Clinical, Histological and Genetic Characterisation of a Disorder of Sexual Development in a Pygmy Goat
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material
2.2. Diagnostic Imaging
2.3. Surgical and Histological Examination
2.4. Samples, DNA Extraction and Sequencing
2.5. Illumina Read Mapping
2.6. Determination of Karyotype from Sequence Data
2.7. SRY Promotor Mutation
3. Results
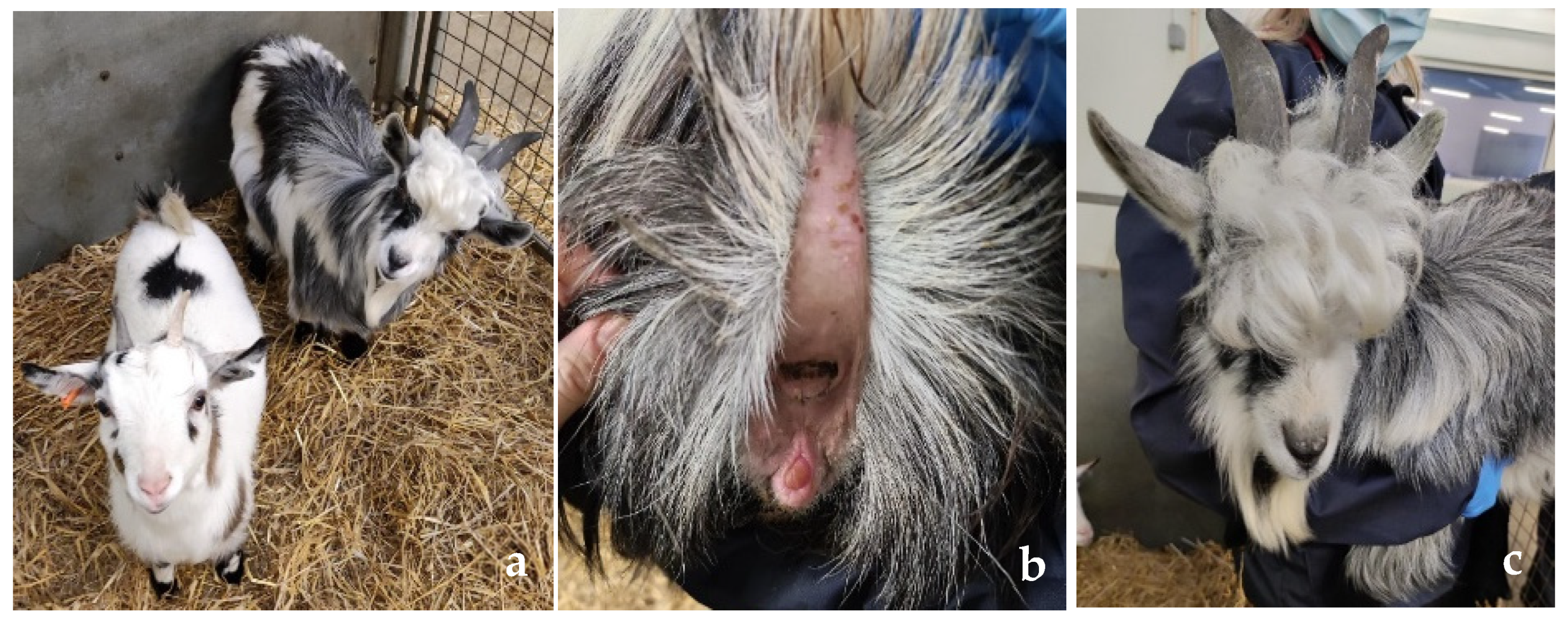
3.1. Clinical Phenotype
3.2. Diagnostic Imaging
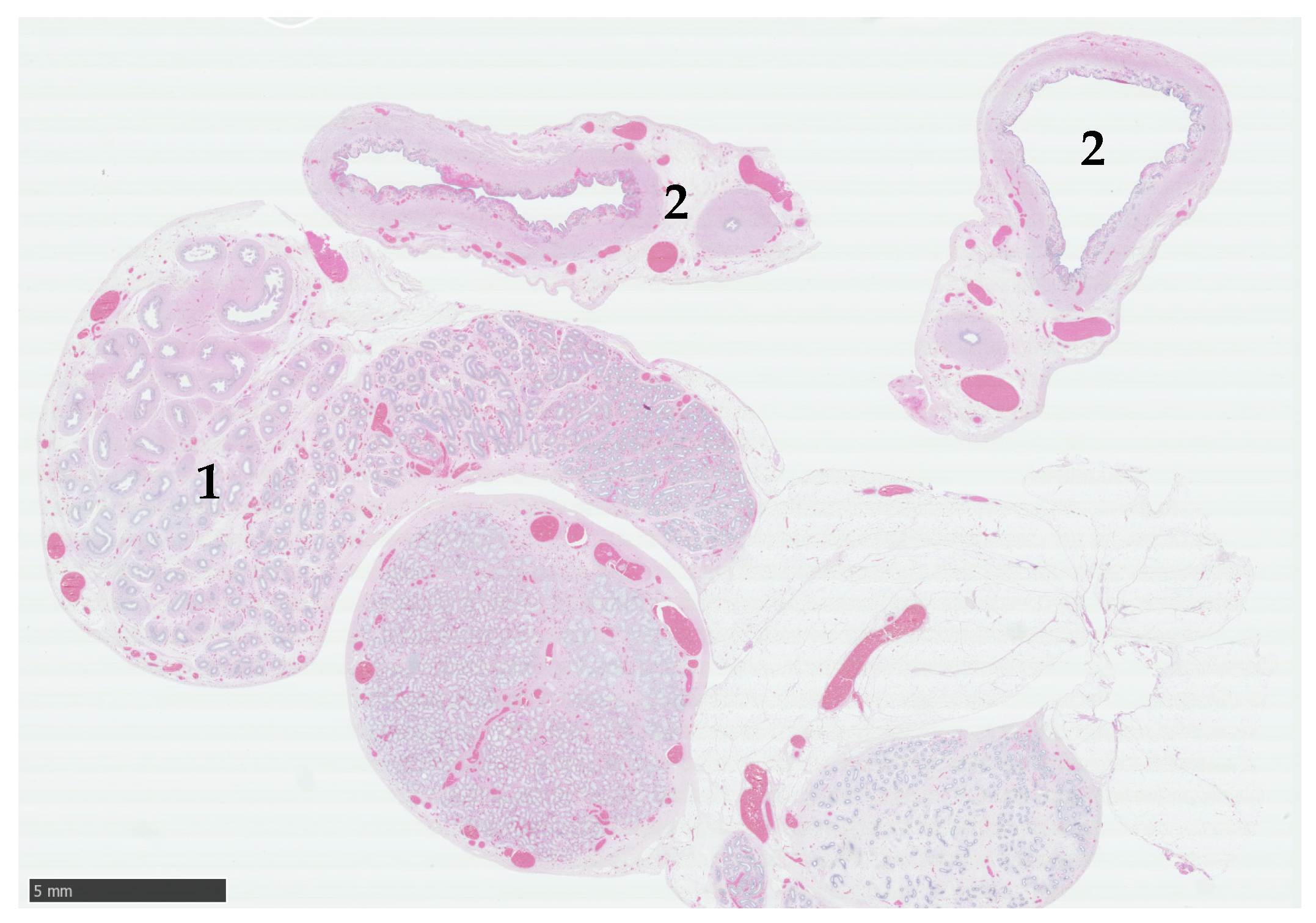
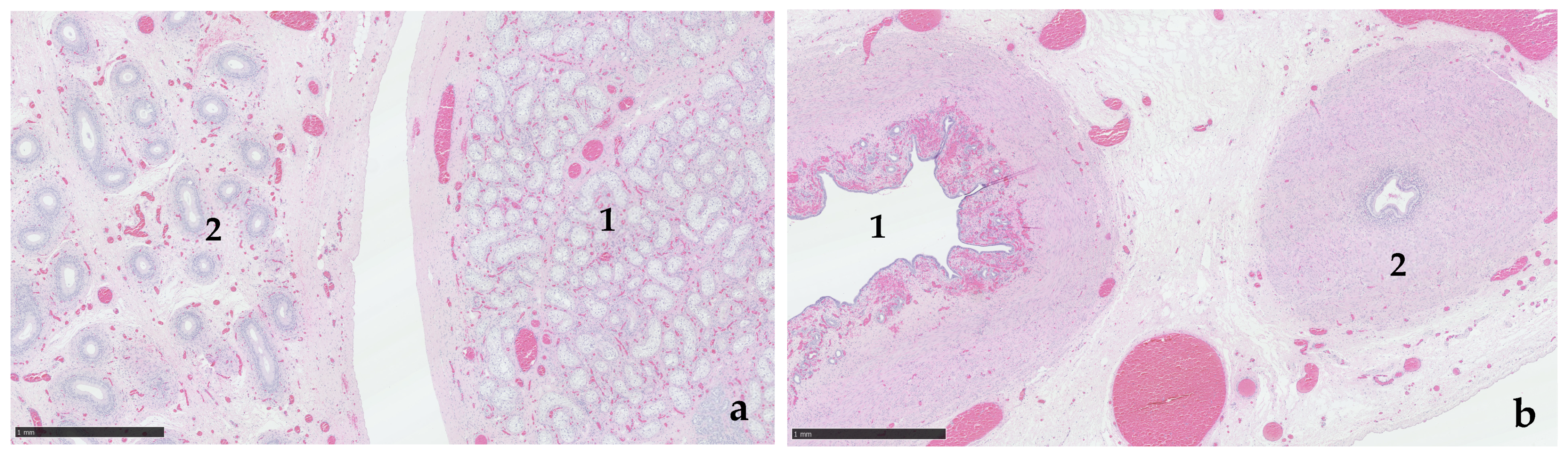
3.3. Exploratory Laparotomy and Histological Examination
3.4. Blood Testosterone Concentrations
3.5. Illumina Sequencing Read Mapping
3.6. SRY Promotor Mutation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, I.A. Consensus statement on management of intersex disorders. Arch. Dis. Child. 2005, 91, 554–563. [Google Scholar] [CrossRef]
- Poth, T.; Breuer, W.; Walter, B.; Hecht, W.; Hermanns, W. Disorders of sex development in the dog—Adoption of a new nomenclature and reclassification of reported cases. Anim. Reprod. Sci. 2010, 121, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rothkopf, A.C.; John, R.M. Understanding Disorders of Sexual Development. J. Pediatr. Nurs. 2014, 29, e23–e34. [Google Scholar] [CrossRef]
- Yang, S.; Han, H.; Li, J.; Zhang, Y.; Zhao, J.; Wei, H.; Hasi, T.; Lv, H.; Zhao, X.; Quan, K. Transcriptomic analysis of gene expression in normal goat ovary and intersex goat gonad. Reprod. Domest. Anim. 2021, 56, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Lischer, H.E.L.; Pieńkowska-Schelling, A.; Keller, I.; Häfliger, I.M.; Letko, A.; Schelling, C.; Lühken, G.; Drögemüller, C. New genomic features of the polled intersex syndrome variant in goats unraveled by long-read whole-genome sequencing. Anim. Genet. 2020, 51, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.-P.; Vaiman, D.; Vigier, B.; Servel, N.; Cribiu, E.P.; Cotinot, C.; Grosclaude, F.; Pailhoux, E.; Taourit, S.; Chaffaux, S.; et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 2001, 29, 453–458. [Google Scholar] [CrossRef]
