Inclusion of Multi-Strained Probiotics Improves the Fecal Microbiota and Carcass Quality of Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Use
2.2. Animals and Experimental Design
2.3. Fecal Sample Collection
2.4. Carcass Traits and Meat Quality
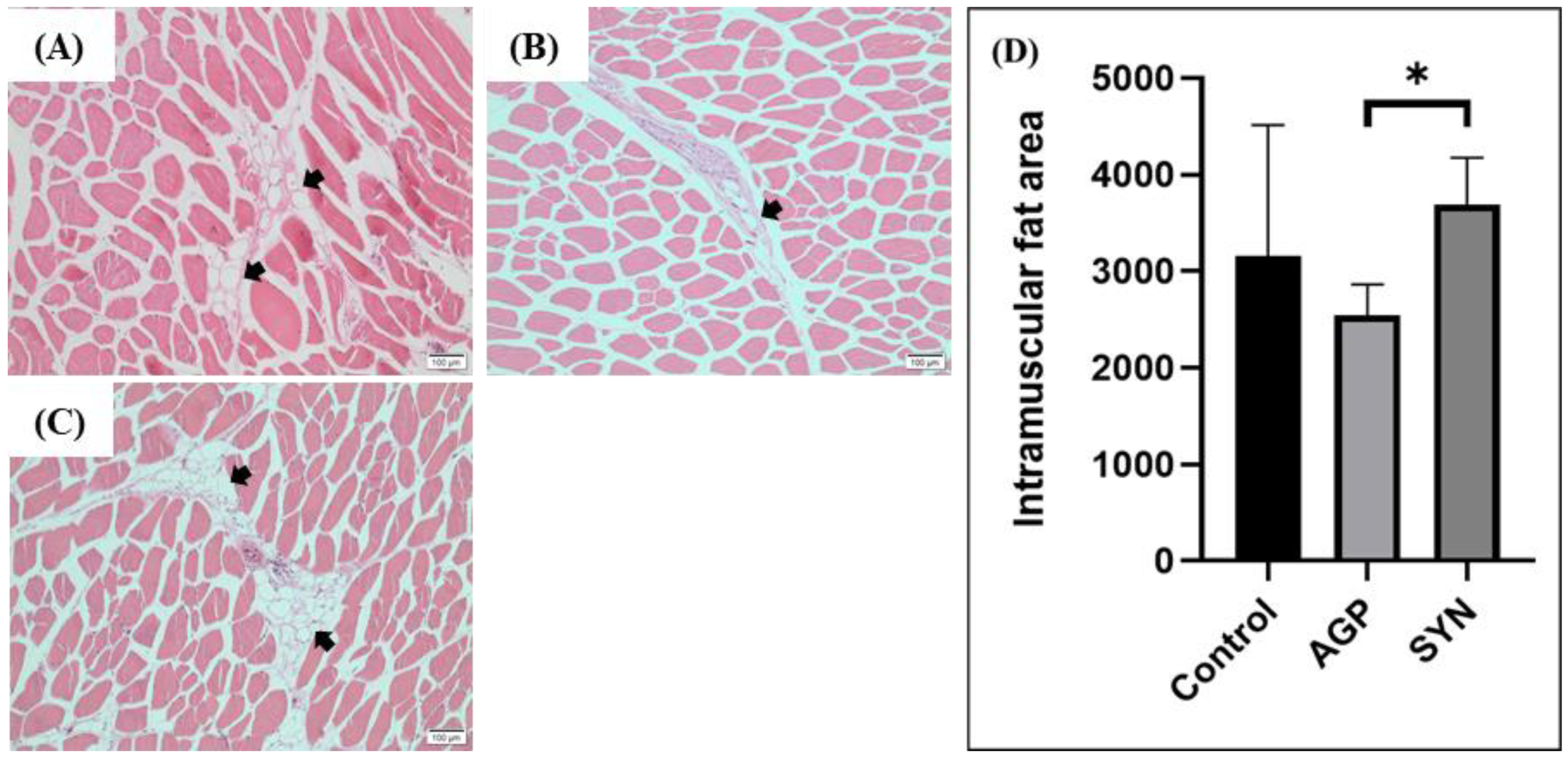
2.5. IMF Distribution in LD Muscle
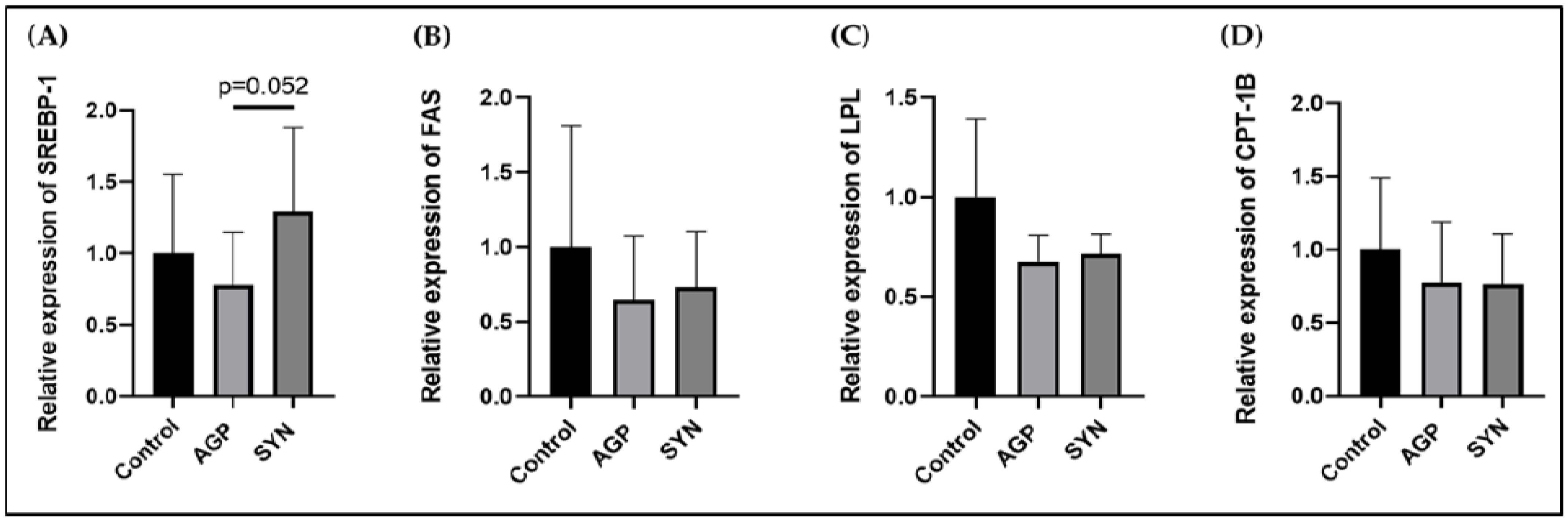
2.6. Expression of Genes Related to Lipid Metabolism in LD Muscle
2.7. Microbial DNA Extraction and 16S rRNA Gene Sequence Analysis
2.8. Statistical Analysis
3. Results
3.1. Carcass Traits and Meat Quality
3.2. IMF Distribution in LD Muscle
3.3. Expression of Genes Related to Lipid Metabolism in LD Muscle
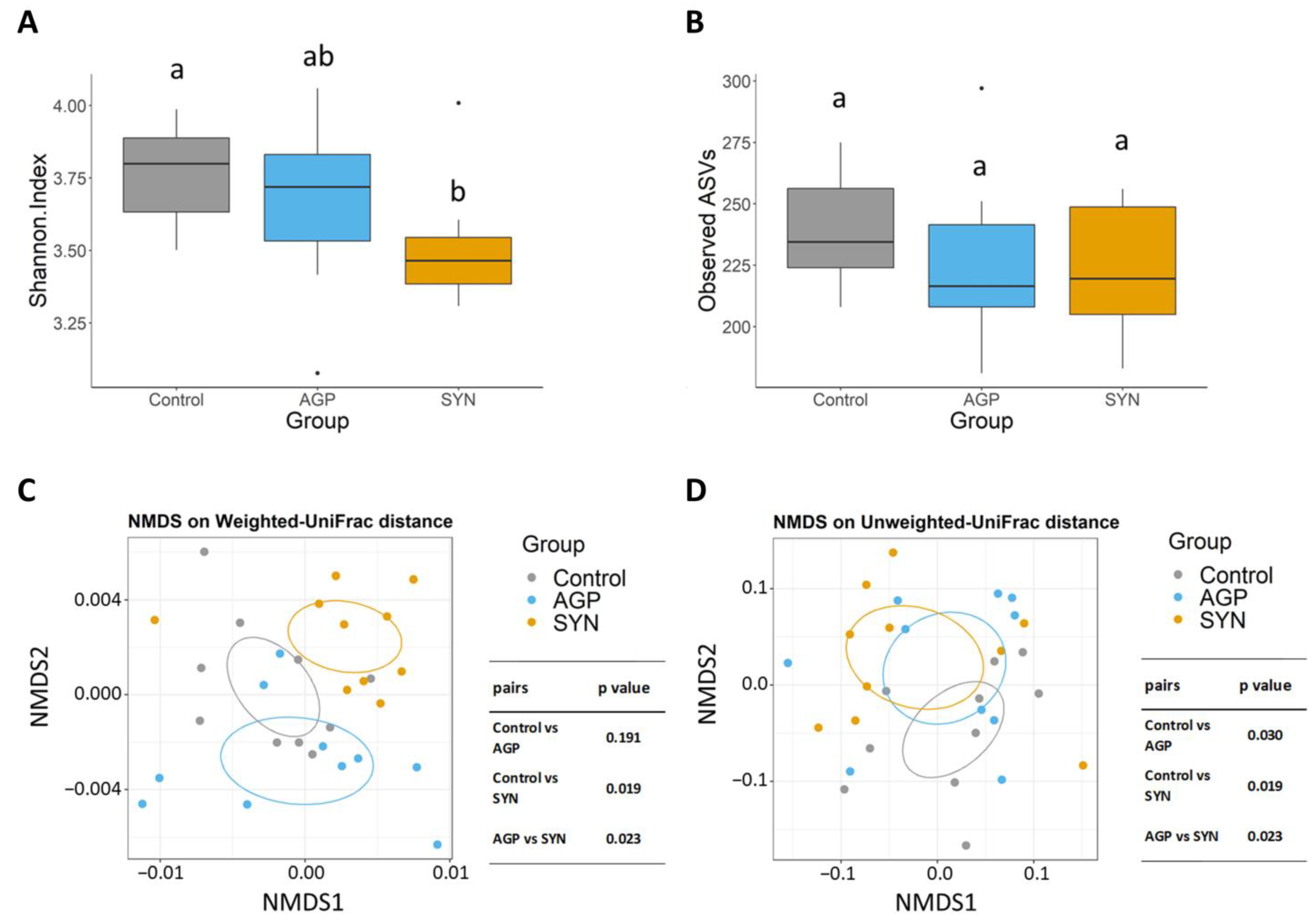
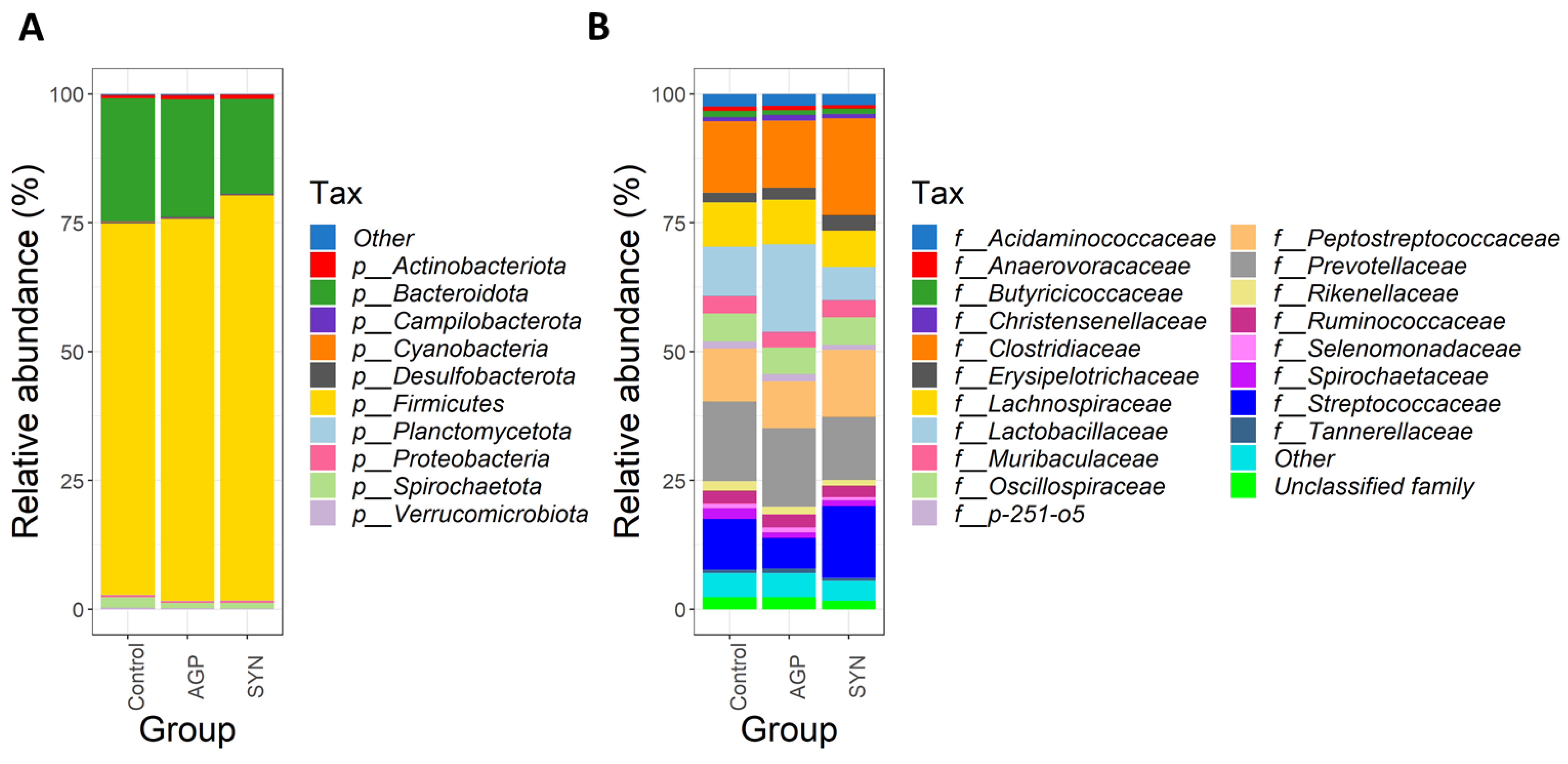
3.4. Composition of Gut Microbiota of Pigs Fed Different Treatments
3.5. Comparison of the Functional Capacity of the Gut Microbiome Under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGP | Antibiotic Growth Promoter |
| BCAA | Branched Chain Amino Acids |
| CPT-1B | Carnitine Palmitoyltransferase 1B |
| FAS | Fatty Acid Synthase |
| FE | Feed Efficiency |
| IACUC | Institutional Animal Care and Use Committee |
| IF | Intramuscular Fat |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LD | Longissimus Dorsi |
| LDA | Linear Discriminant Analysis |
| LEfSe | Linear Discriminant Analysis Effect Size |
| LPL | Lipoprotein Lipase |
| LYD | Landrace × Yorkshire × Duroc |
| NMDS | Non-metric Multi-Dimensional Scaling |
| PCA | Principal Component Analysis |
| SREBP-1 | Sterol Regulatory Element Binding Protein-1 |
References
