better than that of single-strain fermentation, so mixed-strain fermentation is mostly used in practical production applications [75]. The microbial fermentation of plant protein feeds for ruminant production has also made progress with the widespread use of fermented feed technology. Wang et al. found that fermented SBM improved the rumen microecology of lactating dairy cows by increasing the number of copies of filamentous Bacillus succinicus, Leptospira ruminantium, and Prevotella [87]. Kim et al. reported that fermented SBM also reduced diarrhoea in calves [88]. Microbial fermentation not only improves the nutritional value and utilisation of feeds, but also improves the intestinal microbiota of animals, which plays a growth-promoting role [89].
Microbial fermentation can also destroy ANFs in miscellaneous meal. Khalaf et al. found that microbial fermentation significantly reduced FG in CSM by studying the effect of microorganisms screened during the solid-state fermentation of CSM. However, there were differences between different strains of bacteria, among which the optimal conditions for the reduction in FG content by Pseudohyphae tropicalis yeast fermentation were incubation for 48 h, incubation temperature of 30 °C, 55% moisture content of solid substrate, and pH value around 5.2. Under these optimised conditions, the crude protein and amino acid content of fermentation substrate was significantly increased [90]. Olukomaiya et al. used Aspergillus soya and Aspergillus figlii in a complex to ferment RSM and reduced the PA content from 27.06 mg/g to 22.13 mg/g [91]. Bacillus subtilis was able to produce extracellular enzymes, thereby promoting the degradation of acid detergent fibres (ADF) [86]. Lactobacilli were able to reduce the activity of trypsin inhibiting factor and reduce the content of ANFs in feeds [92]. After microbial fermentation, the content of ANFs decreased significantly [93,94]. Studies have shown that fermentation can remove 85% of ANFs and toxic substances from CSM and RSM [95]. However, the mechanism of the microbial fermentation of feeds for the removal of ANFs and the degradation of harmful substances in raw materials is not fully understood, and it may be related to microbial metabolism. Mi et al. showed that the addition of 19.04% fermented RSM to the diet of black goats significantly increased the abundance of propionate, butyrate, and volatile fatty acids (VFA) in the rumen, and it was also able to increase the number of beneficial bacteria in black goat rumen microbiota [96].
It is worth noting that microbial fermentation can not only reduce ANFs in miscellaneous meals, but it can also reduce costs and increase efficiency [97]. In addition, the process of feed fermentation also produces aromatic substances that enhance the flavour of feed ingredients to promote animal feeding, so it is more advantageous to use microbial degradation to treat ANFs. However, it is necessary to assess the safety of certain metabolites produced during fermentation to prevent them from posing any toxicity risks. The application of microbial degradation on different ANFs is shown in Table 2.

Table 2.
Application of microbial degradation to different ANFs.
4. Prospects
In the future, the chemical structure, biological activity, and mechanism of action of ANFs in feeds need to be deeply investigated to explore more efficient inactivation strategies. The currently available elimination methods have their obvious advantages and disadvantages. High-temperature treatments are particularly effective for unstable ANFs such as antigenic proteins and protease inhibitors, and dehulling also facilitates the removal of ANFs, such as tannins, which are mainly found in seed shells. While chemical methods have limited their use due to their toxicity and possible environmental stress, enzymes are more effective in removing stable ANFs and are now widely used in the feed industry. Microbial degradation is more effective than enzymatic degradation, but this depends on the type of microorganism, the amount added, and the fermentation conditions. In addition, certain emerging technologies such as gamma ray and irradiation have multiple benefits, such as the elimination of microbial and fungal contamination of SBM and miscellaneous meal while retaining protein and amino acid integrity, reduced energy consumption to reduce chemical reactions, etc. These should be further investigated in more detail in the future. At present, the specific mechanism of action of strains in the fermentation process has not been fully studied, and the effect of their application in production varies. Therefore, further research on the specific mechanism of action of different strains in the fermentation process, as well as the interaction effects between different strains, will help develop microbial fermented feeds that are more meaningful for ruminant nutrition. Nevertheless, the mechanism of microbial fermentation feed to reduce ANFs and toxic and harmful substances has not been clarified yet. Moreover, the preservation problem of strains used for fermentation has not been effectively solved, the ratio of fermentation of mixed bacteria needs to be studied in depth, and the speed of fermentation is difficult to be controlled. At the same time, the secondary fermentation of feeds will also bring about a certain amount of economic losses [106]. In the future, during the development and utilisation of microbial fermented feed, it is necessary to establish a special microbial fermented feed research database, improve the quality evaluation system, thoroughly study the action mechanism and advantages of microbial fermented feed, and promote the sustainable development of microbial fermented feed. Microbial fermented plant protein feeds have significantly improved the production performance of ruminants, but there is no significant improvement in the quality of ruminant products. Therefore, it will be of great significance to carry out the research work related to bacterial strain research, animal immunity, intestinal flora, nutrient metabolism, etc., in order to gain a deeper understanding of the mechanism of action of microbial fermented plant protein feeds, and to develop different strains of bacterial strains, different roughage, and different animal species fermented feeds according to the local conditions. Various types of miscellaneous meal due to the price advantage in the proportion of feed gradually increased. In order to solve the issue of the accumulation of gossypol, glucosinolates, tannins, and other ANFs, as well as other problems, biological fermentation and bacteria and enzyme synergistic treatment have been widely introduced into the production process, using various types of enzyme specificity and the high efficiency of ANFs for decomposition and transformation [2]. Biological methods have obvious advantages, as they do not need to use difficult to separate solvents, can significantly reduce costs, and can rely on the physiological activities of the bacterial colony to decompose various types of ANFs. Moreover, the bacterial colony can increase the protein content of the product after moderate reproduction to improve the composition of the product’s nutrient composition. However, the degradation rate of ANFs in the biological method process based on strain compounding is lower than that of the chemical method, and the synergistic treatment of bacteria and enzymes can improve the decomposition rate. However, there is no cost advantage compared with the chemical method, so the method urgently needs corresponding high enzyme-producing bacterial strains. The main ANFs of plant protein feeds and the common elimination methods are shown in Figure 1.