- Boulanger, L.; Pannetier, M.; Gall, L.; Allais-Bonnet, A.; Elzaiat, M.; Le Bourhis, D.; Daniel, N.; Richard, C.; Cotinot, C.; Ghyselinck, N.B.; et al. FOXL2 Is a Female Sex-Determining Gene in the Goat. Curr. Biol. 2014, 24, 404–408. [Google Scholar] [CrossRef]
- Vallenzasca, C.; Galli, A. Cytogenetical and histopathologic study of two cases of polled goats. Andrologia 1990, 22, 289–290. [Google Scholar] [CrossRef]
- Rychlik, T.; Kozubska-Sobocinska, A.; Rejduch, B.; Sikora, J. The phenomenon of cell chimerism in goats. Vet. Med. 2005, 50, 311–314. [Google Scholar] [CrossRef]
- Swyer, G.I.M. Male Pseudohermaphroditism: A Hitherto Undescribed Form. Br. Med. J. 1955, 2, 709–712. [Google Scholar] [CrossRef]
- Berry, D.P.; Herman, E.K.; Carthy, T.R.; Jennings, R.; Bandi-Kenari, N.; O’Connor, R.E.; Mee, J.F.; O’Donovan, J.; Mathews, D.; Stothard, P. Characterisation of eight cattle with Swyer syndrome by whole-genome sequencing. Anim. Genet. 2023, 54, 93–103. [Google Scholar] [CrossRef]
- Chapman, H.M.; Bruere, A.N.; Jaine, P.M. XY gonadal dysgenesis in a Charolais heifer. Anim. Reprod. Sci. 1978, 1, 9–18. [Google Scholar] [CrossRef]
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Incarnato, D.; Palo, R.D.; Zicarelli, L. Reproductive disturbances and sex chromosome abnormalities in two female river buffaloes. Vet. Rec. 2004, 154, 823–824. [Google Scholar] [CrossRef]