- Guerra-Ordaz, A.; González-Ortiz, G.; La Ragione, R.; Woodward, M.; Collins, J.; Pérez, J.; Martín-Orúe, S. Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Li, T.; Kim, I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade qualitytraits. Rev. Bras. De Zootec. 2016, 45, 93–100. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Bogusławska-Wąs, E.; Łupkowska, A. Effect of EM® probiotic on gut microbiota, growth performance, carcass and meat quality of pigs. Livest. Sci. 2020, 241, 104206. [Google Scholar] [CrossRef]
- Suda, Y.; Villena, J.; Takahashi, Y.; Hosoya, S.; Tomosada, Y.; Tsukida, K.; Shimazu, T.; Aso, H.; Tohno, M.; Ishida, M. Immunobiotic Lactobacillus jensenii as immune-health promoting factor to improve growth performance and productivity in post-weaning pigs. BMC Immunol. 2014, 15, 24. [Google Scholar] [CrossRef]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat—2. Consumer acceptability of m. longissimus lumborum. Meat Sci. 1999, 53, 67–72. [Google Scholar] [CrossRef]
- Jeong, J.; Kwon, E.; Im, S.; Seo, K.; Baik, M. Expression of fat deposition and fat removal genes is associated with intramuscular fat content in longissimus dorsi muscle of Korean cattle steers. J. Anim. Sci. 2012, 90, 2044–2053. [Google Scholar] [CrossRef]
- Fang, S.; Xiong, X.; Su, Y.; Huang, L.; Chen, C. 16S rRNA gene-based association study identified microbial taxa associated with pork intramuscular fat content in feces and cecum lumen. BMC Microbiol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The association of diet, gut microbiota and colorectal cancer: What we eat may imply what we get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef]
- Krause, T.R.; Lourenco, J.M.; Welch, C.B.; Rothrock, M.J.; Callaway, T.R.; Pringle, T.D. The relationship between the rumen microbiome and carcass merit in Angus steers. J. Anim. Sci. 2020, 98, skaa287. [Google Scholar] [CrossRef]
