Plasma Lipidomics and Proteomics Analyses Pre- and Post-5000 m Race in Yili Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Samples
2.3. Lipid Extraction
2.4. Liquid Chromatography–Mass Spectrometry
2.5. Lipidome Data Processing and Analysis
2.6. Proteomics Sample Preparation
2.7. Data-Independent Acquisition Mass Spectrometry Detection
2.8. Proteomics Data Analysis
2.9. Correlation Analysis Between DALs and DAPs
3. Results
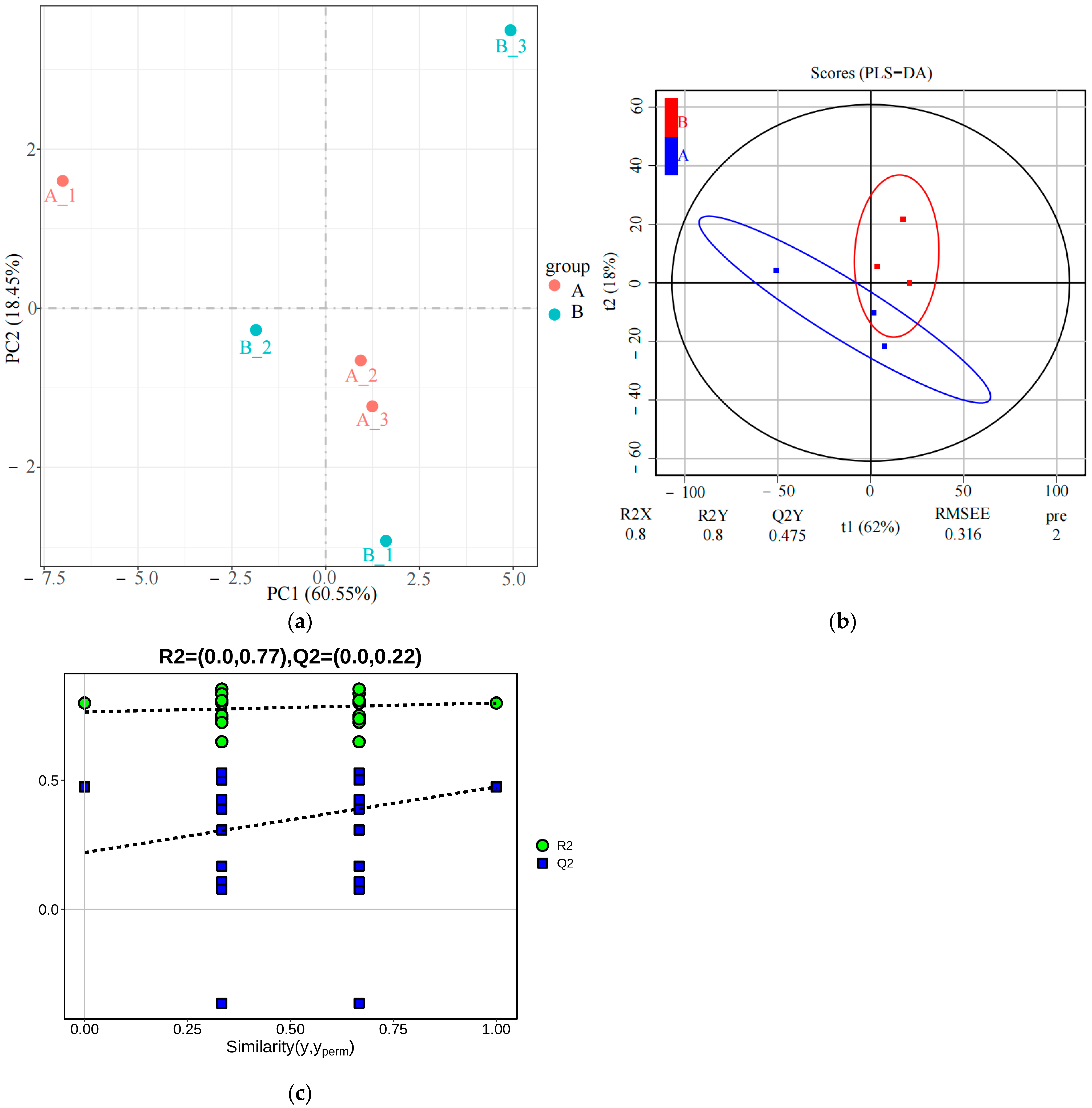
3.1. Lipidomics Detection and Identification of Differential Lipids (DALs)
3.2. Lipid Metabolism Pathways
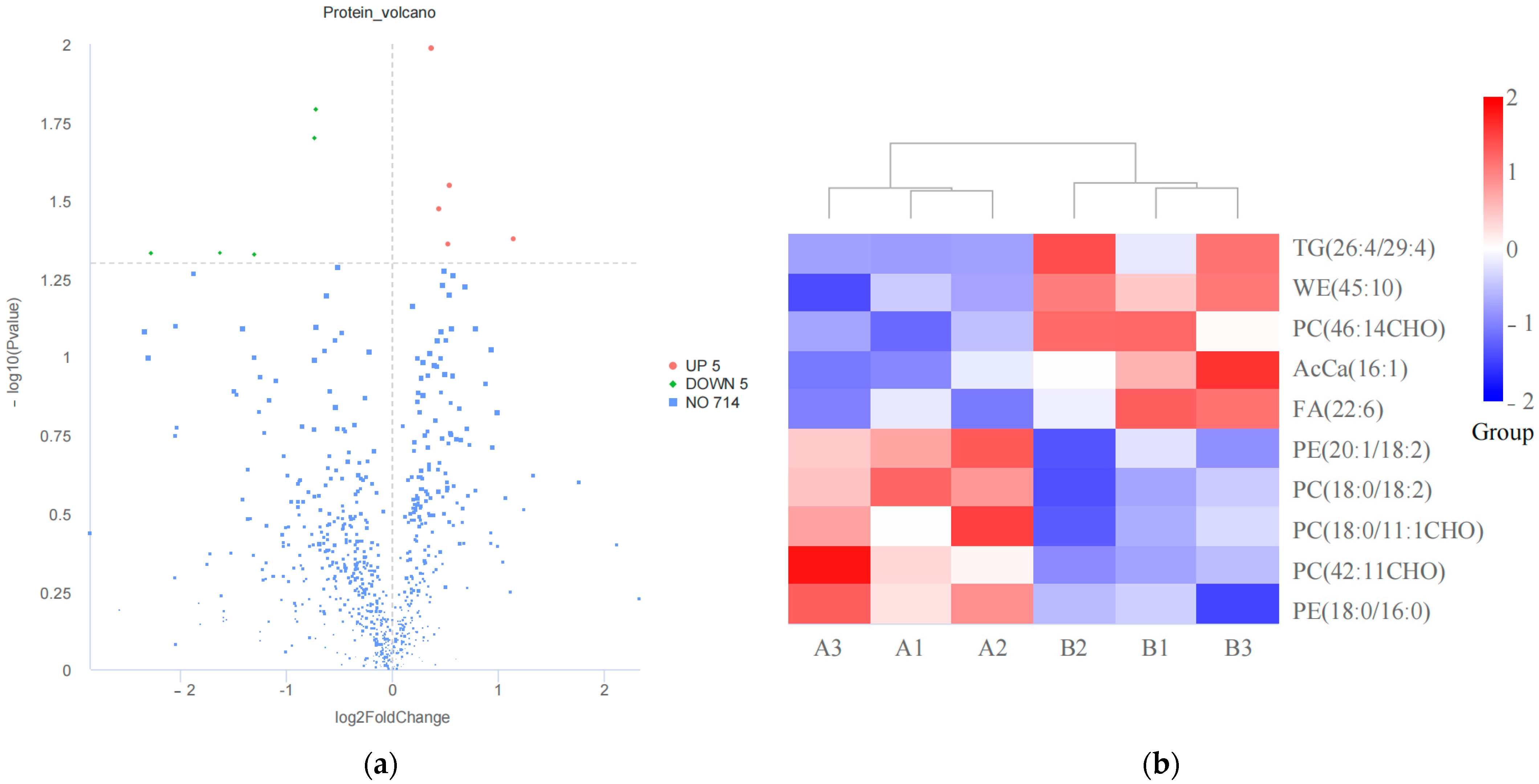
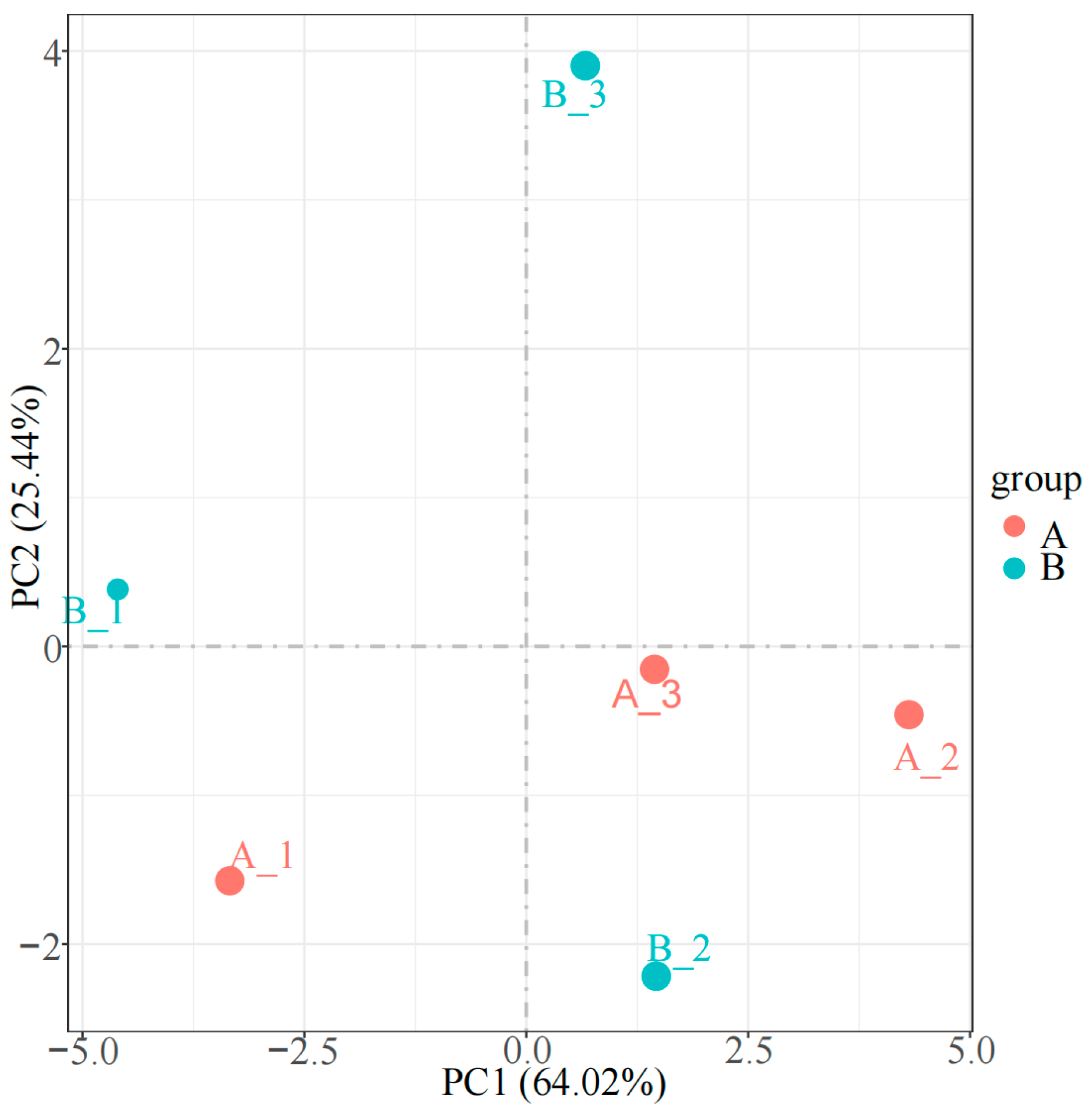
3.3. Proteomics Detection and Identification of Differential Proteins (DAPs)
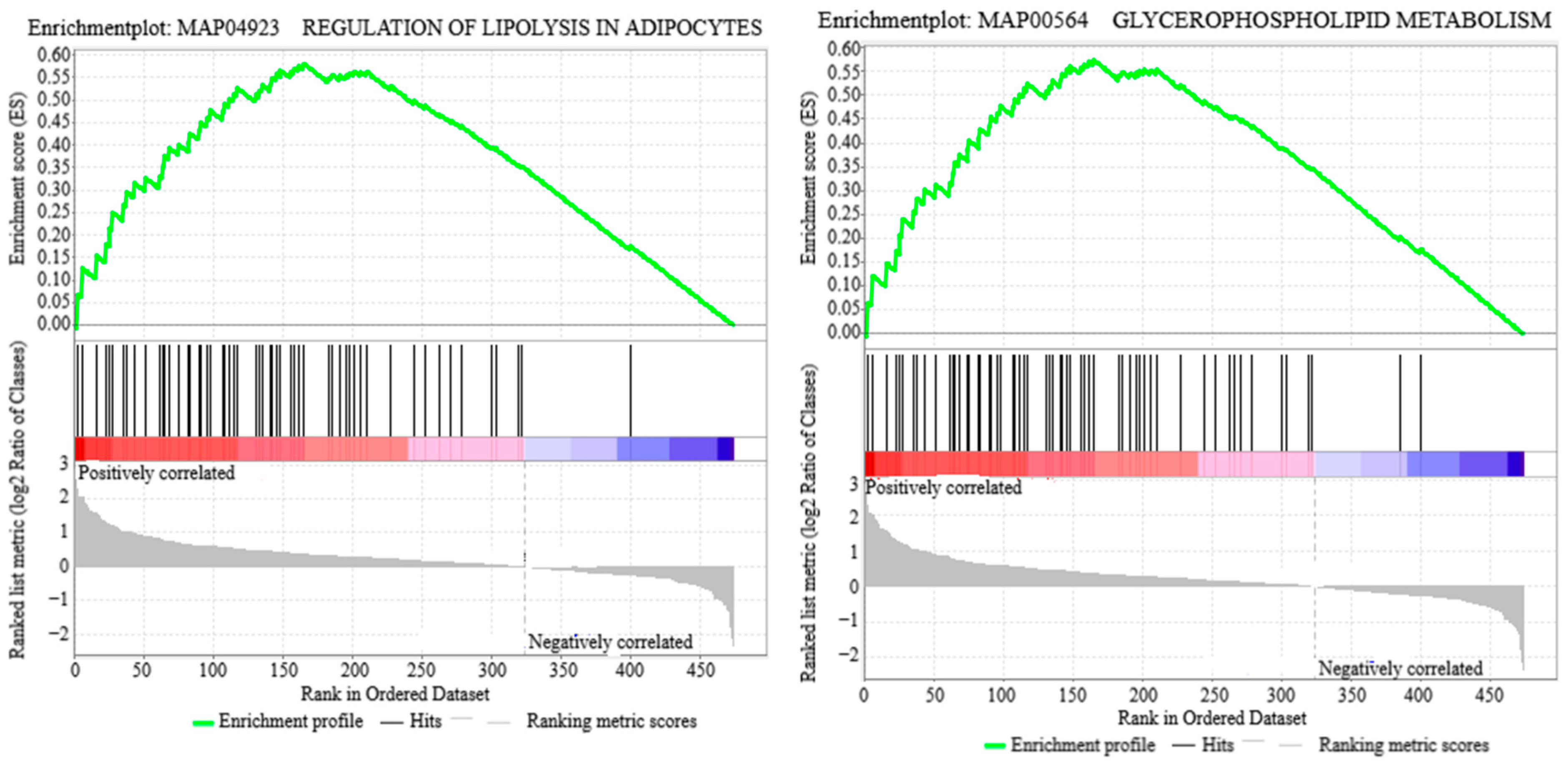
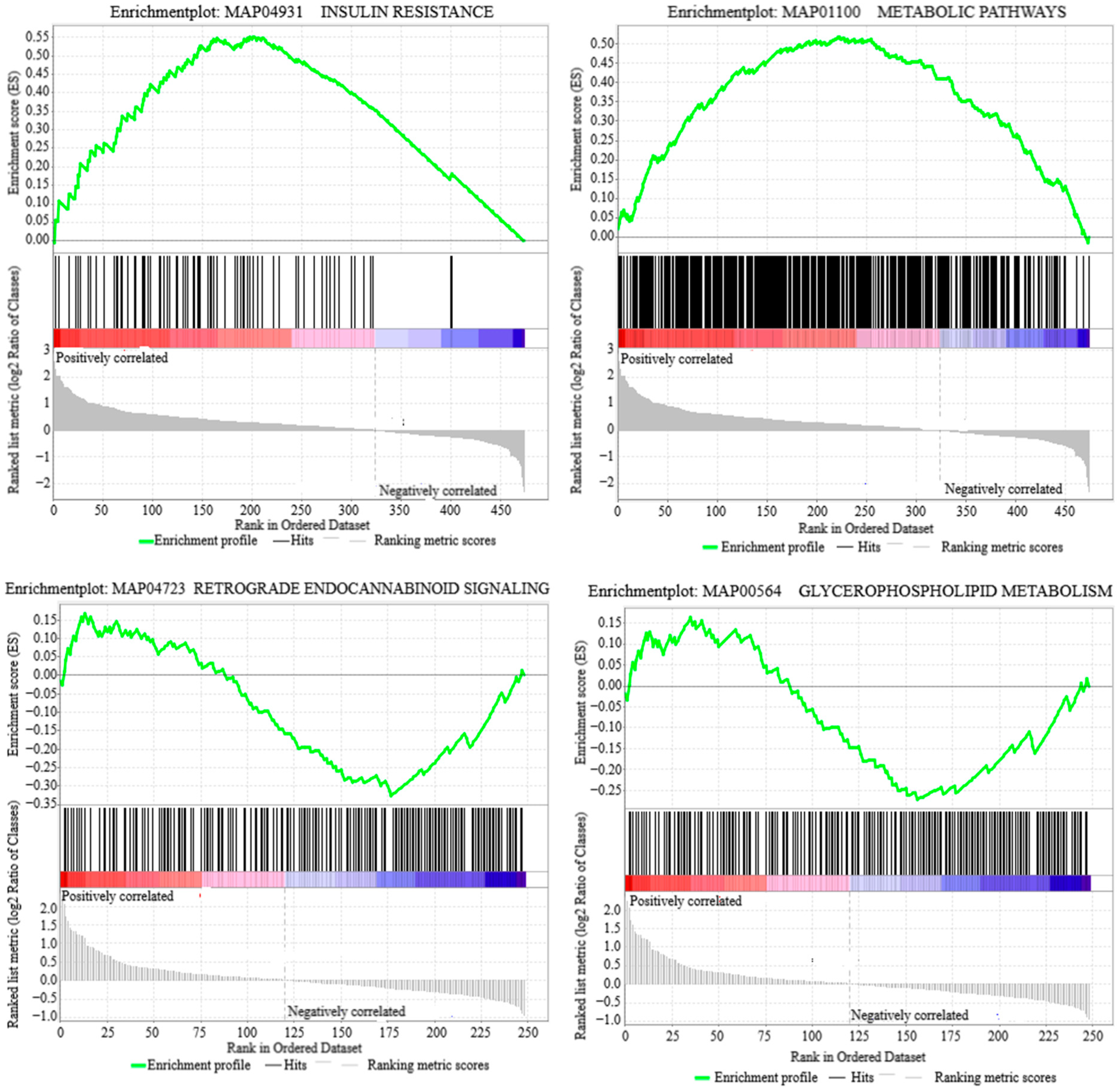
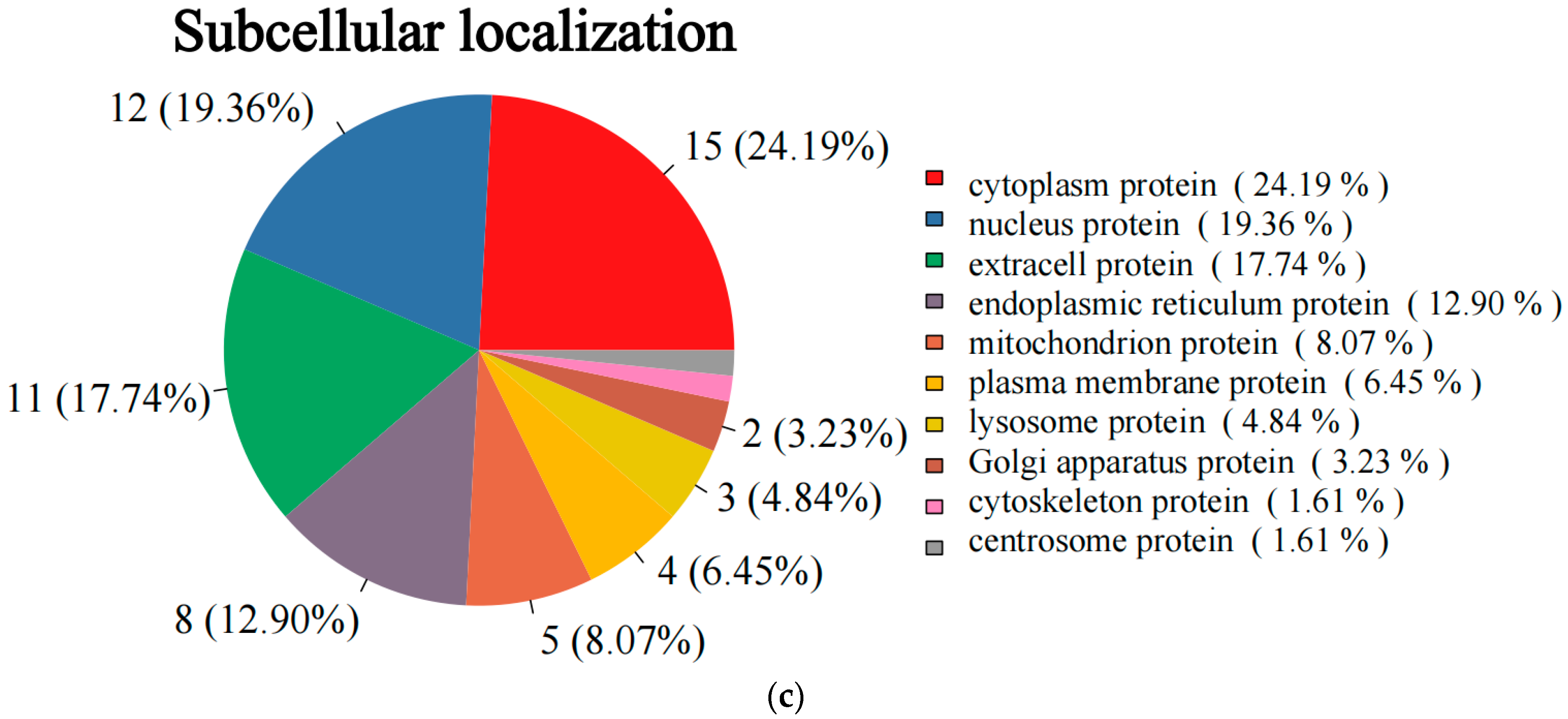
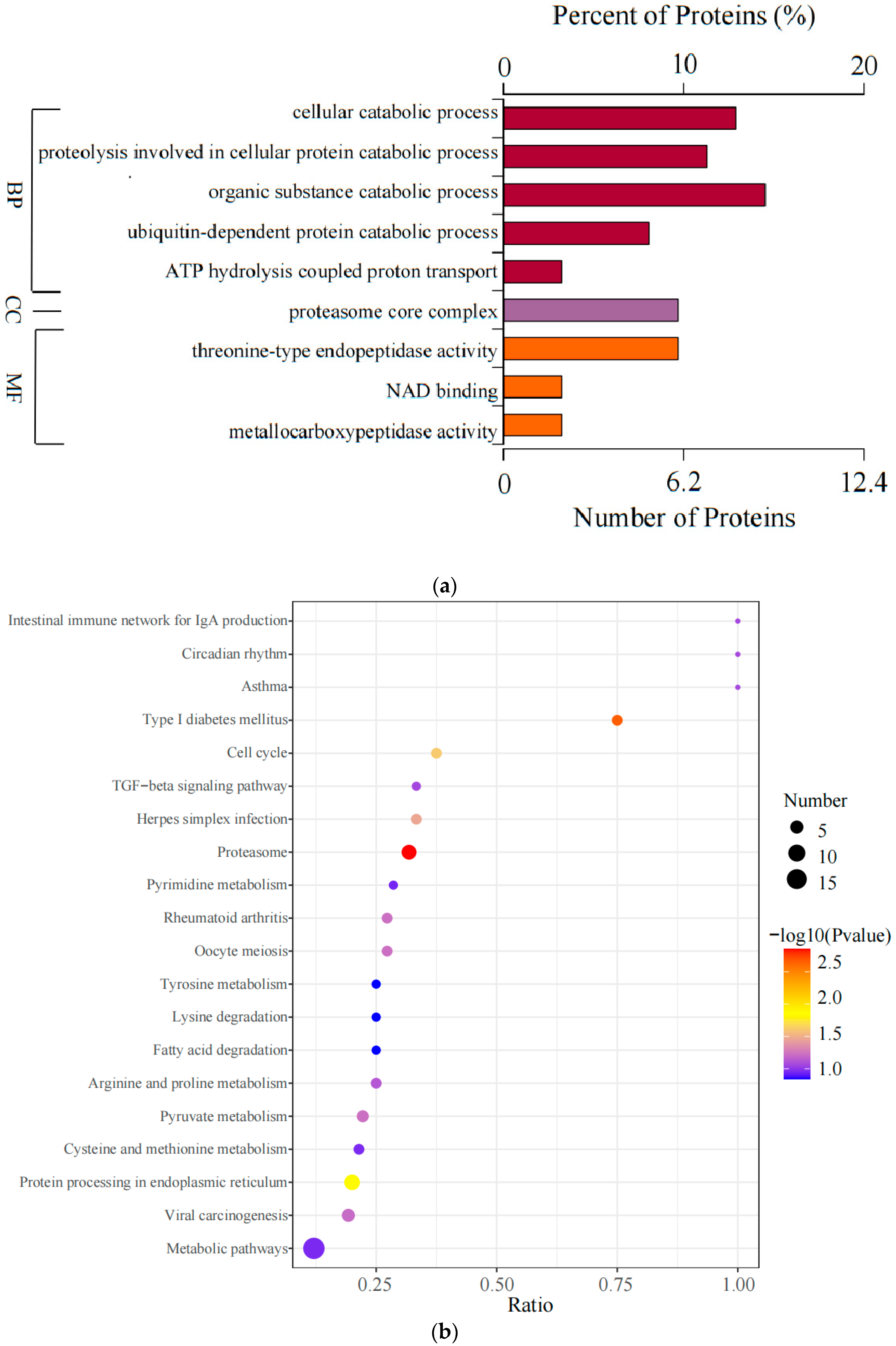
3.4. Enrichment Analysis of DAPs
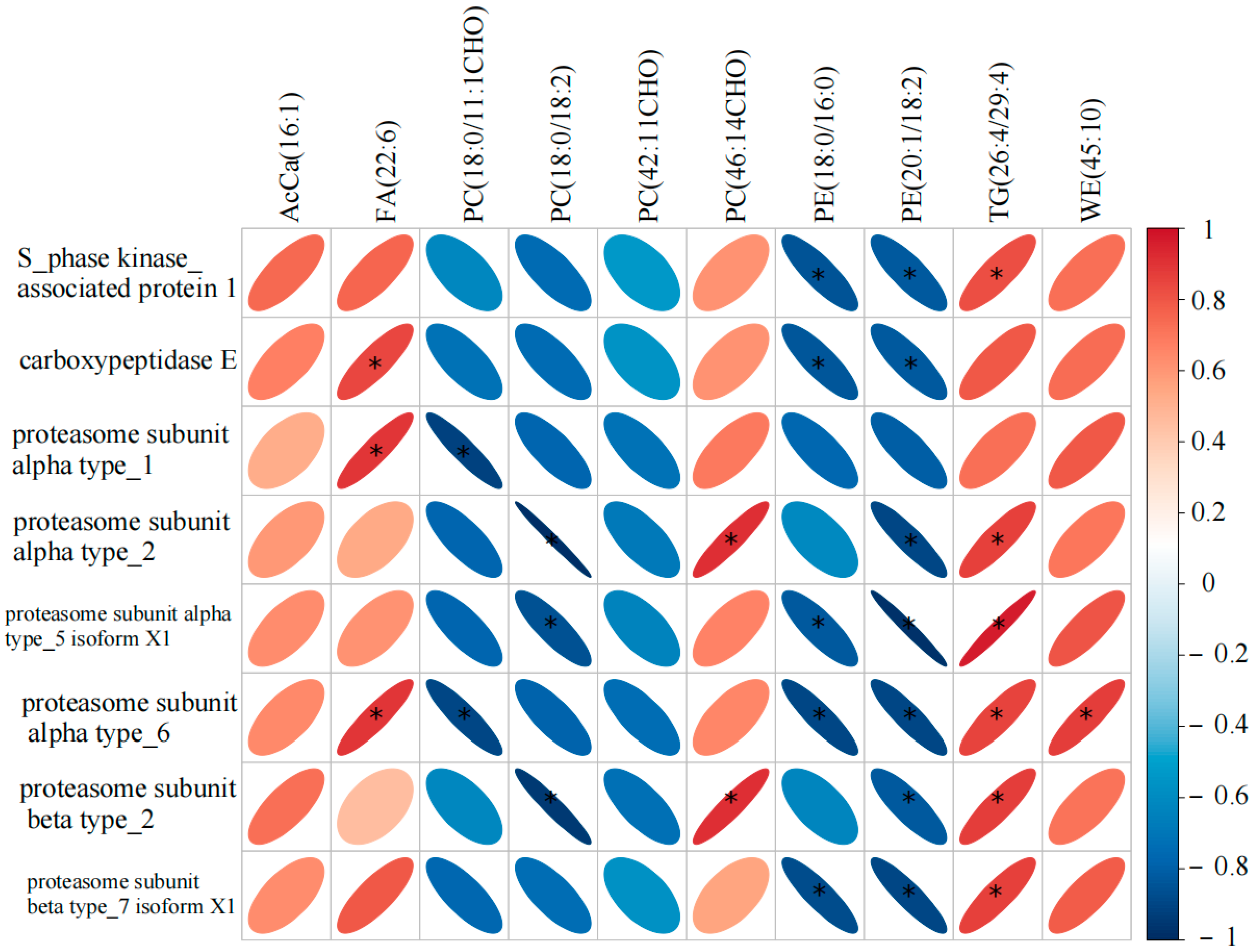
3.5. Correlation Analysis Between DALs and DAPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.W.; Ren, W.L.; Li, Z.X.; Ma, S.K.; Li, L.L.; Wang, R.; Zeng, Y.Q.; Meng, J.; Yao, X.K. Blood-based whole-genome methylation analysis of Yili horses pre- and post-racing. Animals 2025, 15, 326. [Google Scholar] [CrossRef]
- Wang, C.K.; Zeng, Y.Q.; Wang, J.W.; Wang, T.L.; Li, X.Y.; Shen, Z.H.; Meng, J.; Yao, X.K. A genome-wide association study of the racing performance traits in Yili horses based on Blink and FarmCPU models. Sci. Rep. 2024, 14, 27648. [Google Scholar] [CrossRef]
- Ohnuma, K.; Uchida, T.; Leung, G.N.W.; Ueda, T.; Obara, T.; Ishii, H. Establishment of a post-race biomarkers database and application of pathway analysis to identify potential biomarkers in post-race equine plasma. Drug Test. Anal. 2022, 14, 915–928. [Google Scholar] [CrossRef]
- Dunstan, R.H.; Macdonald, M.M.; Thorn, B.; Wood, D.; Roberts, T.K. Modelling of amino acid turnover in the horse during training and racing: A basis for developing a novel supplementation strategy. PLoS ONE 2020, 15, e0226988. [Google Scholar] [CrossRef]
- Blau, L.S.; Gerber, J.; Finkel, A.; Lützow, M.; Maassen, N.; Röhrich, M.A.; Hanff, E.; Tsikas, D.; Shushakov, V.; Jantz, M. Effects of short-term sodium nitrate versus sodium chloride supplementation on energy and lipid metabolism during high-intensity intermittent exercise in athletes. Appl. Sci. 2023, 13, 6849. [Google Scholar] [CrossRef]