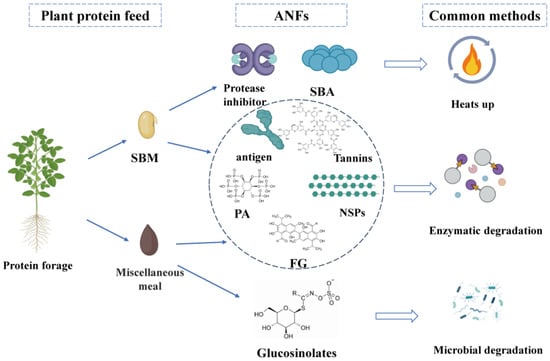
Figure 1.
ANFs in plant protein feeds and common elimination methods (figure created in BioRender, https://biorender.com, accessed on 15 December 2024).
5. Conclusions
The types, anti-nutritional mechanisms and elimination methods of ANFs in major plant protein feeds for ruminants were initially summarised in this review, which provides a reference for antinutritional factor elimination and production of full-price compound feeds for ruminants. Currently, the variable quality of fermentation products from protein feed resources, the unclear association between fermentation effect and microbial strains, and the standardisation of the fermentation process remain the main challenges for the efficient utilisation of protein feed resources for ruminants. With the continuous research on fermentation technology, more effective methods of eliminating ANFs from plant protein feeds will be developed, which is important for alleviating the shortage of high-quality protein resources and enhancing the efficiency of the use of plant protein raw materials for ruminants.
Author Contributions
Conceptualization, Z.Y., Z.L., C.Z. and Z.T.; methodology, Z.Y.; software, Z.Y.; validation, Z.Y., Z.L., C.Z. and Z.T.; formal analysis, Z.Y.; investigation, Z.Y.; resources, Z.Y.; data curation, Z.L.; writing—original draft preparation, Z.Y.; writing—review and editing, Z.Y.; visualization, Z.Y.; supervision, C.Z.; project administration, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the Projects of International Cooperation and Exchanges NSFC (32261143467), the Projects of Hunan Province Science and Technology Innovation Program (2023WK2003), National Key Research and Development Program (2022YFD1300805), and the Opening Fund of the Key Laboratory of South Yuelu Mountain Biological Resources Protection and Utilization (2022HSKFJJ02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANFs | Anti-Nutritional Factors |
| SBM | Soybean Meal |
| RSM | Rapeseed Meal |
| CSM | Cottonseed Meal |
| TIs | Trypsin Inhibitors |
| BBI | Bowman-Birk Inhibitor |
| KTI | Kunitz Inhibitor |
| SBA | Soybean Agglutinin |
| HTs | Hydrolysed Tannins |
| CTs | Condensed Tannins |
| PA | Phytic Acid |
| NSPs | Non-Starch Polysaccharides |
| FG | Free Gossypol |
| OZT | Oxazolidinethione |
| ITC | Isothiocyanate |
| EB | Electron Beam |
| GR | Gamma Ray |
| ADF | Acid Detergent Fibres |
References
- Kim, T.-I.; Mayakrishnan, V.; Lim, D.-H.; Yeon, J.-H.; Baek, K.-S. Effect of Fermented Total Mixed Rations on the Growth Performance, Carcass and Meat Quality Characteristics of Hanwoo Steers. Anim. Sci. J. 2018, 89, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Górka, P.; Penner, G.B. Rapeseed and Canola Meal as Protein Sources in Starter Diets for Calves: Current Knowledge and Directions of Future Studies. Ank. Üniversitesi Vet. Fakültesi Derg. 2020, 67, 313–321. [Google Scholar] [CrossRef]
- Di, D.; He, S.; Zhang, R.; Gao, K.; Qiu, M.; Li, X.; Sun, H.; Xue, S.; Shi, J. Exploring the Dual Role of Anti-Nutritional Factors in Soybeans: A Comprehensive Analysis of Health Risks and Benefits. Crit. Rev. Food Sci. Nutr. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A.; Alumot, E. Anti-Nutritive Factors in Animal Feedstuffs and Their Effects on Livestock. Prog. Food Nutr. Sci. 1987, 11, 115–151. [Google Scholar]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Enhancing the Quality of Total Mixed Ration Containing Cottonseed or Rapeseed Meal by Optimization of Fermentation Conditions. Fermentation 2021, 7, 234. [Google Scholar] [CrossRef]
- Santosh, S.; Raghavendra, K.P.; Velmourougane, K.; Mageshwaran, V.; Blaise, D.; Waghmare, V.N. Microbial Detoxification of Gossypol in Cotton Seed Meal by Solid Substrate Fermentation. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1654–1663. [Google Scholar] [CrossRef]