- Ferrer, L.M.; Monteagudo, L.V.; García de Jalón, J.A.; Tejedor, M.T.; Ramos, J.J.; Lacasta, D. A Case of Ovine Female XY Sex Reversal Syndrome Not Related to Anomalies in the Sex-Determining Region Y (SRY). Cytogenet. Genome Res. 2009, 126, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Rahbaran, M.; Mirzaei, A.; Tabatabaii, M.; Shokrpoor, S.; Mahjoubi, F.; Ara, M.S.; Akbarinejhad, V.; Gharagozloo, F. The study of a hermaphroditic sheep caused by a mutation in the promoter of SRY gene. Vet. Anim. Sci. 2023, 21, 100308. [Google Scholar] [CrossRef] [PubMed]
- King, T.F.J.; Conway, G.S. Swyer syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 504–510. [Google Scholar] [CrossRef]
- Muriuki, C.; Bush, S.J.; Salavati, M.; McCulloch, M.E.B.; Lisowski, Z.M.; Agaba, M.; Djikeng, A.; Hume, D.A.; Clark, E.L. A Mini-Atlas of Gene Expression for the Domestic Goat (Capra hircus). Front. Genet. 2019, 10, 1080. [Google Scholar] [CrossRef]
- Johnsson, M.; Hickey, J.M.; Jungnickel, M.K. Building in vitro tools for livestock genomics: Chromosomal variation within the PK15 cell line. BMC Genom. 2024, 25, 49. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 3 July 2024).
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Toolkit, P. Picard Toolkit. Available online: https://broadinstitute.github.io/picard/ (accessed on 3 July 2024).
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Quinlan, A.R.; Hancock, J. Mosdepth: Quick coverage calculation for genomes and exomes. Bioinformatics 2018, 34, 867–868. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Polat, H.; Dellal, G.; Baritci, I.; Pehlivan, E. Annual Change of the Testosterone Hormone in Male White Goats. Agric. Sci. China 2011, 10, 312–316. [Google Scholar] [CrossRef]
- Singh, P. Testicular Feminizing Syndrome to Androgen Insensitivity Syndrome—A Walk Through Half a Century. Indian Pediatr. Case Rep. 2021, 1, 77–79. [Google Scholar] [CrossRef]
- Alanazi, A.B.; Aldhowayan, A.; Almuhanna, M.M.; Alghamdi, A.M. Persistent Mullerian duct syndrome (PMDS): Case report and review of literature. Urol. Case Rep. 2022, 42, 102031. [Google Scholar] [CrossRef]