- de Hase, E.M.; Petitfils, C.; Alhouayek, M.; Depommier, C.; Le Faouder, P.; Delzenne, N.M.; Van Hul, M.; Muccioli, G.G.; Cenac, N.; Cani, P.D. Dysosmobacter welbionis effects on glucose, lipid, and energy metabolism are associated with specific bioactive lipids. J. Lipid Res. 2023, 64, 100437. [Google Scholar] [CrossRef]
- Koubaa, A.; Koubaa, S.; Elloumi, M. Effect of different warm-up durations on the plasma oxidative stress biomarkers following anaerobic exercise in amateur handball players. Appl. Sci. 2023, 13, 10576. [Google Scholar] [CrossRef]
- Tilekli, M.M.; Yilmaz, A.K.; Yasul, Y.; Çon, N.; Mercan, S.; Tek, N. Influence of diet and exercise on leukocyte telomere length, markers of oxidative stress and inflammation in rats. Exp. Mol. Pathol. 2024, 140, 104947. [Google Scholar] [CrossRef]
- Giers, J.; Bartel, A.; Kirsch, K.; Mueller, S.F.; Horstmann, S.; Gehlen, H. Blood-based assessment of oxidative stress, inflammation, endocrine and metabolic adaptations in eventing horses accounting for plasma volume shift after exercise. Vet. Med. Sci. 2024, 10, e1409. [Google Scholar] [CrossRef]
- Morais, L.V.; dos Santos, S.N.; Gomes, T.H.; Romano, C.M.; Colombo-Souza, P.; Amaral, J.B.; Shio, M.T.; Neves, L.M.; Bachi, A.L.L.; Franca, C.N.; et al. Acute strength exercise training impacts differently the HERV-W expression and inflammatory biomarkers in resistance exercise training individuals. PLoS ONE 2024, 19, e0303798. [Google Scholar] [CrossRef]
- Li, X.B.; Ma, J.; Li, H.Y.; Li, H.; Ma, Y.H.; Deng, H.F.; Yang, K.L. Effect of β-alanine on the athletic performance and blood amino acid metabolism of speed-racing Yili horses. Front. Vet. Sci. 2024, 11, 1339940. [Google Scholar] [CrossRef]
- Wang, T.L.; Zeng, Y.Q.; Ma, C.X.; Meng, J.; Wang, J.W.; Ren, W.L.; Wang, C.K.; Yuan, X.X.; Yang, X.X.; Yao, X.K. Plasma non-targeted metabolomics analysis of Yili horses raced on tracks with different surface hardness. J. Equine Vet. Sci. 2023, 121, 104197. [Google Scholar] [CrossRef]
- Morville, T.; Rosenkilde, M.; Mattsson, N.; Dela, F.; Helge, J.W.; Rasmusen, H.K. 2706 km cycling in 2 weeks: Effects on cardiac function in 6 elderly male athletes. Phys. Sportsmed. 2018, 46, 263–268. [Google Scholar] [CrossRef]