- Heendeniya, R.G.; Christensen, D.A.; Maenz, D.D.; McKinnon, J.J.; Yu, P. Protein Fractionation Byproduct from Canola Meal for Dairy Cattle. J. Dairy Sci. 2012, 95, 4488–4500. [Google Scholar] [CrossRef]
- Enneking, D.; Wink, M. Towards the Elimination of Anti-Nutritional Factors in Grain Legumes. In Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference; Knight, R., Ed.; Springer: Dordrecht, The Netherlands, 2000; pp. 671–683. ISBN 978-94-011-4385-1. [Google Scholar]
- Tao, A.; Wang, J.; Luo, B.; Liu, B.; Wang, Z.; Chen, X.; Zou, T.; Chen, J.; You, J. Research Progress on Cottonseed Meal as a Protein Source in Pig Nutrition: An Updated Review. Anim. Nutr. 2024, 18, 220–233. [Google Scholar] [CrossRef]
- Yin, X.; Chen, M.; Yang, C.; Duan, C.; Ji, S.; Yan, H.; Liu, Y.; Zhang, Y. Effects of Replacing Soybean Meal with Cottonseed Meal, Peanut Meal, Rapeseed Meal, or Distillers’ Dried Grains with Solubles on the Growth Performance, Nutrient Digestibility, Serum Parameters, and Rumen Fermentation in Growing Lambs. Vet. Sci. 2024, 11, 322. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhang, D.; Ding, H.; Feng, S.; Zhao, C.; Wu, J.; Wang, X. Soybean Antigen Protein-Induced Intestinal Barrier Damage by Trigging Endoplasmic Reticulum Stress and Disordering Gut Microbiota in Weaned Piglets. Molecules 2023, 28, 6500. [Google Scholar] [CrossRef]
- Ogawa, T.; Bando, N.; Tsuji, H.; Nishikawa, K.; Kitamura, K. α-Subunit of β-Conglycinin, an Allergenic Protein Recognized by IgE Antibodies of Soybean-Sensitive Patients with Atopic Dermatitis. Biosci. Biotechnol. Biochem. 1995, 59, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Qin, G.; Tian, H.; Zhang, F. Three-Dimensional Structure of Gly m 5 (β-Conglycinin) Plays an Important Role in Its Stability and Overall Allergenicity. Food Chem. 2017, 234, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Arsov, A.; Tsigoriyna, L.; Batovska, D.; Armenova, N.; Mu, W.; Zhang, W.; Petrov, K.; Petrova, P. Bacterial Degradation of Antinutrients in Foods: The Genomic Insight. Foods 2024, 13, 2408. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ye, X.; Ng, T. Purification of Melibiose-Binding Lectins from Two Cultivars of Chinese Black Soybeans. Acta Biochim. Biophys. Sin. 2008, 40, 1029–1038. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and Practical Applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef]
- Tianjiao, E.; Xu, C.; Fan, X.; Liu, J.; Zhao, J.; Bao, N.; Zhao, Y.; Farouk, M.H.; Ji, Y.; Wu, Z.; et al. Soybean Agglutinin Induced Apoptotic Effects by Down-Regulating ANXA2 Through FAK Pathway in IPEC-J2 Cells. J. Anim. Physiol. Anim. Nutr. 2024, 109, 350–361. [Google Scholar] [CrossRef]
- Lalles, J.P.; Tukur, H.M.; Toullec, R.; Miller, B.G. Analytical Criteria for Predicting Apparent Digestibility of Soybean Protein in Preruminant Calves. J. Dairy Sci. 1996, 79, 475–482. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Biagia, G.; Cipollini, I.; Paulicks, B.R.; Roth, F.X. Effect of Tannins on Growth Performance and Intestinal Ecosystem in Weaned Piglets. Arch. Anim. Nutr. 2010, 64, 121–135. [Google Scholar] [CrossRef]
- Khajali, F.; Slominski, B.A. Factors That Affect the Nutritive Value of Canola Meal for Poultry. Poult. Sci. 2012, 91, 2564–2575. [Google Scholar] [CrossRef]
- Sun, X.; Tiffany, D.G.; Urriola, P.E.; Shurson, G.G.; Hu, B. Nutrition Upgrading of Corn-Ethanol Co-Product by Fungal Fermentation: Amino Acids Enrichment and Anti-Nutritional Factors Degradation. Food Bioprod. Process. 2021, 130, 1–13. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Fuerst, E.P.; Morris, C.F. Wheat Arabinoxylan Structure Provides Insight into Function. Cereal Chem. 2013, 90, 387–395. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Henry, C.J. Reducing the Glycemic Impact of Carbohydrates on Foods and Meals: Strategies for the Food Industry and Consumers with Special Focus on Asia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 670–702. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.M.; McConnell, B.R.; Drexler, R.A.; Ng, K.M.; Maldonado-Gomez, M.X.; Conner, A.M.S.; Vierra, C.G.; Krishnakumar, N.; Gerber, H.M.; Garcia, J.K.A.; et al. Highly Soluble β-Glucan Fiber Modulates Mechanisms of Blood Glucose Regulation and Intestinal Permeability. Nutrients 2024, 16, 2240. [Google Scholar] [CrossRef]