- Smit, M.M.; Ekenstedt, K.; Minor, K.; Lim, C.; Leegwater, P.A.J.; Furrow, E. Prevalence of the AMHR2 mutation in Miniature Schnauzers and genetic investigation of a Belgian Malinois with persistent Müllerian duct syndrome. Reprod. Domest. Anim. 2017, 53, 371–376. [Google Scholar] [CrossRef]
- Haibel, G.K.; Rojko, J.L. Persistent müllerian duct syndrome in a goat. Vet. Pathol. 1990, 27, 135–137. [Google Scholar] [CrossRef]
- She, Z.-Y.; Yang, W.-X. Sry and SoxE genes: How they participate in mammalian sex determination and gonadal development? Semin. Cell Dev. Biol. 2017, 63, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Rios, S.; Mazzaro Monteiro, I.C.; Gonçalves Braz dos Santos, L.; Garcia Caldas, N.; Rios Chen, A.C.; Rios Chen, J.; Spindola Camargo Silva, H. A Case of Swyer Syndrome Associated with Advanced Gonadal Dysgerminoma Involving Long Survival. Case Rep. Oncol. 2015, 8, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, T.; Durkin, K.; Lear, T.L.; Das, P.J.; Avila, F.; Kachroo, P.; Chowdhary, B.P. Molecular heterogeneity of XY sex reversal in horses. Anim. Genet. 2010, 41, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, W.V.; Munro, I.B.; Wilkes, P.R. Heifer sterility associated with single-birth freemartinism. Vet. Rec. 1977, 100, 333–336. [Google Scholar] [CrossRef]
- Denoyelle, L.; Talouarn, E.; Bardou, P.; Colli, L.; Alberti, A.; Danchin, C.; Del Corvo, M.; Engelen, S.; Orvain, C.; Palhière, I.; et al. VarGoats project: A dataset of 1159 whole-genome sequences to dissect Capra hircus global diversity. Genet. Sel. Evol. 2021, 53, 86. [Google Scholar] [CrossRef]
- Consortium, V.; Nijman, I.J.; Rosen, B.D.; Bardou, P.; Faraut, T.; Cumer, T.; Daly, K.G.; Zheng, Z.; Cai, Y.; Asadollahpour, H.; et al. Geographical contrasts of Y-chromosomal haplogroups from wild and domestic goats reveal ancient migrations and recent introgressions. Mol. Ecol. 2022, 31, 4364–4380. [Google Scholar] [CrossRef]