- Cieminski, K.; Flis, D.J.; Dzik, K.; Kaczor, J.J.; Czyrko, E.; Halon-Golabek, M.; Wieckowski, M.R.; Antosiewicz, J.; Ziolkowski, W. Swim training affects Akt signaling and ameliorates loss of skeletal muscle mass in a mouse model of amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 20899. [Google Scholar] [CrossRef]
- Delsmann, M.M.; Stürznickel, J.; Amling, M.; Ueblacker, P.; Rolvien, T. Musculoskeletal laboratory diagnostics in competitive sport. Orthopade 2021, 50, 700–712. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.J.; Zhang, H.; Feng, M.W.; Ding, J.J.; Zhang, B.; Cheng, Z.J.; Qian, L.M. Exercise-induced peptide EIP-22 protect myocardial from ischaemia/reperfusion injury via activating JAK2/STAT3 signalling pathway. J. Cell. Mol. Med. 2021, 25, 3560–3572. [Google Scholar] [CrossRef]
- Panagoulias, I.; Charokopos, N.; Thomas, I.; Spantidea, P.I.; de Lastic, A.L.; Rodi, M.; Anastasopoulou, S.; Aggeletopoulou, I.; Lazaris, C.; Karkoulias, K.; et al. Shifting gears: Study of immune system parameters of male habitual marathon runners. Front. Immunol. 2023, 13, 1009065. [Google Scholar] [CrossRef]
- Bi, Y.X.; Liu, X.J.; Liu, Y.; Wang, M.Y.; Shan, Y.M.; Yin, Y.H.; Meng, X.L.; Sun, F.J.; Li, H.; Li, Z.D. Molecular and biochemical investigations of the anti-fatigue effects of tea polyphenols and fruit extracts of Lycium ruthenicum Murr. on mice with exercise-induced fatigue. Front. Mol. Biosci. 2023, 10, 1223411. [Google Scholar] [CrossRef]
- Nemkov, T.; Cendali, F.; Stefanoni, D.; Martinez, J.L.; Hansen, K.C.; San-Millán, I.; D’Alessandro, A. Metabolic signatures of performance in elite world tour professional male cyclists. Sports Med. 2023, 53, 1651–1665. [Google Scholar] [CrossRef]
- Dearlove, D.J.; Holdsworth, D.; Kirk, T.; Hodson, L.; Charidemou, E.; Kvalheim, E.; Stubbs, B.; Beevers, A.; Griffin, J.L.; Evans, R.; et al. β-Hydroxybutyrate oxidation in exercise is impaired by low-carbohydrate and high-fat availability. Front. Med. 2021, 8, 721673. [Google Scholar] [CrossRef]
- López-Soldado, I.; Guinovart, J.J.; Duran, J. Increased liver glycogen levels enhance exercise capacity in mice. J. Biol. Chem. 2021, 297, 100976. [Google Scholar] [CrossRef] [PubMed]