- Recharla, N.; Kim, D.; Ramani, S.; Song, M.; Park, J.; Balasubramanian, B.; Puligundla, P.; Park, S. Dietary Multi-Enzyme Complex Improves In Vitro Nutrient Digestibility and Hind Gut Microbial Fermentation of Pigs. PLoS ONE 2019, 14, e0217459. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Baioni, L.; Grasselli, F. Gossypol, a Polyphenolic Aldehyde from Cotton Plant, Interferes with Swine Granulosa Cell Function. Domest. Anim. Endocrinol. 2009, 37, 30–36. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhao, L.; Zhang, Y. Structure, Properties of Gossypol and Its Derivatives—From Physiological Activities to Drug Discovery and Drug Design. Nat. Prod. Rep. 2022, 39, 1282–1304. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Y.; Xie, W.; Zhou, D.; Tang, S.; Kuang, M.; Wang, Y.; Du, S. Physicochemical and Functional Properties of Protein Isolate Obtained from Cottonseed Meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef]
- Noftsger, S.M.; Hopkins, B.A.; Diaz, D.E.; Brownie, C.; Whitlow, L.W. Effect of Whole and Expanded-Expelled Cottonseed on Milk Yield and Blood Gossypol. J. Dairy Sci. 2000, 83, 2539–2547. [Google Scholar] [CrossRef]
- Paim, T.; Viana, P.; Brandão, E.; Amador, S.; Barbosa, T.; Cardoso, C.; Lucci, C.; DeSouza, J.; Mcmanus, C.; Abdalla, A.; et al. Impact of Feeding Cottonseed Coproducts on Reproductive System of Male Sheep during Peripubertal Period. Sci. Agric. 2016, 73, 489–497. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.; Teng, P.-Y.; Choi, J.; Singh, A.K.; Vaddu, S.; Thippareddi, H.; Kim, W.K. Influence of Rapeseed, Canola Meal and Glucosinolate Metabolite (AITC) as Potential Antimicrobials: Effects on Growth Performance, and Gut Health in Salmonella Typhimurium Challenged Broiler Chickens. Poult. Sci. 2022, 101, 101551. [Google Scholar] [CrossRef] [PubMed]
- Wlazło, Ł.; Kowalska, D.; Bielański, P.; Chmielowiec-Korzeniowska, A.; Ossowski, M.; Łukaszewicz, M.; Czech, A.; Nowakowicz-Dębek, B. Effect of Fermented Rapeseed Meal on the Gastrointestinal Microbiota and Immune Status of Rabbit (Oryctolagus cuniculus). Animals 2021, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, I.H.; Woyengo, T.A. Toxicity of Canola-Derived Glucosinolate Degradation Products in Pigs—A Review. Animals 2020, 10, 2337. [Google Scholar] [CrossRef]
- Hamadi, S.; Salari, S.; Aghaei, A.; Ghorbani, M.R. Changes in Performance and Apparent Ileal Digestibility of Broiler Chickens Fed Diets Containing Electron-Irradiated Full-Fat Canola Seed. Radiat. Phys. Chem. 2023, 210, 111046. [Google Scholar] [CrossRef]
- Hajiazizi, F.; Sadeghi, A.; Ibrahim, S. Camelina sativa (L. Crantz) Products; an Alternative Feed Ingredient for Poultry Diets with Its Nutritional and Physiological Consequences. Trop. Anim. Health Prod. 2024, 56, 59. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A Fermented Rapeseed Meal Additive: Effects on Production Performance, Nutrient Digestibility, Colostrum Immunoglobulin Content and Microbial Flora in Sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef]
- Kelly, D.; O’Brien, J.J.; McCracken, K.J. Effect of Creep Feeding on the Incidence, Duration and Severity of Post-Weaning Diarrhoea in Pigs. Res. Vet. Sci. 1990, 49, 223–228. [Google Scholar] [CrossRef]
- Hampson, D.J.; Smith, W.C. Influence of Creep Feeding and Dietary Intake after Weaning on Malabsorption and Occurrence of Diarrhoea in the Newly Weaned Pig. Res. Vet. Sci. 1986, 41, 63–69. [Google Scholar] [CrossRef]