- Wu, C.; Lyu, W.; Hong, Q.; Zhang, X.; Yang, H.; Xiao, Y. Gut microbiota influence lipid metabolism of skeletal muscle in pigs. Front. Nutr. 2021, 8, 675445. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Chlebicz-Wójcik, A.; Nowak, A. Probiotic properties of new Lactobacillus strains intended to be used as feed additives for monogastric animals. Probiotics Antimicrob. Proteins 2021, 13, 146–162. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Islam, M.A.; Takahashi, H.; Villena, J.; Kitazawa, H. Exopolysaccharides from Streptococcus thermophilus ST538 modulate the antiviral innate immune response in porcine intestinal epitheliocytes. Front. Microbiol. 2020, 11, 528770. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.; Ghareeb, K.; Böhm, J. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int. J. Mol. Sci. 2008, 9, 2205–2216. [Google Scholar] [CrossRef]
- National Pork Producers Council. Official Color and Marbling Standards; In Proceedings of the Natl; Pork Producers Council: Des Moines, IA, USA, 1999. [Google Scholar]
- Van Laack, R.; Stevens, S.; Stalder, K. The influence of ultimate pH and intramuscular fat content on pork tenderness and tenderization. J. Anim. Sci. 2001, 79, 392–397. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2000; Volume 17. [Google Scholar]
- Falkeholm, L.; Grant, C.A.; Magnusson, A.; Möller, E. Xylene-free method for histological preparation: A multicentre evaluation. Lab. Investig. 2001, 81, 1213–1221. [Google Scholar] [CrossRef]
- Qi, K.; Men, X.; Wu, J.; Xu, Z. Rearing pattern alters porcine myofiber type, fat deposition, associated microbial communities and functional capacity. BMC Microbiol. 2019, 19, 181. [Google Scholar] [CrossRef]
- Zhao, S.; Ren, L.; Chen, L.; Zhang, X.; Cheng, M.; Li, W.; Zhang, Y.; Gao, S. Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 2009, 44, 1029–1037. [Google Scholar] [CrossRef]