- Binz, R.L.; Burns, K.; Pathak, R. Protocol for preparation and staining of chromosomes isolated from mouse and human tissues for conventional and molecular cytogenetic analysis. STAR Protoc. 2024, 5, 102897. [Google Scholar] [CrossRef]
- Braudeau, C.; Salabert-Le Guen, N.; Chevreuil, J.; Rimbert, M.; Martin, J.C.; Josien, R. An easy and reliable whole blood freezing method for flow cytometry immuno-phenotyping and functional analyses. Cytom. Part B Clin. Cytom. 2021, 100, 652–665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque Castro, A.; Marr, M.M.; Clark, E.L.; Poldy, J.; Liu, L.; Daniel, C.; Malbon, A.; Kelly, R.; Murdoch, F.; Macrae, A.; et al. Clinical, Histological and Genetic Characterisation of a Disorder of Sexual Development in a Pygmy Goat. Animals 2025, 15, 976. https://doi.org/10.3390/ani15070976
Luque Castro A, Marr MM, Clark EL, Poldy J, Liu L, Daniel C, Malbon A, Kelly R, Murdoch F, Macrae A, et al. Clinical, Histological and Genetic Characterisation of a Disorder of Sexual Development in a Pygmy Goat. Animals. 2025; 15(7):976. https://doi.org/10.3390/ani15070976
Chicago/Turabian StyleLuque Castro, Alberto, Melissa M. Marr, Emily L. Clark, Jacqueline Poldy, Lily Liu, Carola Daniel, Alexandra Malbon, Robert Kelly, Fraser Murdoch, Alastair Macrae, and et al. 2025. "Clinical, Histological and Genetic Characterisation of a Disorder of Sexual Development in a Pygmy Goat" Animals 15, no. 7: 976. https://doi.org/10.3390/ani15070976
APA StyleLuque Castro, A., Marr, M. M., Clark, E. L., Poldy, J., Liu, L., Daniel, C., Malbon, A., Kelly, R., Murdoch, F., Macrae, A., & Sargison, N. (2025). Clinical, Histological and Genetic Characterisation of a Disorder of Sexual Development in a Pygmy Goat. Animals, 15(7), 976. https://doi.org/10.3390/ani15070976