- Peng, C.; Cao, C.; He, M.; Shu, Y.; Tang, X.; Wang, Y.; Zhang, Y.; Xia, X.; Li, Y.; Wu, J. Soybean Glycinin- and β-Conglycinin-Induced Intestinal Damage in Piglets via the P38/JNK/NF-κB Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9534–9541. [Google Scholar] [CrossRef]
- Deng, H.; Ye, T.; Deng, Y.; Cui, Y.; Guo, H.; Deng, J. miRNA Expression Analysis of IPEC-J2 Cells Damaged by Soybean 7S Globulin Reveals Ssc-miR-221-5p as the Factor Alleviating Cell Damage. J. Agric. Food Chem. 2024, 72, 11694–11705. [Google Scholar] [CrossRef] [PubMed]
- Gillman, J.D.; Kim, W.-S.; Krishnan, H.B. Identification of a New Soybean Kunitz Trypsin Inhibitor Mutation and Its Effect on Bowman-Birk Protease Inhibitor Content in Soybean Seed. J. Agric. Food Chem. 2015, 63, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Moreau, T.; Recoules, E.; De Pauw, M.; Labas, V.; Réhault-Godbert, S. Evidence That the Bowman-Birk Inhibitor from Pisum Sativum Affects Intestinal Proteolytic Activities in Chickens. Poult. Sci. 2024, 103, 103182. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Rahman, M.M.; Amin, R.; Karim, M.R.; Mahmud, Z.H.; Hossain, M.T. Solanum Tuberosum Lectin Inhibits Ehrlich Ascites Carcinoma Cells Growth by Inducing Apoptosis and G2/M Cell Cycle Arrest. Tumour Biol. 2016, 37, 8437–8444. [Google Scholar] [CrossRef]
- Qiao, W.-L.; Hu, H.-Y.; Shi, B.-W.; Zang, L.-J.; Jin, W.; Lin, Q. Lentivirus-Mediated Knockdown of TSP50 Suppresses the Growth of Non-Small Cell Lung Cancer Cells via G0/G1 Phase Arrest. Oncol. Rep. 2016, 35, 3409–3418. [Google Scholar] [CrossRef][Green Version]
- Xu, H.; Fu, J.; Luo, Y.; Li, P.; Song, B.; Lv, Z.; Guo, Y. Effects of Tannic Acid on the Immunity and Intestinal Health of Broiler Chickens with Necrotic Enteritis Infection. J. Anim. Sci. Biotechnol. 2023, 14, 72. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Bee, G.; Silacci, P.; Ampuero-Kragten, S.; Čandek-Potokar, M.; Wealleans, A.L.; Litten-Brown, J.; Salminen, J.-P.; Mueller-Harvey, I. Hydrolysable Tannin-Based Diet Rich in Gallotannins Has a Minimal Impact on Pig Performance but Significantly Reduces Salivary and Bulbourethral Gland Size. Animal 2017, 11, 1617–1625. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Hao, Z.; Guo, X.; Zhu, B. Anaerobic Solid-State Fermentation with Bacillus Subtilis for Digesting Free Gossypol and Improving Nutritional Quality in Cottonseed Meal. Front. Nutr. 2022, 9, 1017637. [Google Scholar] [CrossRef]
- Rathore, K.; Pandeya, D.; Campbell, L.; Wedegaertner, T.; Puckhaber, L.; Stipanovic, R.; Thenell, S.; Hague, S.; Hake, K. Ultra-Low Gossypol Cottonseed: Selective Gene Silencing Opens Up a Vast Resource of Plant-Based Protein to Improve Human Nutrition. Crit. Rev. Plant Sci. 2020, 39, 2539–2547. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan Structural Characteristics, Interaction with Gut Microbiota and Potential Health Functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Ravindran, V.; Cabahug, S.; Ravindra, G.; Selle, P.H.; Bryden, W.L. Response of Broiler Chickens to Microbial Phytase Supplementation as Influenced by Dietary Phytic Acid and Non-Phytate Phosphorous Levels. II. Effects on Apparent Metabolisable Energy, Nutrient Digestibility and Nutrient Retention. Br. Poult. Sci. 2000, 41, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, O.O.; Bello, A.; Dersjant-Li, Y.; Adeola, O. Evaluation of the Responses of Broiler Chickens to Varying Concentrations of Phytate Phosphorus and Phytase. Ⅱ. Grower Phase (Day 12–23 Post Hatching). Poult. Sci. 2022, 101, 101616. [Google Scholar] [CrossRef] [PubMed]