- Wang, W.; Xue, W.; Jin, B.; Zhang, X.; Ma, F.; Xu, X. Candidate gene expression affects intramuscular fat content and fatty acid composition in pigs. J. Appl. Genet. 2013, 54, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Baas, T.J.; Malek, M.; Dekkers, J.C.; Prusa, K.; Rothschild, M. Correlations among selected pork quality traits. J. Anim. Sci. 2002, 80, 617–627. [Google Scholar] [CrossRef]
- Tyra, M.; Ropka-Molik, K.; Terman, A.; Piórkowska, K.; Oczkowicz, M.; Bereta, A. Association between subcutaneous and intramuscular fat content in porcine ham and loin depending on age, breed and FABP3 and LEPR genes transcript abundance. Mol. Biol. Rep. 2013, 40, 2301–2308. [Google Scholar] [CrossRef]
- Goerl, K.; Eilert, S.; Mandigo, R.; Chen, H.; Miller, P. Pork characteristics as affected by two populations of swine and six crude protein levels. J. Anim. Sci. 1995, 73, 3621–3626. [Google Scholar]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [PubMed]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of intestinal microbiota on growth and feed efficiency in pigs: A review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, L.; Zhou, Y.; Wang, S.; Zhou, L. Analysis of the duodenal microbiotas of weaned piglet fed with epidermal growth factor-expressed Saccharomyces cerevisiae. BMC Microbiol. 2016, 16, 166. [Google Scholar]
- Han, G.G.; Lee, J.-Y.; Jin, G.-D.; Park, J.; Choi, Y.H.; Chae, B.J.; Kim, E.B.; Choi, Y.-J. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16S rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar]
- Cui, C.; Shen, C.; Jia, G.; Wang, K. Effect of dietary Bacillus subtilis on proportion of Bacteroidetes and Firmicutes in swine intestine and lipid metabolism. Genet. Mol. Res. 2013, 12, 1766–1776. [Google Scholar]