- Beghelli, D.; Zallocco, L.; Angeloni, C.; Bistoni, O.; Ronci, M.; Cavallucci, C.; Mazzoni, M.R.; Nuccitelli, A.; Catalano, C.; Hrelia, S.; et al. Dietary supplementation with boswellia serrata, verbascum thapsus, and curcuma longa in show jumping horses: Effects on serum proteome, antioxidant status, and anti-inflammatory gene expression. Life 2023, 13, 750. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Ohmura, H.; Takahashi, Y.; Ebisuda, Y.; Yoneda, K.; Miyata, H. Physiological and skeletal muscle responses to high-intensity interval exercise in Thoroughbred horses. Front. Vet. Sci. 2023, 10, 1241266. [Google Scholar] [CrossRef] [PubMed]
- Acri, G.; Testagrossa, B.; Piccione, G.; Arfuso, F.; Giudice, E.; Giannetto, C. Central and peripheral fatigue evaluation during physical exercise in athletic horses by means of raman spectroscopy. Animals 2023, 13, 2201. [Google Scholar] [CrossRef]
- Bonhomme, M.M.; Patarin, F.; Kruse, C.J.; François, A.C.; Renaud, B.; Couroucé, A.; Leleu, C.; Boemer, F.; Toquet, M.P.; Richard, E.A.; et al. Untargeted metabolomics profiling reveals exercise intensity-dependent alterations in Thoroughbred racehorses’ plasma after routine conditioning sessions. ACS Omega 2023, 8, 48557–48571. [Google Scholar] [CrossRef]
- de Siqueira, R.F.; Silva, B.O.; Fernandes, M.L.; Fernandes, W.R. Evaluation basal lipid metabolism components and adiposity in trained Arabian horses for Endurance and Racing. Cienc. Rural 2024, 54, e20230373. [Google Scholar] [CrossRef]
- Kiely, M.; Warrington, G.D.; McGoldrick, A.; Pugh, J.; Cullen, S. Physiological demands of professional flat and jump horse racing. J. Strength. Cond. Res. 2020, 34, 2173–2177. [Google Scholar] [CrossRef]
- Yang, L.P.; Li, P.C.; Huang, X.X.; Wang, C.K.; Zeng, Y.Q.; Wang, J.W.; Yao, X.K.; Meng, J. Effects of combined transcriptome and metabolome analysis training on athletic performance of 2-year-old trot-type Yili horses. Genes 2025, 16, 197. [Google Scholar] [CrossRef]
- Perera, T.R.W.; Bromfield, E.G.; Gibb, Z.; Nixon, B.; Sheridan, A.R.; Rupasinghe, T.; Skerrett-Byrne, D.A.; Swegen, A. Plasma lipidomics reveals lipid signatures of early pregnancy in mares. Int. J. Mol. Sci. 2024, 25, 11073. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.X.; Cheng, X.M.; Liu, X.Y.; Sun, H.D.; Guo, Z.G.; Li, J.; Tang, X.Y.; Wang, Z.; Sun, W.; et al. LC-MS-based plasma metabolomics and lipidomics analyses for differential diagnosis of bladder cancer and renal cell carcinoma. Front. Oncol. 2020, 10, 717. [Google Scholar] [CrossRef]