- Darambazar, E.; Damiran, D.; Beaulieu, D. Effect of Hydrothermal, Phytase, or Organic Acid Pretreatments of Canola Meal on Phytate Level of the Meal. Sustain. Agric. Res. 2019, 8, 35. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant Food Anti-Nutritional Factors and Their Reduction Strategies: An Overview. Food Prod. Process Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Xie, C.; Li, W.; Gao, R.; Yan, L.; Wang, P.; Gu, Z.; Yang, R. Determination of Glucosinolates in Rapeseed Meal and Their Degradation by Myrosinase from Rapeseed Sprouts. Food Chem. 2022, 382, 132316. [Google Scholar] [CrossRef]
- Korompokis, K.; De Brier, N.; Delcour, J.A. Differences in Endosperm Cell Wall Integrity in Wheat (Triticum aestivum L.) Milling Fractions Impact on the Way Starch Responds to Gelatinization and Pasting Treatments and Its Subsequent Enzymatic in Vitro Digestibility. Food Funct. 2019, 10, 4674–4684. [Google Scholar] [CrossRef]
- Andrade, J.C.; Mandarino, J.M.G.; Kurozawa, L.E.; Ida, E.I. The Effect of Thermal Treatment of Whole Soybean Flour on the Conversion of Isoflavones and Inactivation of Trypsin Inhibitors. Food Chem. 2016, 194, 1095–1101. [Google Scholar] [CrossRef]
- Jensen, S.K.; Liu, Y.-G.; Eggum, B.O. The Effect of Heat Treatment on Glucosinolates and Nutritional Value of Rapeseed Meal in Rats. Anim. Feed Sci. Technol. 1995, 53, 17–28. [Google Scholar] [CrossRef]
- Duodu, C.P.; Adjei-Boateng, D.; Edziyie, R.E.; Agbo, N.W.; Owusu-Boateng, G.; Larsen, B.K.; Skov, P.V. Processing Techniques of Selected Oilseed By-Products of Potential Use in Animal Feed: Effects on Proximate Nutrient Composition, Amino Acid Profile and Antinutrients. Anim. Nutr. 2018, 4, 442–451. [Google Scholar] [CrossRef]
- Acosta, J.A.; Petry, A.L.; Gould, S.A.; Jones, C.K.; Stark, C.R.; Fahrenholz, A.; Patience, J.F. Effects of Grinding Method and Particle Size of Wheat Grain on Energy and Nutrient Digestibility in Growing and Finishing Pigs. Transl. Anim. Sci. 2020, 4, txaa062. [Google Scholar] [CrossRef] [PubMed]
- Bornaei, L.; Salari, S.; Erfani Majd, N. Effect of Electron Beam Irradiated Barley Grains on Growth Performance, Blood Parameters, Nutrient Digestibility, Microbial Population, and Intestinal Histomorphometry in Broiler Chickens. J. Appl. Anim. Res. 2022, 50, 408–419. [Google Scholar] [CrossRef]
- Bahraini, Z.; Salari, S.; Sari, M.; Fayazi, J.; Behgar, M. Effect of Radiation on Chemical Composition and Protein Quality of Cottonseed Meal. Anim. Sci. J. 2017, 88, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Soetan, K.O.; Oyewole, O.E. The Need for Adequate Processing to Reduce the Anti-Nutritional Factors in Plants Used as Human Foods and Animal Feeds: A Review. Afr. J. Food Sci. 2009, 3, 223–232. [Google Scholar]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Oey, I.; Roohinejad, S. Effect of Extrusion on the Anti-Nutritional Factors of Food Products: An Overview. Food Control 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Alonso, R.; Aguirre, A.; Marzo, F. Effects of Extrusion and Traditional Processing Methods on Antinutrients and in Vitro Digestibility of Protein and Starch in Faba and Kidney Beans. Food Chem. 2000, 68, 159–165. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Lee, S.A.; Jones, C.K.; Htoo, J.K.; Stein, H.H. Digestibility of Amino Acids, Fiber, and Energy by Growing Pigs, and Concentrations of Digestible and Metabolizable Energy in Yellow Dent Corn, Hard Red Winter Wheat, and Sorghum May Be Influenced by Extrusion. Anim. Feed Sci. Technol. 2020, 268, 114602. [Google Scholar] [CrossRef]
- Barraza, M.L.; Coppock, C.E.; Brooks, K.N.; Wilks, D.L.; Saunders, R.G.; Latimer, G.W. Iron Sulfate and Feed Pelleting to Detoxify Free Gossypol in Cottonseed Diets for Dairy Cattle1. J. Dairy Sci. 1991, 74, 3457–3467. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Singhal, R.S.; Tiwari, S.R. Supercritical Carbon Dioxide Extraction of Cottonseed Oil. J. Food Eng. 2007, 79, 892–898. [Google Scholar] [CrossRef]