- Leng, Y.; Yi, M.; Fan, J.; Bai, Y.; Ge, Q.; Yao, G. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci. Rep. 2016, 6, 22814. [Google Scholar] [CrossRef]
- Quan, J.; Cai, G.; Ye, J.; Yang, M.; Ding, R.; Wang, X.; Zheng, E.; Fu, D.; Li, S.; Zhou, S. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci. Rep. 2018, 8, 4536. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.; Ramana, J. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar]
- Motato, K.E.; Milani, C.; Ventura, M.; Valencia, F.E.; Ruas-Madiedo, P.; Delgado, S. Bacterial diversity of the Colombian fermented milk “Suero Costeño” assessed by culturing and high-throughput sequencing and DGGE analysis of 16S rRNA gene amplicons. Food Microbiol. 2017, 68, 129–136. [Google Scholar]
- Hugenholtz, F.; Davids, M.; Schwarz, J.; Müller, M.; Tomé, D.; Schaap, P.; Hooiveld, G.J.; Smidt, H.; Kleerebezem, M. Metatranscriptome analysis of the microbial fermentation of dietary milk proteins in the murine gut. PLoS ONE 2018, 13, e0194066. [Google Scholar]
- Barker, H. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 1981, 50, 23–40. [Google Scholar] [PubMed]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Li, T.; Yin, Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids 2016, 48, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, C.K.; Huebel, N.; Abd El-Bary, M.M.; Loh, G.; Klaus, S.; Blaut, M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 2010, 104, 919–929. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, X.; Yu, X.; Hu, C.; Zhang, X. The family Coriobacteriaceae is a potential contributor to the beneficial effects of Roux-en-Y gastric bypass on type 2 diabetes. Surg. Obes. Relat. Dis. 2018, 14, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Desmarchelier, C.; Haller, D.; Gérard, P.; Rohn, S.; Lepage, P.; Daniel, H. Intestinal microbiota in metabolic diseases: From bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes 2014, 5, 544–551. [Google Scholar]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut microbiota and metabolic health among overweight and obese individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar]
- Liu, Y.; Li, Y.; Feng, X.; Wang, Z.; Xia, Z. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult. Sci. 2018, 97, 3218–3229. [Google Scholar]
- Murakami, Y.; Ojima-Kato, T.; Saburi, W.; Mori, H.; Matsui, H.; Tanabe, S.; Suzuki, T. Supplemental epilactose prevents metabolic disorders through uncoupling protein-1 induction in the skeletal muscle of mice fed high-fat diets. Br. J. Nutr. 2015, 114, 1774–1783. [Google Scholar] [CrossRef]
- Kim, M.; Park, T.; Jeong, J.Y.; Baek, Y.; Lee, H.-J. Association between rumen microbiota and marbling score in Korean native beef cattle. Animals 2020, 10, 712. [Google Scholar] [CrossRef] [PubMed]


| Items | Week 4–6 | Week 7–12 | Week 13–22 |
|---|---|---|---|
| Cooked corn | 50.00 | - | - |
| Corn | - | 55.00 | 60.50 |
| Soybean meal | 11.00 | 25.00 | 18.00 |
| Whole soy flour | 15.00 | - | - |
| Fish Meal | 6.00 | 4.00 | 2.50 |
| Wheat | - | 7.00 | 10.00 |
| Whey Powder | 5.00 | - | - |
| Skim Milk Powder | 5.00 | - | - |
| Soybean Oil | 2.00 | 3.00 | 3.00 |
| Commercial Premix | 6.00 | 6.00 | 6.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated composition | |||
| Digestible energy, kcal/kg | 3619.02 | 3518.93 | 3396.31 |
| Metabolic energy, kcal/kg | 3403.17 | 3188.39 | 3046.70 |
| Crude protein, % | 20.48 | 18.79 | 16.29 |
| Crude fat, % | 6.60 | 6.14 | 5.57 |