- Tanaka, R.; Fuse, S.; Kuroiwa, M.; Amagasa, S.; Endo, T.; Ando, A.; Kime, R.; Kurosawa, Y.; Hamaoka, T. Vigorous-intensity physical activities are associated with high brown adipose tissue density in humans. Int. J. Environ. Res. Public. Health 2020, 17, 2796. [Google Scholar] [CrossRef]
- Ogasawara, J.; Izawa, T.; Sakurai, T.; Shirato, K.; Ishibashi, Y.; Ohira, Y.; Ishida, H.; Ohno, H.; Kizaki, T. Habitual exercise training acts as a physiological stimulator for constant activation of lipolytic enzymes in rat primary white adipocytes. Biochem. Biophys. Res. Commun. 2015, 464, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Whytock, K.L.; Shepherd, S.O.; Wagenmakers, A.J.M.; Strauss, J.A. Hormone-sensitive lipase preferentially redistributes to lipid droplets associated with perilipin-5 in human skeletal muscle during moderate-intensity exercise. J. Physiol. 2018, 596, 2077–2090. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, G.F.; Zhao, X.B.; Huang, Z.R.; Li, P.; Zhang, H.F. Post-exercise effects and long-term training adaptations of hormone sensitive lipase lipolysis induced by high-intensity interval training in adipose tissue of mice. Front. Physiol. 2020, 11, 535722. [Google Scholar] [CrossRef]
- Stokie, J.R.; Abbott, G.; Howlett, K.F.; Hamilton, D.L.; Shaw, C.S. Intramuscular lipid utilization during exercise: A systematic review, meta-analysis, and meta-regression. J. Appl. Physiol. 2023, 134, 581–592. [Google Scholar] [CrossRef] [PubMed]
- El Ouali, E.; Bosquet, L.; Elgharbaoui, B.; Laziri, F.; Laher, I.; Hackney, A.C.; Ibrahimi, A.; Taib, B.; El Harane, S.; Weiss, K.; et al. Association between “cluster of differentiation 36 (CD36)” and adipose tissue lipolysis during exercise training: A systematic review. Front. Physiol. 2023, 14, 1256440. [Google Scholar] [CrossRef]
- Silva, A.A.R.; Bertolucci, V.; Scariot, P.P.M.; da Cruz, J.P.; Mendes, F.M.M.; de Oliveira, D.C.; Plumari, C.D.; Dos Reis, I.G.M.; Porcari, A.M.; Messias, L.H.D. Glycerophospholipids in red blood cells are associated with aerobic performance in young swimmers. Nutrients 2024, 16, 765. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.J.; McWilliams, S.R. The fat of the matter: How dietary fatty acids can affect exercise performance. Integr. Comp. Biol. 2014, 54, 903–912. [Google Scholar] [CrossRef]