- Pelitire, S.M.; Dowd, M.K.; Cheng, H.N. Acidic Solvent Extraction of Gossypol from Cottonseed Meal. Anim. Feed Sci. Technol. 2014, 195, 120–128. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in Non-Ruminant Animal Nutrition: A Critical Review on Phytase Activities in the Gastrointestinal Tract and Influencing Factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, V.S.; Glebova, I.V.; Zinoviev, S.V. Reevaluation of Phytase Action Mechanism in Animal Nutrition. Biochemistry 2021, 86, S152–S165. [Google Scholar] [CrossRef]
- Jorquera, M.; Martínez, O.; Maruyama, F.; Marschner, P.; de la Luz Mora, M. Current and Future Biotechnological Applications of Bacterial Phytases and Phytase-Producing Bacteria. Microbes Environ. 2008, 23, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-State Fermented Plant Protein Sources in the Diets of Broiler Chickens: A Review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Missotten, J.A.; Michiels, J.; Degroote, J.; De Smet, S. Fermented Liquid Feed for Pigs: An Ancient Technique for the Future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented Liquid Feed—Microbial and Nutritional Aspects and Impact on Enteric Diseases in Pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in Research on Solid-State Fermented Feed and Its Utilization: The Pioneer of Private Customization for Intestinal Microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Li, Z.-L.; Kang, L.-N.; Jiang, Y.-Y.; Liu, X.-Y.; Chi, Y.-P.; Li, Y.-Q.; Wang, J.-H. Effects of Bacillus Fermentation on the Protein Microstructure and Anti-Nutritional Factors of Soybean Meal. Lett. Appl. Microbiol. 2017, 65, 520–526. [Google Scholar] [CrossRef]
- O’Meara, F.M.; Gardiner, G.E.; O’Doherty, J.V.; Clarke, D.; Cummins, W.; Lawlor, P.G. Effect of Wet/Dry, Fresh Liquid, Fermented Whole Diet Liquid, and Fermented Cereal Liquid Feeding on Feed Microbial Quality and Growth in Grow-Finisher Pigs. J. Anim. Sci. 2020, 98, skaa166. [Google Scholar] [CrossRef]
- Dujardin, M.; Elain, A.; Lendormi, T.; Le Fellic, M.; Le Treut, Y.; Sire, O. Keeping under Control a Liquid Feed Fermentation Process for Pigs: A Reality Scale Pilot Based Study. Anim. Feed Sci. Technol. 2014, 194, 81–88. [Google Scholar] [CrossRef]
- Pandey, A. Solid-State Fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing Global Ruminant Agricultural Challenges Through Understanding the Rumen Microbiome: Past, Present, and Future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The Potential of Fermentation on Nutritional and Technological Improvement of Cereal and Legume Flours: A Review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Chi, C.-H.; Cho, S.-J. Improvement of Bioactivity of Soybean Meal by Solid-State Fermentation with Bacillus Amyloliquefaciens versus Lactobacillus Spp. and Saccharomyces Cerevisiae. LWT Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Li, X.; Xiao, H.; Zhang, P.; Shen, W.; Wan, F.; He, J.; Tang, S.; Tan, Z.; et al. Fermented Soybean Meal Replacement in the Diet of Lactating Holstein Dairy Cows: Modulated Rumen Fermentation and Ruminal Microflora. Front. Microbiol. 2021, 12, 625857. [Google Scholar] [CrossRef]
- Kim, M.H.; Yun, C.H.; Kim, H.S.; Kim, J.H.; Kang, S.J.; Lee, C.H.; Ko, J.Y.; Ha, J.K. Effects of Fermented Soybean Meal on Growth Performance, Diarrheal Incidence and Immune-Response of Neonatal Calves. Anim. Sci. J. 2010, 81, 475–481. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Yin, Y.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Amino Acid and Phosphorus Digestibility of Fermented Corn-Soybean Meal Mixed Feed with Bacillus Subtilis and Enterococcus Faecium Fed to Pigs. J. Anim. Sci. 2017, 95, 3996–4004. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Meleigy, S.A. Reduction of Free Gossypol Levels in Cottonseed Meal by Microbial Treatment. Int. J. Agric. Biol. 2008, 10, 1560–8530. [Google Scholar]