| Crude fiber, % | 1.39 | 2.57 | 3.72 |
| Crude ash, % | 5.37 | 5.61 | 5.38 |
| Lysine, % | 1.25 | 1.16 | 1.00 |
| Methionine + cystine, % | 0.62 | 0.59 | 0.53 |
| Calcium, % | 0.80 | 0.90 | 0.81 |
| Available phosphorus, % | 0.54 | 0.44 | 0.42 |
| Samples | Primer | Gene Sequence |
|---|---|---|
| Gene Expression | ||
| CPT-1B | Forward | ATG GTG GGC GAC TAA CT |
| Reverse | TGC CTG CTG TCT GTG AG | |
| FAS | Forward | AGC CTA ACT CCT CGC TGC AAT |
| Reverse | TCC TTG GAA CCG TCT GTG TTC | |
| LPL | Forward | TCC TTG GAA CCG TCT GTG TTC |
| Reverse | CAC CAC AGC CAC AGC AAC TC | |
| SREBP-1 | Forward | GCG ACG GTG CCT CTG GTA GT |
| Reverse | CGC AAG ACG GCG GAT TTA | |
| β-actin | Forward | CAC CAC AGC CAC AGC AAC TC |
| Reverse | CAT CGT CGC CCG CAA AGC |
| Traits | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | AGP | SYN | |||
| Live body weight (kg) | 118.00 | 119.67 | 119.00 | 3.54 | 0.913 |
| Carcass length (cm) | 74.12 | 71.37 | 73.46 | 1.74 | 0.343 |
| Carcass weight (kg) | 92.01 | 92.67 | 93.4 | 2.88 | 0.910 |
| Carcass percentage (%) | 78.2 | 77.45 | 78.43 | 1.66 | 0.470 |
| Back fat (cm) | 2.56 | 2.27 | 2.47 | 0.24 | 0.538 |
| Subcutaneous fat (%) | 20.50 | 19.43 | 18.98 | 2.27 | 0.803 |
| Bone (%) | 17.04 | 17.39 | 16.83 | 0.64 | 0.833 |
| Lean (%) | 53.46 | 54.42 | 55.82 | 1.94 | 0.798 |
| Longissimus muscle (kg) | 3.19 | 3.28 | 3.44 | 0.16 | 0.470 |
| LD muslce area (cm2) | 74.05 | 74.24 | 81.51 | 4.59 | 0.323 |
| Traits 2 | Treatments 3 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | AGP | SYN | |||
| Meat pH | 5.61 | 5.86 | 5.62 | 0.14 | 0.235 |
| Marbling score | 2.96 a | 2.26 b | 3.08 a | 0.21 | 0.030 |
| L | 42.59 | 39.89 | 44.5 | 2.92 | 0.468 |
| a | 5.79 a | 4.71 a | 6.96 a* | 0.77 | 0.160 |
| b | 2.15 | 1.51 | 2.89 | 1.06 | 0.811 |