- Sharpe, A.J.; McKenzie, M. Mitochondrial fatty acid oxidation disorders associated with short-chain enoyl-coa hydratase (echs1) deficiency. Cells 2018, 7, 46. [Google Scholar] [CrossRef]
- Mao, Y.Z.; Zhang, J.J.; Zhou, Q.; He, X.D.; Zheng, Z.F.; Wei, Y.; Zhou, K.Q.; Lin, Y.; Yu, H.W.; Zhang, H.H.; et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024, 34, 13–30. [Google Scholar] [CrossRef]
- Imdad, S.; Lim, W.; Kim, J.H.; Kang, C. Intertwined relationship of mitochondrial metabolism, gut microbiome and exercise potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.; Galano, M.; Chan, R.; Sanyal, E.; Issop, L.; Lee, S.; Taylor, L.; Kaur, P.; Daly, E.; Papadopoulos, V. Dynamic remodeling of membranes and their lipids during acute hormone-induced steroidogenesis in ma-10 mouse leydig tumor cells. Int. J. Mol. Sci. 2021, 22, 2554. [Google Scholar] [CrossRef]
- Mateos, M.V.; Giusto, N.M.; Salvador, G.A. Distinctive roles of PLD signaling elicited by oxidative stress in synaptic endings from adult and aged rats. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1823, 2136–2148. [Google Scholar] [CrossRef]
- Anderson, K.C.; Liu, J.; Liu, Z.Q. Interplay of fatty acids, insulin and exercise in vascular health. Lipids Health Dis. 2025, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.N.; Zhu, S.; He, Y.; Gong, J.J.; Zou, W.J.; Tan, C.P.; Liu, Y.F.; Xu, Y.J. The comparison of arachidonic acid-enriched phosphatidylcholine and triglyceride on defending nerve injury. Food Biosci. 2024, 59, 104101. [Google Scholar] [CrossRef]
- He, B.; Xu, J.; Pang, S.S.; Tang, H.Q. Phosphatidylcholine mediates the crosstalk between LET-607 and DAF-16 stress response pathways. PLoS Genet. 2021, 17, e1009573. [Google Scholar] [CrossRef]
- Saare, M.; Tserel, L.; Haljasmägi, L.; Taalberg, E.; Peet, N.; Eimre, M.; Vetik, R.; Kingo, K.; Saks, K.; Tamm, R.; et al. Monocytes present age-related changes in phospholipid concentration and decreased energy metabolism. Aging Cell 2020, 19, e13127. [Google Scholar] [CrossRef]