- Olukomaiya, O.O.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-State Fermentation of Canola Meal with Aspergillus Sojae, Aspergillus Ficuum and Their Co-Cultures: Effects on Physicochemical, Microbiological and Functional Properties. LWT 2020, 127, 109362. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, Ecology and Industrial Applications of Aroma Formation in Yeast. FEMS Microbiol. Rev. 2017, 41, S95–S128. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Murekatete, N.; Hua, Y.; Kong, X.; Zhang, C. Effects of Fermentation on Nutritional and Functional Properties of Soybean, Maize, and Germinated Sorghum Composite Flour. Int. J. Food Eng. 2012, 8, 1–15. [Google Scholar] [CrossRef]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The Probiotic Bacillus Licheniformis Ameliorates Heat Stress-Induced Impairment of Egg Production, Gut Morphology, and Intestinal Mucosal Immunity in Laying Hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef]
- Mi, H.; Ren, A.; Zhu, J.; Ran, T.; Shen, W.; Zhou, C.; Zhang, B.; Tan, Z. Effects of Different Protein Sources on Nutrient Disappearance, Rumen Fermentation Parameters and Microbiota in Dual-Flow Continuous Culture System. AMB Express 2022, 12, 15. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Liu, L.; Chang, Z.; Peng, N. Effective Gossypol Removal from Cottonseed Meal through Optimized Solid-State Fermentation by Bacillus Coagulans. Microb. Cell Factories 2022, 21, 252. [Google Scholar] [CrossRef]
- Qi, N.; Zhan, X.; Milmine, J.; Sahar, M.; Chang, K.-H.; Li, J. Isolation and Characterization of a Novel Hydrolase-Producing Probiotic Bacillus Licheniformis and Its Application in the Fermentation of Soybean Meal. Front. Nutr. 2023, 10, 1123422. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional Quality Improvement of Soybean Meal by Bacillus Velezensis and Lactobacillus Plantarum during Two-Stage Solid-State Fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef]
- Ranjan, A.; Sahu, N.P.; Deo, A.D.; Kumar, S. Solid State Fermentation of De-Oiled Rice Bran: Effect on in Vitro Protein Digestibility, Fatty Acid Profile and Anti-Nutritional Factors. Food Res. Int. 2019, 119, 1–5. [Google Scholar] [CrossRef]
- Jain, J.; Kumar, A.; Singh, D.; Singh, B. Purification and Kinetics of a Protease-Resistant, Neutral, and Thermostable Phytase from Bacillus Subtilis Subsp. Subtilis JJBS250 Ameliorating Food Nutrition. Prep. Biochem. Biotechnol. 2018, 48, 718–724. [Google Scholar] [CrossRef]
- Ashayerizadeh, A.; Dastar, B.; Shams Shargh, M.; Sadeghi Mahoonak, A.; Zerehdaran, S. Fermented Rapeseed Meal Is Effective in Controlling Salmonella Enterica Serovar Typhimurium Infection and Improving Growth Performance in Broiler Chicks. Vet. Microbiol. 2017, 201, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Puyo, M.; Simonin, S.; Klein, G.; David-Vaizant, V.; Quijada-Morín, N.; Alexandre, H.; Tourdot-Maréchal, R. Use of Oenological Tannins to Protect the Colour of Rosé Wine in a Bioprotection Strategy with Metschnikowia Pulcherrima. Foods 2023, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Refstie, S.; Sahlström, S.; Bråthen, E.; Baeverfjord, G.; Krogedal, P. Lactic Acid Fermentation Eliminates Indigestible Carbohydrates and Antinutritional Factors in Soybean Meal for Atlantic Salmon (Salmo Salar). Aquaculture 2005, 246, 331–345. [Google Scholar] [CrossRef]
- Wang, W.-K.; Li, W.-J.; Wu, Q.-C.; Wang, Y.-L.; Li, S.-L.; Yang, H.-J. Isolation and Identification of a Rumen Lactobacillus Bacteria and Its Degradation Potential of Gossypol in Cottonseed Meal during Solid-State Fermentation. Microorganisms 2021, 9, 2200. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.I.; Ezeokoli, O.T.; Sulyok, M.; van Wyk, D.A.B.; Oyedele, O.A.; Akinyemi, O.M.; Chibuzor-Onyema, I.E.; Adeleke, R.A.; Nwangburuka, C.C.; et al. High-Throughput Sequence Analyses of Bacterial Communities and Multi-Mycotoxin Profiling During Processing of Different Formulations of Kunu, a Traditional Fermented Beverage. Front. Microbiol. 2018, 9, 3282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).