| Cooking loss (%) | 24.73 | 25.31 | 24.86 | 0.88 | 0.990 |
| Firmness (kg) | 9.07 a | 9.99 a | 6.56 a* | 1.29 | 0.099 |
| Toughness (kg/s) | 16.18 a | 18.14 a | 10.98 a* | 2.80 | 0.114 |
| Item | Treatments 2 | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | AGP | SYN | |||
| Moisture | 70.95 | 72.48 | 71.32 | 0.56 | 0.088 |
| Ash | 1.07 | 1.1 | 1.07 | 0.02 | 0.297 |
| Lipid | 6.42 | 4.22 | 5.57 | 0.87 | 0.159 |
| Protein | 21.77 b | 22.78 ab | 23.07 a | 0.42 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-Y.; Liao, Y.-C.; Chang, H.-T.; Lin, H.-C.; Weng, H.-M.; Chang, I.-J.; Young, S.-L.; Shen, P.-C.; Bhattarai, B.P.; Lin, J.-S.; et al. Inclusion of Multi-Strained Probiotics Improves the Fecal Microbiota and Carcass Quality of Pigs. Animals 2025, 15, 993. https://doi.org/10.3390/ani15070993
Lee T-Y, Liao Y-C, Chang H-T, Lin H-C, Weng H-M, Chang I-J, Young S-L, Shen P-C, Bhattarai BP, Lin J-S, et al. Inclusion of Multi-Strained Probiotics Improves the Fecal Microbiota and Carcass Quality of Pigs. Animals. 2025; 15(7):993. https://doi.org/10.3390/ani15070993
Chicago/Turabian StyleLee, Ting-Yu, Yi-Chu Liao, Hsiao-Tung Chang, Hsiao-Ching Lin, Hsiu-Ming Weng, I-Ju Chang, San-Land Young, Perng-Chih Shen, Bishnu Prasad Bhattarai, Jin-Seng Lin, and et al. 2025. "Inclusion of Multi-Strained Probiotics Improves the Fecal Microbiota and Carcass Quality of Pigs" Animals 15, no. 7: 993. https://doi.org/10.3390/ani15070993
APA StyleLee, T.-Y., Liao, Y.-C., Chang, H.-T., Lin, H.-C., Weng, H.-M., Chang, I.-J., Young, S.-L., Shen, P.-C., Bhattarai, B. P., Lin, J.-S., & Lee, J.-W. (2025). Inclusion of Multi-Strained Probiotics Improves the Fecal Microbiota and Carcass Quality of Pigs. Animals, 15(7), 993. https://doi.org/10.3390/ani15070993