- Borges, I.B.P.; de Oliveira, D.S.; Marie, S.K.N.; Lenario, A.M.; Oba-Shinjo, S.M.; Shinjo, S.K. Exercise training attenuates ubiquitin-proteasome pathway and increases the genes related to autophagy on the skeletal muscle of patients with inflammatory myopathies. J. Clin. Rheumatol. 2021, 27, S224–S231. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, X.Y.; Zhang, X.Y.; Jin, Z.; Liu, S.J.; Fu, L.; Niu, Y.M. Exercise improves high-fat diet-induced metabolic disorder by promoting HDAC5 degradation through the ubiquitin-proteasome system in skeletal muscle. Appl. Physiol. Nutr. Metab. 2022, 46, 1322–1330. [Google Scholar] [CrossRef]
- Castro-Gomes, T.; Corrotte, M.; Tam, C.; Andrews, N.W. Plasma membrane repair is regulated extracellularly by proteases released from lysosomes. PLoS ONE 2016, 11, e0152583. [Google Scholar] [CrossRef]
- Maruyama, T.; Noda, N.N. Autophagy-regulating protease Atg4, structure, function, regulation and inhibition. J. Antibiot. 2018, 71, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ouzounian, M.; de Couto, G.; Chen, M.Y.; Yan, R.; Fukuoka, M.; Li, G.H.; Moon, M.; Liu, Y.; Gramolini, A.; et al. Cathepsin-l ameliorates cardiac hypertrophy through activation of the autophagy-lysosomal dependent protein processing pathways. J. Am. Heart Assoc. 2013, 2, e000191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.A.; Li, S.J.; Wu, H.J. Ubiquitination-proteasome system (U.S) and autophagy two main protein degradation machineries in response to cell stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef]
- Jo, S.; Lockridge, A.; Alejandro, E.U. eIF4G1 and carboxypeptidase E axis dysregulation in O-GlcNAc transferase- deficient pancreatic β-cells contributes to hyperproinsulinemia in mice. J. Biol. Chem. 2019, 294, 13040–13050. [Google Scholar] [CrossRef]
- Petrera, A.; Kern, U.; Linz, D.; Gomez-Auli, A.; Hohl, M.; Gassenhuber, J.; Sadowski, T.; Schilling, O. Proteomic profiling of cardiomyocyte-specific cathepsin a overexpression links cathepsin a to the oxidative stress response. J. Proteome Res. 2016, 15, 3188–3195. [Google Scholar] [CrossRef] [PubMed]
- Kachariya, N.N.; Dantu, S.C.; Kumar, A. Backbone and side chain assignments of human cell cycle regulatory protein S-phase kinase-associated protein 1. Biomol. NMR Assign. 2016, 10, 351–355. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Busino, L. The biology of f-box proteins: The SCF family of e3 ubiquitin ligases. Adv. Exp. Med. Biol. 2020, 1217, 111–122. [Google Scholar] [CrossRef]
- Janson, C.; Kasahara, N.; Prendergast, G.C.; Colicelli, J. Rin3 is a negative regulator of mast cell responses to SCF. PLoS ONE 2012, 7, e49615. [Google Scholar] [CrossRef]
- Molfetta, R.; Lecce, M.; Milito, N.D.; Putro, E.; Pietropaolo, G.; Marangio, C.; Scarno, G.; Moretti, M.; De Smaele, E.; Santini, T.; et al. SCF and IL-33 regulate mouse mast cell phenotypic and functional plasticity supporting a pro-inflammatory microenvironment. Cell Death Dis. 2023, 14, 616. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Rees, J.C.; Parks, B.A.; Andrews, M.; Gardner, M.; Grigorutsa, E.; Kuklenyik, Z.; Pirkle, J.L.; Barr, J.R. Integrated quantitative targeted lipidomics and proteomics reveal unique fingerprints of multiple metabolic conditions. Biomolecules 2022, 12, 1439. [Google Scholar] [CrossRef]
- Mach, M.; Plachta, L.; Wydro, P. Study of the correlation between the structure of selected triester of phosphatidylcholine and their impact on physicochemical properties of model mammalian membranes. BBA-Biomembranes 2024, 1866, 184254. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport. Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kawano, S.; Endo, T. Lipid homeostasis in mitochondria. Biol. Chem. 2020, 401, 821–833. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ren, W.; Li, Z.; Li, L.; Wang, R.; Ma, S.; Zeng, Y.; Meng, J.; Yao, X. Plasma Lipidomics and Proteomics Analyses Pre- and Post-5000 m Race in Yili Horses. Animals 2025, 15, 994. https://doi.org/10.3390/ani15070994
Wang J, Ren W, Li Z, Li L, Wang R, Ma S, Zeng Y, Meng J, Yao X. Plasma Lipidomics and Proteomics Analyses Pre- and Post-5000 m Race in Yili Horses. Animals. 2025; 15(7):994. https://doi.org/10.3390/ani15070994
Chicago/Turabian StyleWang, Jianwen, Wanlu Ren, Zexu Li, Luling Li, Ran Wang, Shikun Ma, Yaqi Zeng, Jun Meng, and Xinkui Yao. 2025. "Plasma Lipidomics and Proteomics Analyses Pre- and Post-5000 m Race in Yili Horses" Animals 15, no. 7: 994. https://doi.org/10.3390/ani15070994
APA StyleWang, J., Ren, W., Li, Z., Li, L., Wang, R., Ma, S., Zeng, Y., Meng, J., & Yao, X. (2025). Plasma Lipidomics and Proteomics Analyses Pre- and Post-5000 m Race in Yili Horses. Animals, 15(7), 994. https://doi.org/10.3390/ani15070994





